Alternative Methods to Animal Testing for the Safety Evaluation of Cosmetic Ingredients: An Overview
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Acute Toxicity
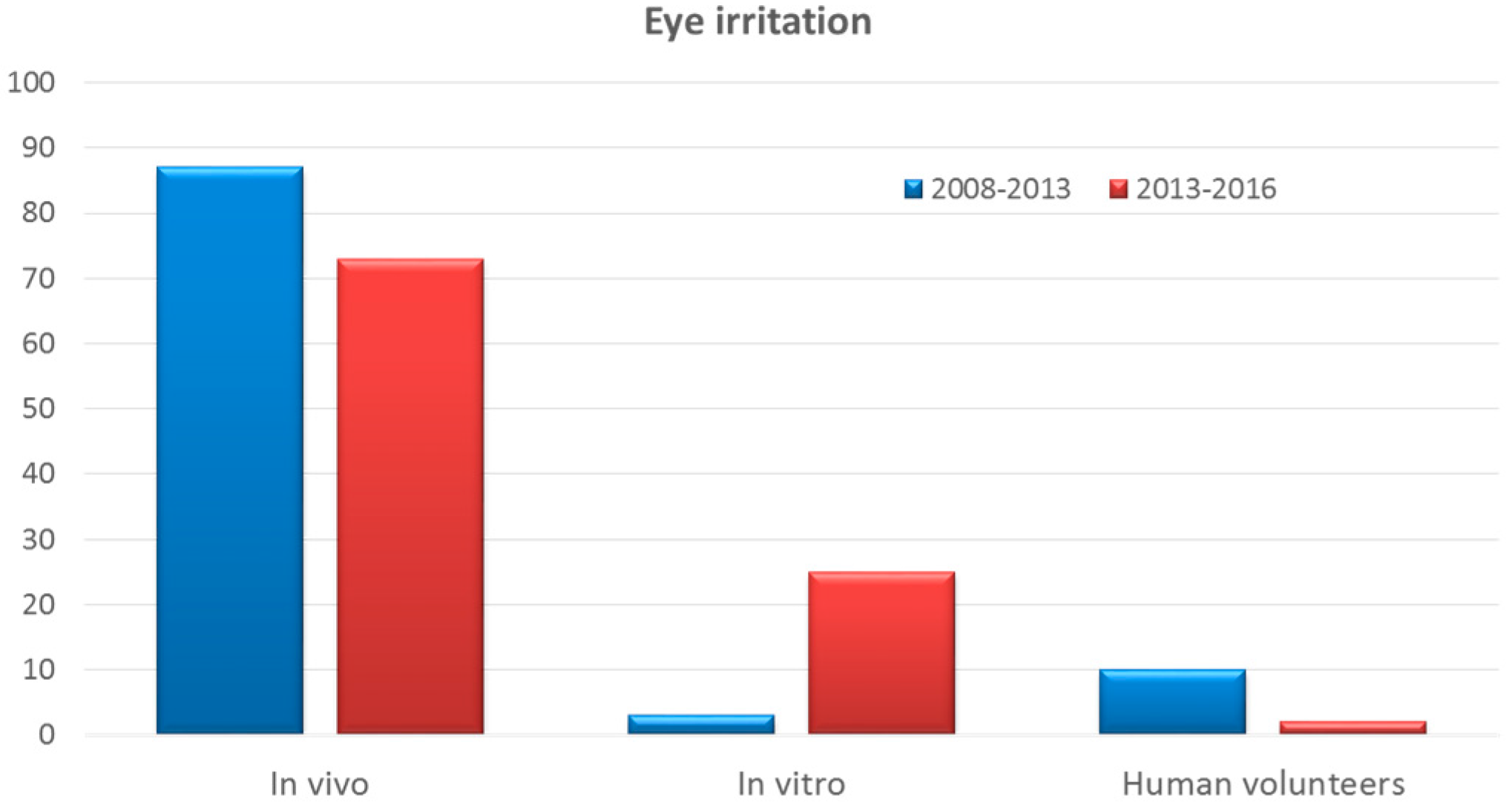
3.2. Eye Irritation
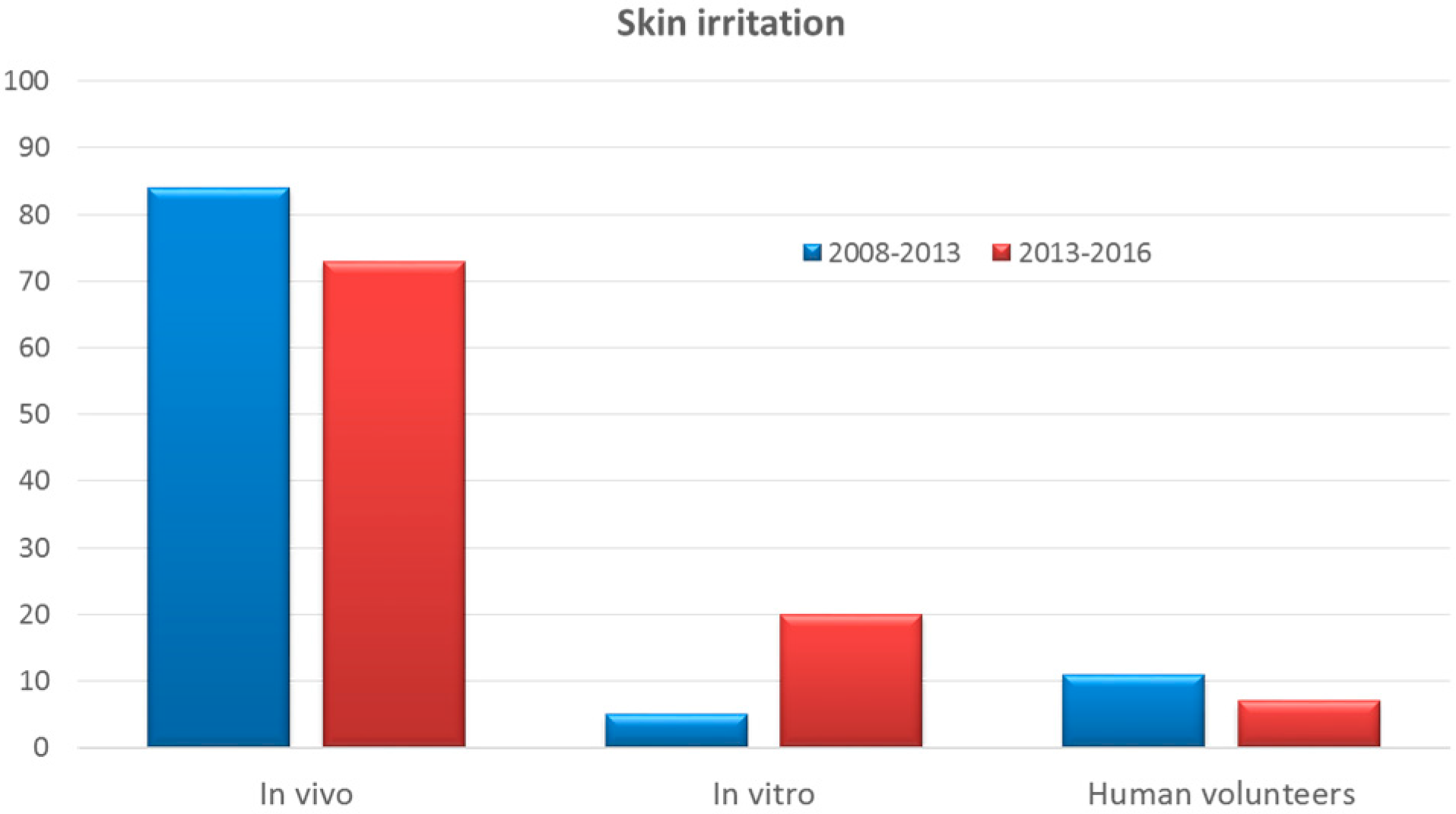
3.3. Skin Irritation
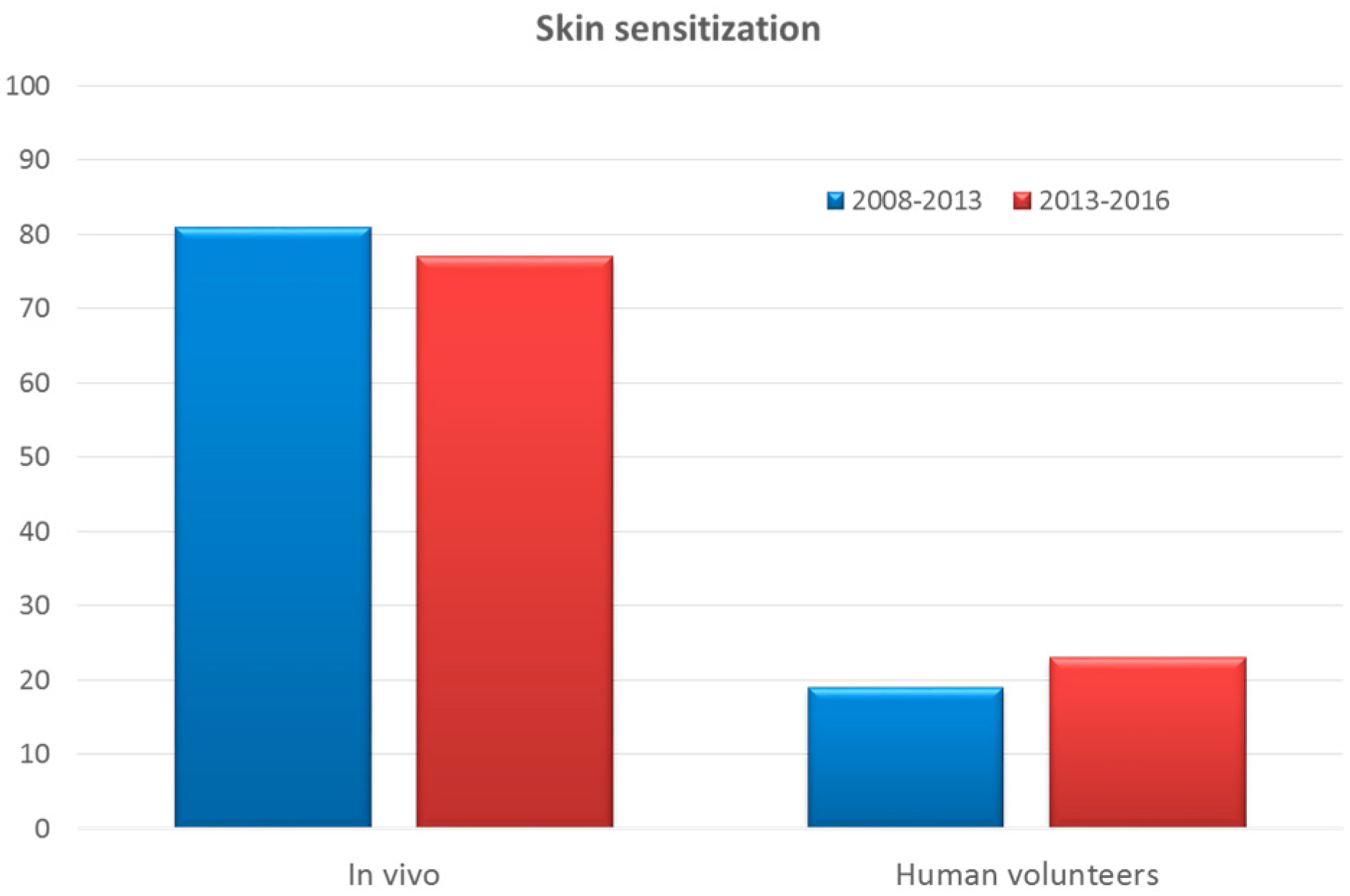
3.4. Skin Sensitization
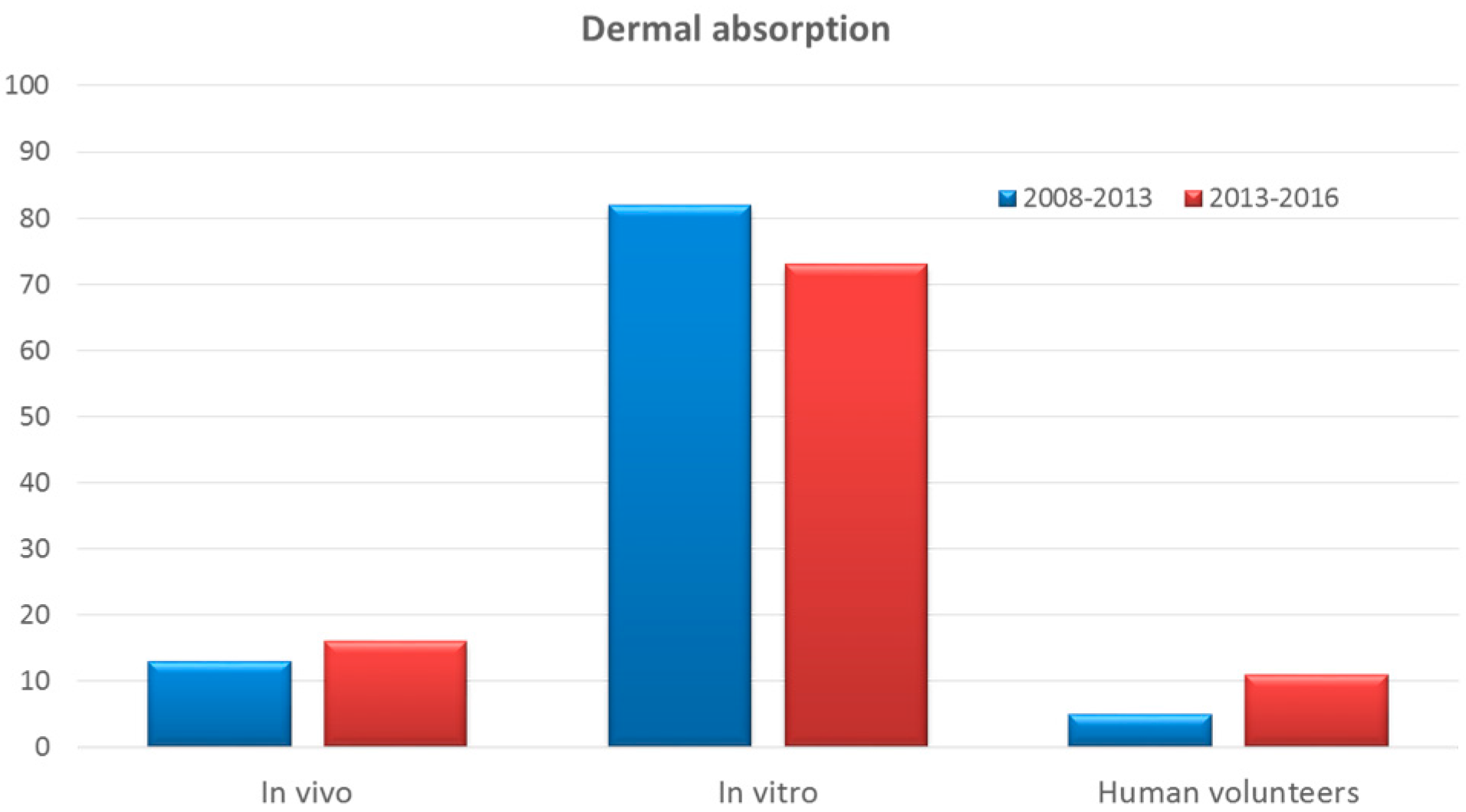
3.5. Dermal Absorption
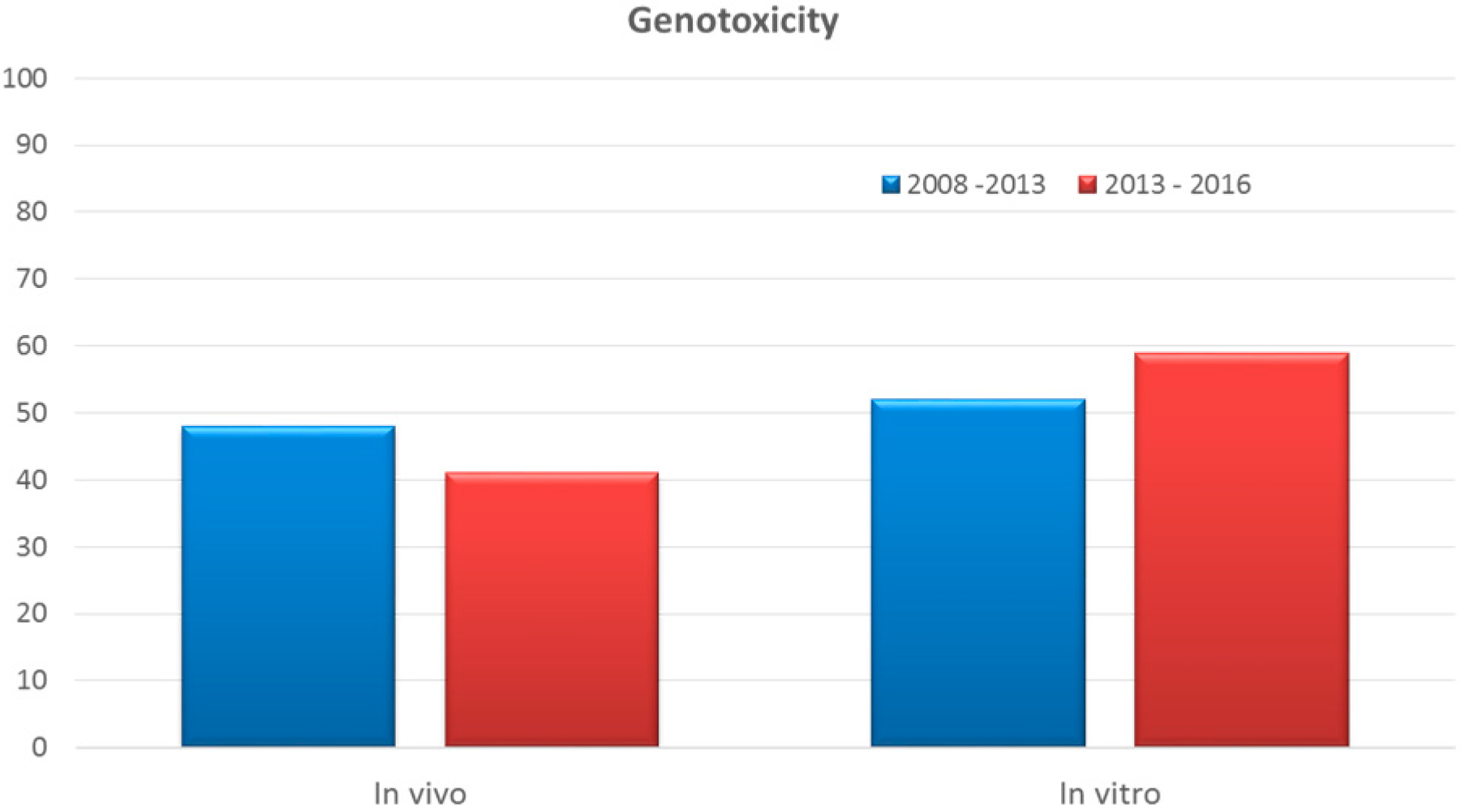
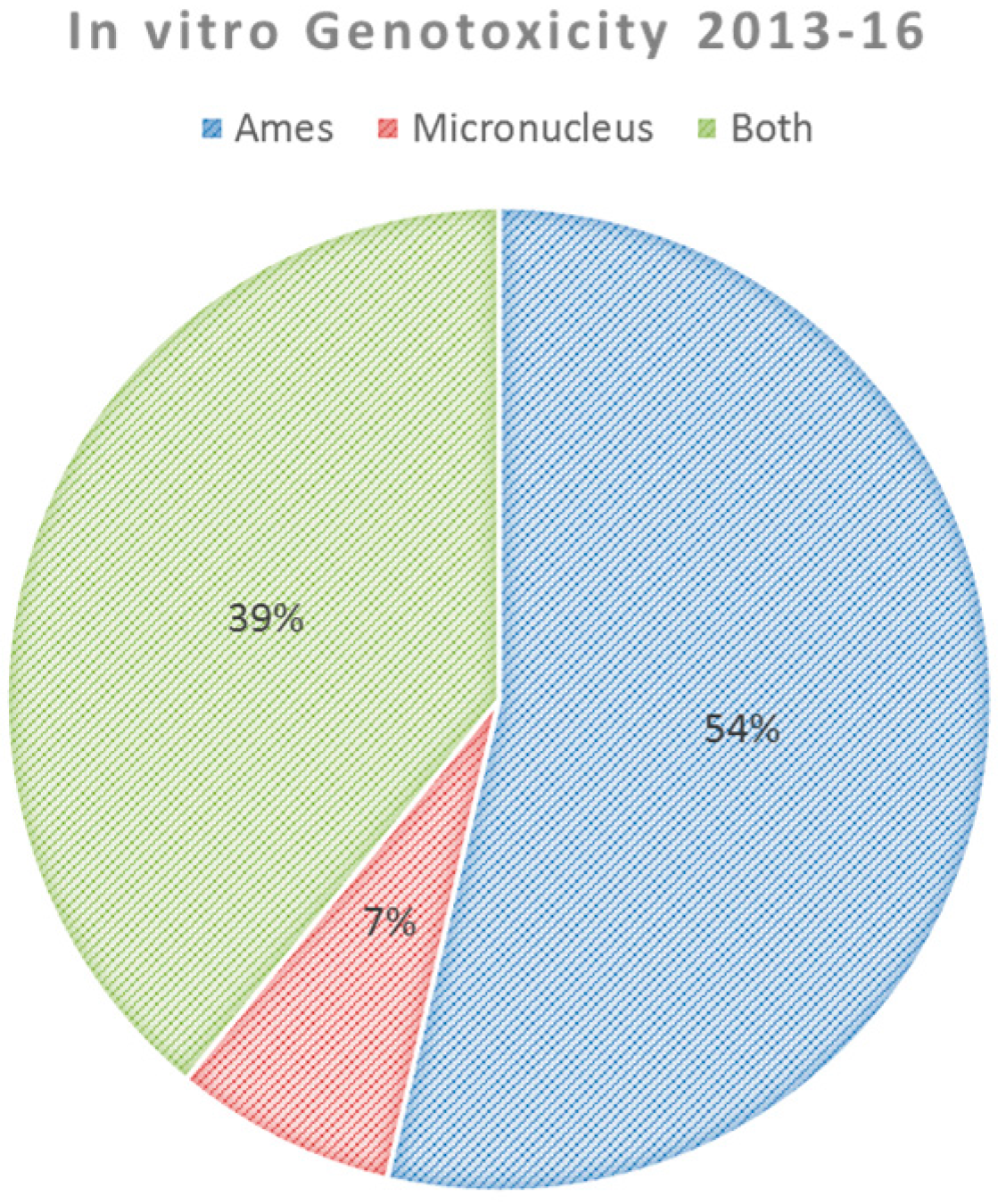
3.6. Genotoxicity
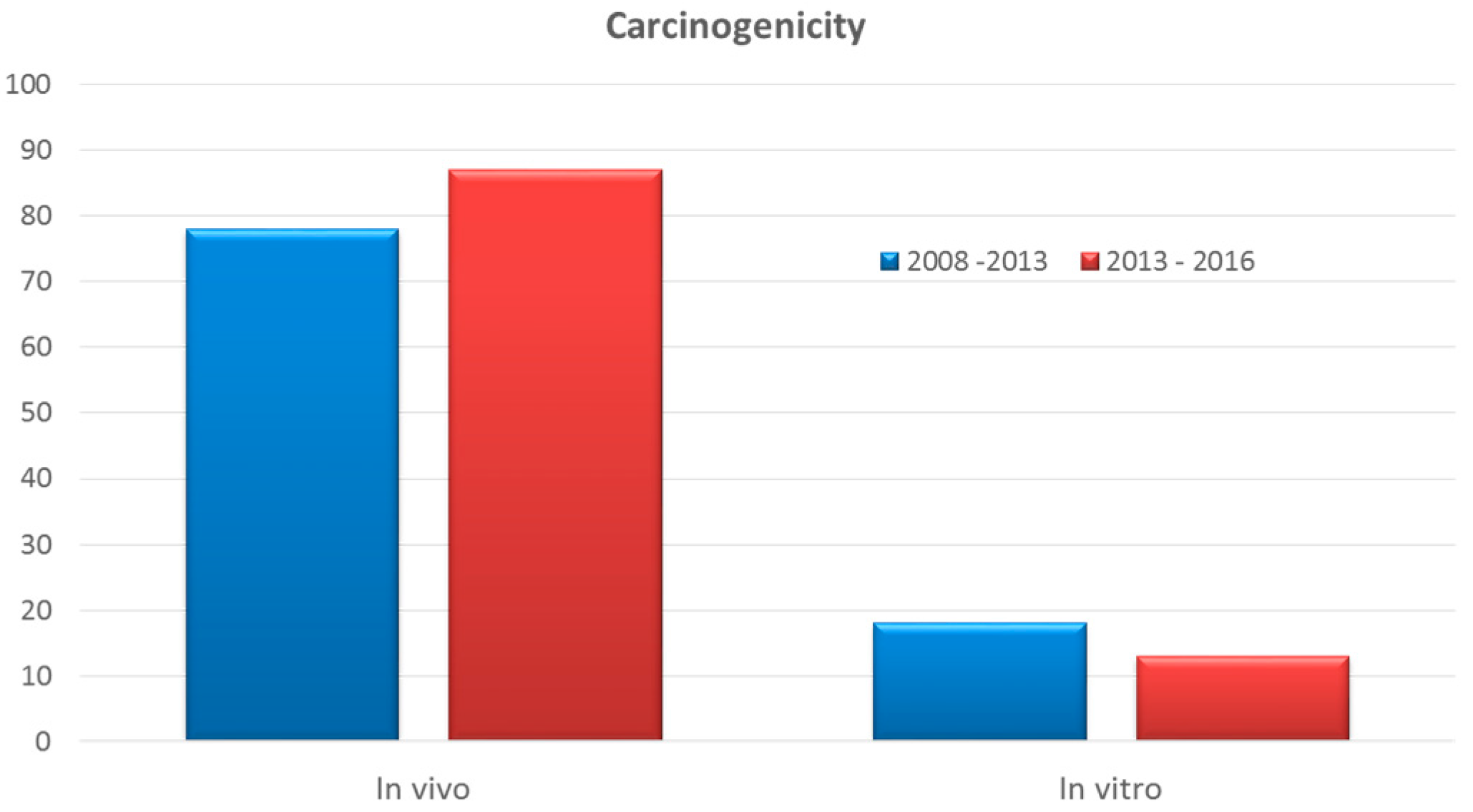
3.7. Carcinogenicity
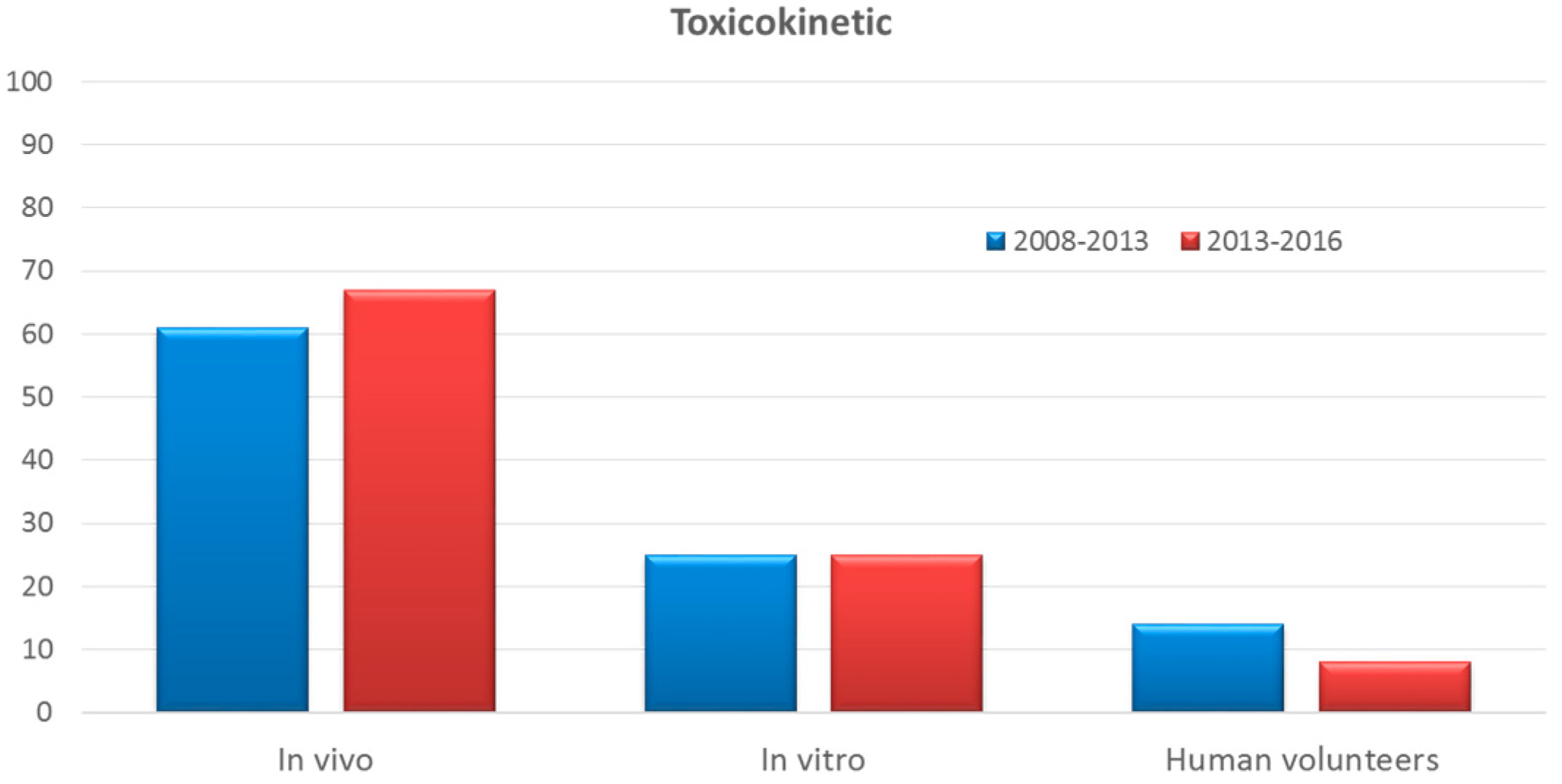
3.8. Toxicokinetic Studies
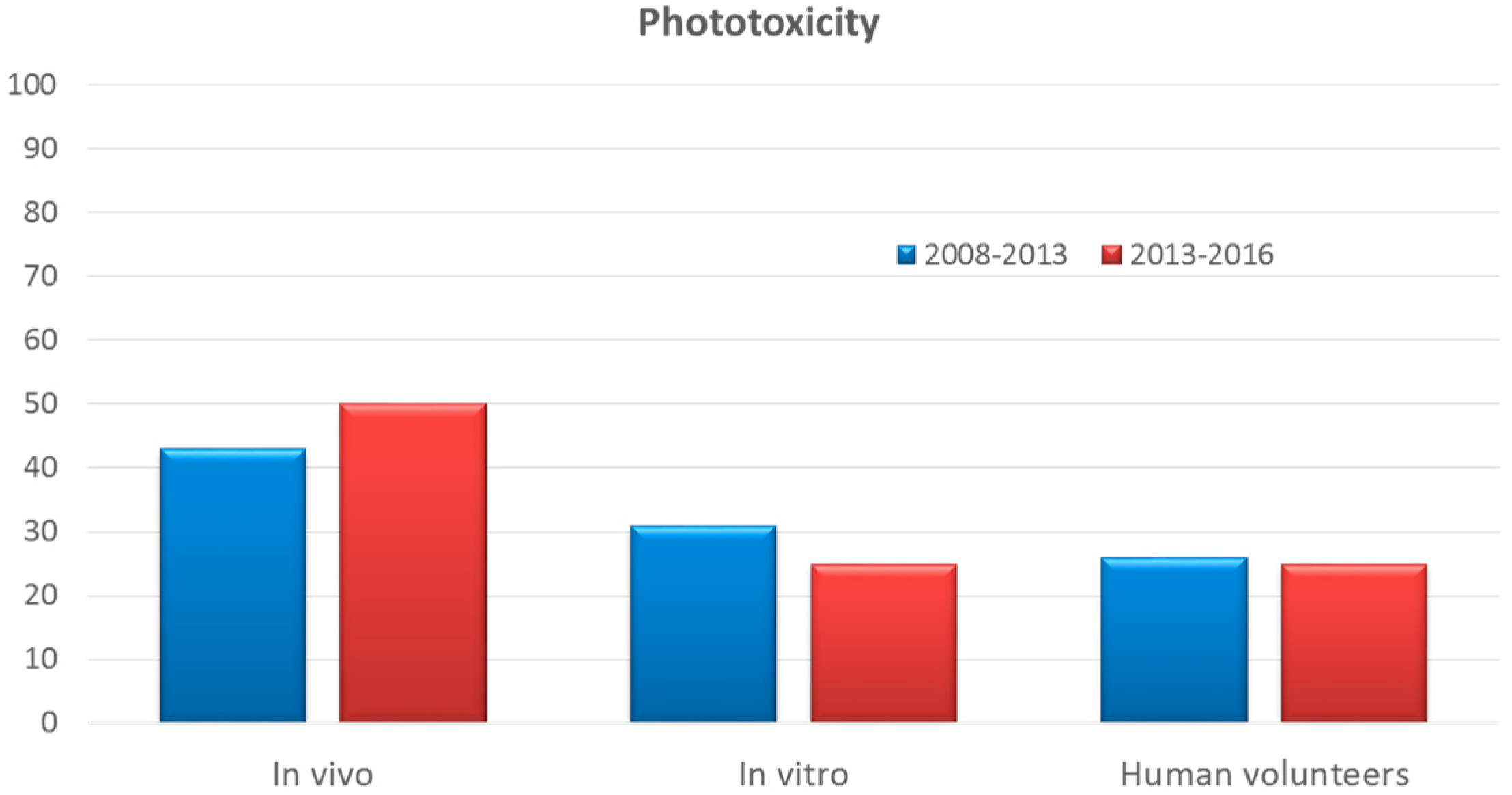
3.9. Phototoxicity
