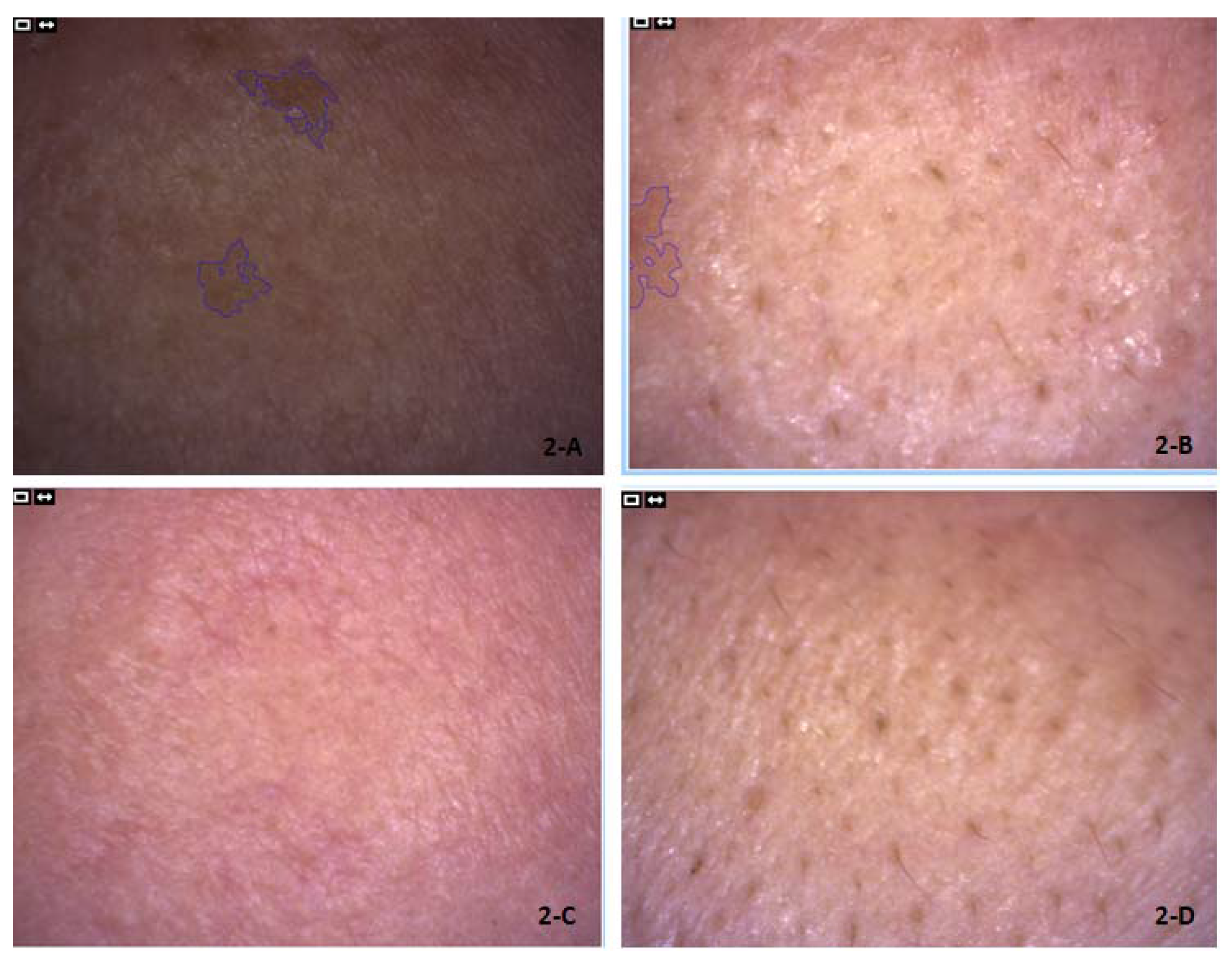
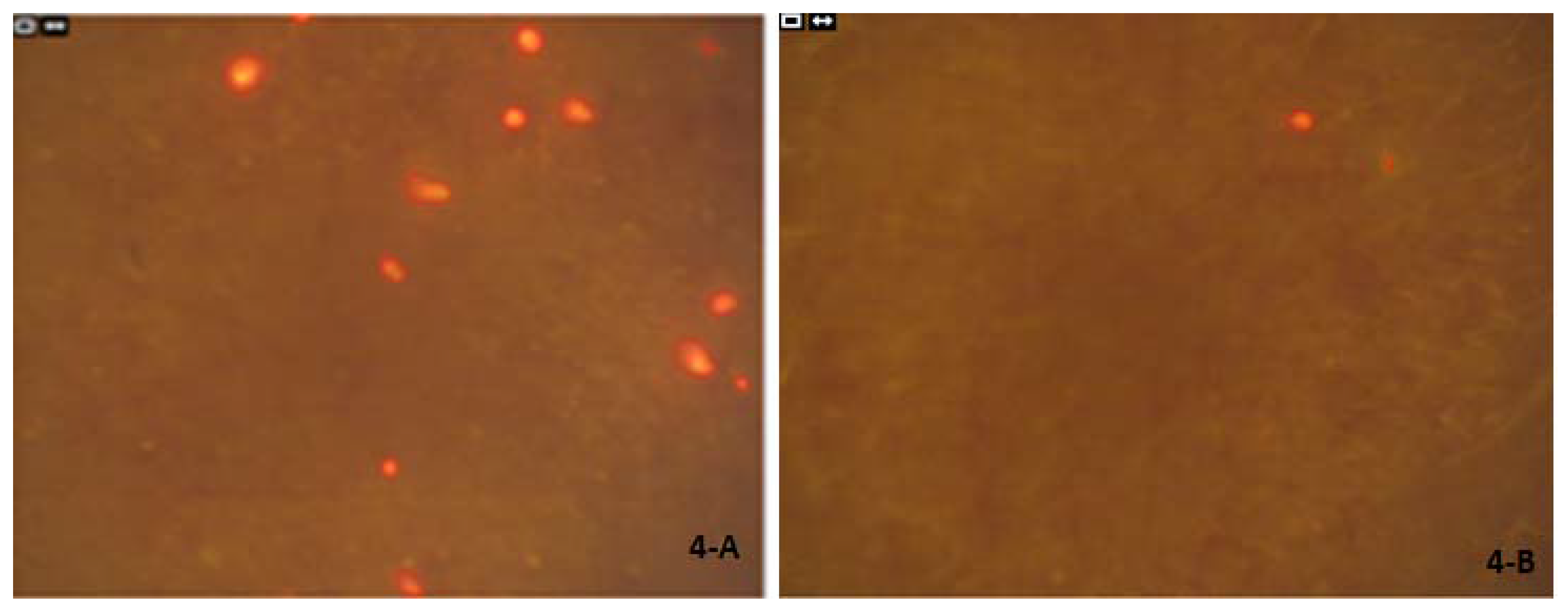
1. Introduction
Healthy skin is determined mainly by percutaneous absorption, plasticity, and cellular renovation [
1]. During the aging process, there is a lack of organization of these characteristics along with the loss of its antioxidant defense capacity, which causes an imbalance in the epithelium, resulting in an increase of free radical levels [
1,
2]. Currently, these factors are considered the main causes of the aging process. Free radical species causes oxidative stress in biological structures, resulting in the appearance of both physiological and biochemical changes leading to phenotypic consequences such as expression lines, flaccidity, low hydration, and skin pigmentation [
2,
3].