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Vinardell, M.P. The use of non-animal alternatives in the safety evaluations of cosmetics ingredients by the Scientific Committee on Consumer Safety (SCCS). Regul. Toxicol. Pharmacol. 2015, 71, 198–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF (accessed on 18 May 2017).
- Prieto, P.; Cole, T.; Curren, R.; Gibson, R.M.; Liebsch, M.; Raabe, H.; Tuomainen, A.M.; Whelan, M.; Kinsner-Ovaskainen, A. Assessment of the predictive capacity of the 3T3 Neutral Red Uptake cytotoxicity test method to identify substances not classified for acute oral toxicity (LD50 > 2000 mg/kg): Results of an ECVAM validation study. Regul. Toxicol. Pharmacol. 2013, 65, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Test No. 405: Acute Eye Irritation/Corrosion. Available online: http://dx.doi.org/10.1787/9789264185333-en (accessed on 18 May 2017).
- Draize, J.H.; Woodward, G.; Calvery, H.O. Method for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Vinardell, M.P.; Mitjans, M. Alternative methods for eye and skin irritation tests: An overview. J. Pharm. Sci. 2008, 97, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Test No. 438: Isolated Chicken Eye Test Method for Identifying i) Chemicals Inducing Serious Eye Damage and ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. Available online: http://dx.doi.org/10.1787/9789264203860-en (accessed on 18 May 2017).
- Test No. 437: Bovine Corneal Opacity and Permeability Test Method for Identifying i) Chemicals Inducing Serious Eye Damage and ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. Available online: http://dx.doi.org/10.1787/9789264203846-en (accessed on 18 May 2017).
- Jírová, D.; Kejlová, K.; Janoušek, S.; Bendová, H.; Malý, M.; Kolářová, H.; Dvořáková, M. Eye irritation hazard of chemicals and formulations assessed by methods in vitro. Neuro Endocrinol. Lett. 2014, 35, 133–140. [Google Scholar] [PubMed]
- Test No. 492: Reconstructed human Cornea-like Epithelium (RhCE) test method for identifying chemicals not requiring classification and labelling for eye irritation or serious eye damage. Available online: http://dx.doi.org/10.1787/9789264242548-en (accessed on 20 May 2017).
- McNamee, P.; Hibatallah, J.; Costabel-Farkas, M.; Goebel, C.; Araki, D.; Dufour, E.; Hewitt, N.J.; Jones, P.; Kirst, A.; Le Varlet, B.; et al. A tiered approach to the use of alternatives to animal testing for the safety assessment of cosmetics: Eye irritation. Regul. Toxicol. Pharmacol. 2009, 54, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, E.; Barroso, J.; Eskes, C.; Hoffmann, S.; McNamee, P.; Alépée, N.; Bessou-Touya, S.; De Smedt, A.; De Wever, B.; Pfannenbecker, U.; et al. Retrospective analysis of the Draize test for serious eye damage/eye irritation: Importance of understanding the in vivo endpoints under UN GHS/EU CLP for the development and evaluation of in vitro test methods. Arch. Toxicol. 2014, 88, 701–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, J.; Pfannenbecker, U.; Adriaens, E.; Alépée, N.; Cluzel, M.; De Smedt, A.; Hibatallah, J.; Klaric, M.; Mewes, K.R.; Millet, M.; et al. Cosmetics Europe compilation of historical serious eye damage/eye irritation in vivo data analysed by drivers of classification to support the selection of chemicals for development and evaluation of alternative methods/strategies: The Draize eye test Reference Database (DRD). Arch. Toxicol. 2017, 91, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Test No. 404: Acute Dermal Irritation/Corrosion. Available online: http://dx.doi.org/10.1787/9789264070622-en (accessed on 24 May 2017).