In this regard, there is widespread interest in the development of cosmetics that could act to decrease effects of free radicals in the skin, as the human skin ages possibly because the cells accumulate free radical damage throughout time. However, the development of antiaging cosmetic emulsions for topical use is, in general terms, a big challenge [
3]. First, the system is inherently unstable once it is made since it is composed of a blend of two insoluble liquids united by a surfactant layer [
2,
3]. Another important point to be considered is that for this type of formulation to have the desired effect, the substance must be both absorbed by the skin and released at the target tissue in its active form [
2]. Such a process is related to the molecular arrangement, the physicochemical properties of the active pharmaceutical ingredients (API), and the vehicle used in the formulation. In this context, one of the molecules that has been a current target of research in the cosmetic field is resveratrol [
1,
2,
3,
4]. Resveratrol (3,4,5–trihydroxy-trans-stilbene) is a natural phenolic compound produced by some spermatophyte species in response to attacks by pathogens or exposure to ultraviolet radiation or ozone. This molecule has been studied by many researchers for having a variety of beneficial effects on health. Resveratrol acts in a preventive and healing way in many diseases because of its anti-inflammatory, antioxidant, neuroprotective, cardioprotective, antiviral, anticarcinogenic, photoprotective, antimicrobial, and antiaging effects [
4,
5,
6,
7]. The antioxidant effect of resveratrol seems to be related to the inhibition of cyclooxygenase, hydroperoxidase, kinase C protein, Bcl-2 phosphorylation, kinase B protein (PKB or Akt), focal adhesion kinase, NF-kB, matrix metalloproteinase 9, and cell cycle regulators [
5,
6]. It has demonstrated great potential for use as a more efficient means to combat lipid peroxidation when compared with vitamins C and E (95%, 65%, and 37%, respectively) [
8]. Besides its antioxidant effect, resveratrol has been reported to be an important selective inhibitor of tyrosine kinase, a key melanogenesis catalyst enzyme, suggesting that it can also be used as a skin-lightening agent [
8,
9,
10,
11]. Recent studies have shown that this substance has an important photoprotective effect once it acts on cell signaling mechanisms related to photoaging caused by ultraviolet radiation (UVA and UVB rays) [
7,
8,
9,
10]. According to Polonini et al., when applied topically, the chemopreventive, antiproliferative, and photoprotective properties of resveratrol are emphasized [
4].
Among the different cosmetics produced, emulsions are widely used to incorporate incompatible cosmetic components [
1]. Despite the existence of some commercial products containing resveratrol, there are no studies on its incorporation into emulsions made with liotropic liquid crystals [
4,
6,
7,
8]. Liquid crystal systems are more stable when compared with other emulsions because they promote higher cutaneous hydration by retaining water in the corneal layer and delivering the sustained release of active ingredients to target areas. When the chemical structure is compared with the composition and disposition of both lipids and water present in the epidermis, an emulsion is the best option to address cutaneous issues in the cosmetic field, in particular, as an antiaging treatment [
9,
10,
11,
12,
13,
14].
In this context, several advantages of using the liquid crystal formulation in cosmetics include: (i) visual features, some crystals possess properties such as thermochromism (some substances change their colors at different temperatures); (ii) protective properties, substances incorporated in the liquid crystal matrix, or the formulation made with it, have protection against photo- or thermo-degradation because of its microstructure; (iii) hydrating properties, promote an increase in water retention in the stratum corneum layer resulting in a higher cutaneous hydration and an extended release of substances because its chemical structure is similar to the composition arrangement of lipids and water in the human epidermis; and (iv) stability, have a higher physicochemical stability when compared with traditional emulsions [
12,
13,
14].
In this sense, a liotropic liquid crystal emulsion containing resveratrol is hypothesized to act as an effective antiaging cosmetic that could work on both molecular and cellular changes that occur in aging processes. These changes are visible mainly because of the action of free radicals that cause a decrease in cell proliferation, elasticity, and capability of realizing gas exchange. Ultimately, free radicals result in the appearance of expression lines, skin pigmentation, a decrease in skin thickness, and dry skin [
15,
16,
17]. Besides these phenotypic changes, psychosocial changes may occur because high self-esteem bolsters good mental and physical health [
17].
Among the many procedures that can be performed, namely laser sessions, intensely pulsed light treatments, photodynamic therapy, facial filling with hyaluronic acid, botulinum toxin injections, chemical peels, radiofrequency sessions, and dermabrasion procedures, one can also consider antiaging creams [
16]. These creams are used as a home care treatment, present a lower risk when compared with other procedures, and can delay and/or improve the signs of aging skin.
The aim of this work is to evaluate the cosmetic potential of a multipurpose liotropic liquid crystal emulsion containing resveratrol for use in human volunteers, as well as to determine some relevant physicochemical properties of the product.