- Test No. 430: In Vitro Skin Corrosion: Transcutaneous Electrical Resistance Test Method (TER). Available online: http://dx.doi.org/10.1787/9789264203808-en (accessed on 24 May 2017).
- Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. Available online: http://dx.doi.org/10.1787/9789264242845-en (accessed on 24 May 2017).
- SCCS/1392/10, SCCS (Scientific Committee on Consumer Safety), Memorandum (addendum) on the in vitro test EPISKIN™ for skin irritation testing, 14 December 2010.
- Alépée, N.; Barroso, J.; De Smedt, A.; De Wever, B.; Hibatallah, J.; Klaric, M.; Mewes, K.R.; Millet, M.; Pfannenbecker, U.; Tailhardat, M.; et al. Use of HPLC/UPLC-spectrophotometry for detection of formazan in in vitro Reconstructed human Tissue (RhT)-based test methods employing the MTT-reduction assay to expand their applicability to strongly coloured test chemicals. Toxicol. In Vitro 2015, 29, 741–761. [Google Scholar] [CrossRef]
- Test No. 431: In Vitro Skin Corrosion: Reconstructed Human Epidermis (RHE) Test Method. Available online: http://dx.doi.org/10.1787/9789264242753-en (accessed on 24 May 2017).
- Macfarlane, M.; Jones, P.; Goebel, C.; Dufour, E.; Rowland, J.; Araki, D.; Costabel-Farkas, M.; Hewitt, N.J.; Hibatallah, J.; Kirst, A.; et al. A tiered approach to the use of alternatives to animal testing for the safety assessment of cosmetics: Skin irritation. Regul. Toxicol. Pharmacol. 2009, 54, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zug, K.A. Fragrance allergic contact dermatitis. Dermatitis 2014, 25, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Bensefa-Colas, L.; Frosch, P.; Giménez-Arnau, A.; John, S.M.; Lepoittevin, J.P.; Lidén, C.; White, I.R.; Duus Johansen, J. Patch testing with hair cosmetic series in Europe: A critical review and recommendation. Contact Dermat. 2015, 73, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Lidén, C.; Yazar, K.; Johansen, J.D.; Karlberg, A.T.; Uter, W.; White, I.R. Comparative sensitizing potencies of fragrances, preservatives, and hair dyes. Contact Dermat. 2016, 75, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, L.; Goossens, A. Cosmetic components causing contact urticaria: A review and update. Contact Dermat. 2016, 75, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Test No. 442B: Skin Sensitization: Local Lymph Node Assay: BrdU-ELISA. Available online: http://dx.doi.org/10.1787/9789264090996-en (accessed on 30 May 2017).
- Test No. 406: Skin Sensitisation. Available online: http://dx.doi.org/10.1787/9789264070660-en (accessed on 30 May 2017).
- Test No. 442E: In Vitro Skin Sensitisation: Human Cell Line Activation Test (h-CLAT). Available online: http://dx.doi.org/10.1787/9789264264359-en (accessed on 30 May 2017).
- Ashikaga, T.; Yoshida, Y.; Hirota, M.; Yoneyama, K.; Itagaki, H.; Sakaguchi, H.; Miyazawa, M.; Ito, Y.; Suzuki, H.; Toyoda, H. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT) I. Optimization of the h-CLAT protocol. Toxicol. In Vitro 2006, 20, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Test No. 442C: In Chemico Skin Sensitisation: Direct Peptide Reactivity Assay (DPRA). Available online: http://dx.doi.org/10.1787/9789264229709-en (accessed on 30 May 2017).
- Farkas, M.; Hewitt, N.J.; Hibatallah, J.; Kirst, A.; McNamee, P.; Schellauf, F.; Scheel, J.; Gerberick, G.F.; Vassallo, J.D.; Balley, R.E.; Chaney, J.G.; Morrall, S.W.; Lepoittevin, J.P. Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci. 2004, 81, 332–343. [Google Scholar]
- Gerberick, G.F.; Vassallo, J.D.; Foertsch, L.M.; Price, B.B.; Chaney, J.G.; Lepoittevin, J.P. Quantification of chemical peptide reactivity for screening contact allergens: A classification tree model approach. Toxicol. Sci. 2007, 97, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Test No. 442D: In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method. Available online: http://dx.doi.org/10.1787/9789264229822-en (accessed on 30 May 2017).
- Natsch, A. The Nrf2-Keap1-ARE Toxicity Pathway as a Cellular Sensor for Skin Sensitizers-Functional Relevance and Hypothesis on Innate Reactions to Skin Sensitizers. Toxicol. Sci. 2010, 113, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Papale, A.; Galbiati, V.; Roggen, E.L. Safety Evaluation of Cosmetic Ingredients: In Vitro Opportunities for the Identification of Contact Allergens. Cosmetics 2014, 1, 61–74. [Google Scholar] [CrossRef]
- SCCS/1358/10 Basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients, 22 June 2010.
- Tay, S.L.; Heng, P.W.; Chan, L.W. An investigation of the chick Chorioallantoic membrane as an alternative model to various biological tissues for permeation studies. J. Pharm. Pharmacol. 2011, 63, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- SCCS/1564/15, revision of 25 April 2016. SCCS (Scientific Committee on Consumer Safety), SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation 9 th revision.
- Bouwstra, J.A.; Groenink, H.W.; Kempenaar, J.A.; Romeijn, S.G.; Ponec, M. Water distribution and natural moisturizer factor content in human skin equivalents are regulated by environmental relative humidity. J. Investig. Dermatol. 2008, 128, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Eckl, KM.; Weindl, G.; Ackermann, K.; Küchler, S.; Casper, R.; Radowski, M.R.; Haag, R.; Hennies, H.C.; Schäfer-Korting, M. Increased cutaneous absorption reflects impaired barrier function of reconstructed skin models mimicking keratinisation disorders. Exp. Dermatol. 2014, 23, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Ates, G.; Steinmetz, F.P.; Doktorova, T.Y.; Madden, J.C.; Rogiers, V. Linking existing in vitro dermal absorption data to physicochemical properties: Contribution to the desing of a weight-of-evidence approach for the safety evaluation of cosmetic ingredients with low dermal bioavailability. Regul. Toxicol. Pharmacol. 2016, 76, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Test No. 471: Bacterial Reverse Mutation Test. Available online: http://dx.doi.org/10.1787/9789264071247-en (accessed on 5 June 2017).
- Test No. 487: In Vitro Mammalian Cell Micronucleus Test. Available online: http://dx.doi.org/10.1787/9789264264861-en (accessed on 5 June 2017).
- SCCS/1532/14. SCCS (Scientific Committee on Consumer Safety), Addendum to the SCCS’s Notes of Guidance (NoG) for the Testing of Cosmetic Ingredients and their Safety Evaluation 8th Revision (SCCS/1501/12).
- Sasaki, Y.F.; Nakamura, T.; Kawaguchi, S. What is better experimental design for in vitro comet assay to detect chemical genotoxicity? AATEX 2007, 14, 499–504. [Google Scholar]
- Reus, A.A.; Reisinger, K.; Downs, T.R.; Carr, G.J.; Zeller, A.; Corvi, R.; Krul, C.A.; Pfuhler, S. Comet assay in reconstructed 3D human epidermal skin models—Investigation of intra- and inter-laboratory reproducibility with coded chemicals. Mutagenesis 2013, 28, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.E.; Thomas, A.D.; Wills, J.W.; Pfuhler, S.; Doak, S.H.; Jenkins, G.J. Automation and validation of micronucleus detection in the 3D EpiDerm™ human reconstructed skin assay and correlation with 2D dose responses. Mutagenesis 2014, 29, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Test No. 476: In Vitro Mammalian Cell Gene Mutation Tests using the Hprt and xprt genes. Available online: http://dx.doi.org/10.1787/9789264264809-en (accessed on 5 June 2017).
- Ates, G.; Doktorova, T.Y.; Pauwels, M.; Rogieres, V. Retrosptective analysis of the mutagenicity/genotoxicity data of the cosmetic ingredients present on the Annexes of the Cosmetic EU legislation (2000–12). Mutagenesis 2014, 29, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Test No. 451: Carcinogenicity Studies. Available online: http://dx.doi.org/10.1787/9789264071186-en (accessed on 5 June 2017).
- Lilienblum, W.; Dekant, W.; Foth, H.; Gebel, T.; Hengstler, J.G.; Kahl, R.; Kramer, P.J.; Schweinfurth, H.; Wollin, K.-M. Alternative methods to safety studies in experimental animals: role in the risk assessment of chemicals under the new European chemicals legislation (REACH). Arch. Toxicol. 2008, 82, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Sasaki, K.; Hayashi, K.; Muramatsu, D.; Arai, S.; Endou, N.; Kuroda, S.; Poth, A.; Bohnenberger, S.; Kunkelmann, T.; et al. An international validation study of a Bhas 42 cell transformation assay for the prediction of chemical carcinogenicity. Mutat. Res. 2011, 725, 57–77. [Google Scholar]
- HRI Draft Guidance Document. In Vitro Bhas 42 Cell Transformation Assay. Available online: http://www.oecd.org/env/ehs/testing/Bhas%2042%20CTA%20GD%20after%203rd%20comments-F_CLEAN.pdf (accessed on 30 May 2017).
- Organisation de Coopération et de Développement Économiques; Organisation for Economic Cooperation and Development. Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2016)1&docLanguage=En (accessed on 30 May 2017).
- Sasaki, K.; Umeda, M.; Sakai, A.; Yamazaki, S.; Tanaka, N. Transformation assay in Bhas 42 cells: A model using initiated cells to study mechanisms of carcinogenesis and predict carcinogenic potential of chemicals. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Bruce, S.W.; Sly, J.E.; Kunkelmann, T.; Kunz-Bohnenberger, S.; Poth, A.; Engelhardt, G.; Schulz, M.; Schwind, K.R. Prevalidation study of the Syrian hamster embryo (SHE) cell transformation assay at pH 6.7 for assessment of carcinogenic potential of chemicals. Mutat. Res. 2012, 744, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.; Bremm, K.D.; Alépée, N.; Bessems, J.G.M.; Blaauboer, B.; Boehn, S.N.; Burek, C.; Coecke, S.; Gombau, L.; Hewittj, N.J.; et al. Report from the EPAA workshop: In vitro ADME in safety testing used by EPAA industry sectors. Toxicol. In Vitro 2011, 25, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Mohamed, L.A.; Kaddoumi, A. Experimental models for predicting drug absorption and metabolism. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.A. Drug-permeability and transporter assays in Caco-2 and MDCK cell lines. Future Med. Chem. 2011, 3, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Carrola, J.; Bastos, V.; Jarak, I.; Oliveira-Silva, R.; Malheiro, E.; Daniel-da-Silva, A.L.; Oliveira, H.; Santos, C.; Gil, A.M.; Duarte, I.F. Metabolomics of silver nanoparticles toxicity in HaCaT cells: Structure-activity relationships and role of ionic silver and oxidative stress. Nanotoxicology 2016, 10, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, N.J.; Edwards, R.J.; Fritsche, E.; Goebel, C.; Aeby, P.; Scheel, J.; Reisinger, K.; Ouédraogo, G.; Duche, D.; Eilstein, J.; et al. Use of human in vitro skin models for accurate and ethical risk assessment: Metabolic considerations. Toxicol. Sci. 2013, 133, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Manwaring, J.; Rothe, H.; Obringer, C.; Foltz, D.J.; Baker, T.R.; Troutman, J.A.; Hewitt, N.J.; Goebel, C. Extrapolation of systemic bioavailability assessing skin absorption and epidermal and hepatic metabolism of aromatic amine hair dyes in vitro. Toxicol. Appl. Pharmacol. 2015, 287, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Test No. 432: In Vitro 3T3 NRU Phototoxicity Test. Available online: http://dx.doi.org/10.1787/9789264071162-en (accessed on 5 June 2017).
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinardell, M.P.; Mitjans, M. Alternative Methods to Animal Testing for the Safety Evaluation of Cosmetic Ingredients: An Overview. Cosmetics 2017, 4, 30. https://doi.org/10.3390/cosmetics4030030
Vinardell MP, Mitjans M. Alternative Methods to Animal Testing for the Safety Evaluation of Cosmetic Ingredients: An Overview. Cosmetics. 2017; 4(3):30. https://doi.org/10.3390/cosmetics4030030
Chicago/Turabian StyleVinardell, Maria Pilar, and Montserrat Mitjans. 2017. "Alternative Methods to Animal Testing for the Safety Evaluation of Cosmetic Ingredients: An Overview" Cosmetics 4, no. 3: 30. https://doi.org/10.3390/cosmetics4030030





