Empirical Analysis Revealing Privileged Chemical Space of Cosmetic Preservatives
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Substance Set Description
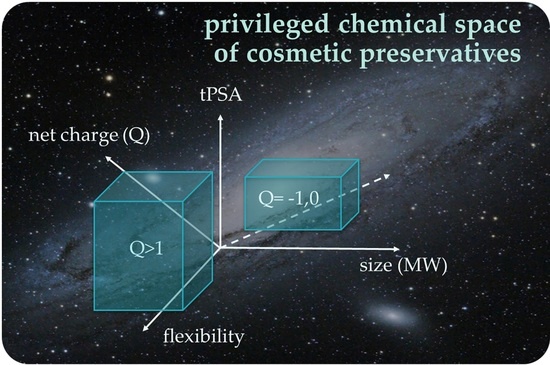
3.2. Evaluation of Chemical Space Reveals Two Distinctive Areas
3.3. Characterization of Molecular Chemical Space: Structural Descriptors
3.3.1. Molecular Size
3.3.2. Flexibility
| Annex V Ref. No. | Preservative 1 | Q 2,3 | MSA 1 (Å2) | tPSA 1 (Å2) | MW 1 (g/mol) | nrotb 1 | HBD 1 | HBA 1 | HBDnA 1 | logD | logS 1 | MoA 3 | Group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Benzoic acid and its salts | −1 (0) | 119 | 40 | 121 | 1 | 0 | 2 | 2 | −0.29 | −1.39 | Protonophores; causing membrane depolariz. [21] | A |
| 2 | Propionic acid and its salts | −1 (0) | 89 | 40 | 73 | 1 | 0 | 2 | 2 | −0.79 | 0.12 | ||
| 3 | Salicylic acid and its salts | −1 (0) | 108 | 60 | 137 | 1 | 1 | 3 | 3 | −1.06 | −1.55 | ||
| 4 | Sorbic acid and its salts | −1 (0) | 129 | 40 | 111 | 2 | 0 | 2 | 2 | 0.20 | −1.07 | ||
| 8 | Pyrithione zinc (here: pyrithione) | −1 (0) | 115 | 35 | 162 | 0 | 0 | 2 | 2 | 0.14 | −0.64 | ||
| 13 | Dehydroacetic acid and its salts | −1 (0) | 155 | 66 | 167 | 1 | 0 | 4 | 4 | −0.16 | −0.73 | ||
| 14 | Formic acid and its salts | −1 (0) | 49 | 40 | 45 | 0 | 0 | 2 | 2 | −2.00 | 0.54 | ||
| 18 | Undec-10-enoic acid and its salts | −1 4 | 232 | 40 | 183 | 9 | 0 | 2 | 2 | 2.71 | −3.01 | - 4 | A |
| 12 bis | Butyl 4-hydroxybenzoate | 0 | 209 | 47 | 192 | 5 | 1 | 3 | 4 | 3.00 | −2.64 | Mechanism is unclear 5 | A |
| 12 bis | Propyl 4-hydroxybenzoate | 0 | 190 | 47 | 180 | 4 | 1 | 3 | 4 | 2.55 | −2.22 | ||
| 12 | Methylparaben | 0 | 151 | 47 | 152 | 2 | 1 | 3 | 4 | 1.67 | −1.60 | ||
| 12 | Ethylparaben | 0 | 171 | 47 | 166 | 3 | 1 | 3 | 4 | 2.03 | −1.94 | ||
| 7 | Biphenyl-2-ol | 0 | 169 | 20 | 170 | 1 | 1 | 1 | 2 | 3.32 | −3.34 | Solubilization of lipids and denaturing proteins [21] | A |
| 11 | Chlorobutanol | 0 | 125 | 20 | 177 | 1 | 1 | 1 | 2 | 1.75 | −1.95 | ||
| 22 | 2,4-Dichlorobenzyl alcohol | 0 | 153 | 20 | 177 | 1 | 1 | 1 | 2 | 2.41 | −2.44 | ||
| 24 | Chlorocresol | 0 | 137 | 20 | 142 | 0 | 1 | 1 | 2 | 2.79 | −2.21 | ||
| 26 | Chloroxylenol | 0 | 154 | 20 | 157 | 0 | 1 | 1 | 2 | 3.30 | −2.44 | ||
| 29 | 2-Phenoxyethanol | 0 | 153 | 29 | 138 | 3 | 1 | 2 | 3 | 1.13 | −1.10 | ||
| 34 | Benzyl alcohol | 0 | 125 | 20 | 108 | 1 | 1 | 1 | 2 | 1.21 | −1.02 | ||
| 37 | Bromochlorophen | 0 | 262 | 40 | 427 | 2 | 2 | 2 | 4 | 5.48 | −5.91 | ||
| 38 | 4-Isopropyl-m-cresol | 0 | 171 | 20 | 150 | 1 | 1 | 1 | 2 | 3.43 | −2.79 | ||
| 40 | Chlorophene | 0 | 205 | 20 | 219 | 2 | 1 | 1 | 2 | 4.37 | −3.92 | ||
| 43 | 1-Phenoxypropan-2-ol | 0 | 169 | 29 | 152 | 3 | 1 | 2 | 3 | 1.54 | −1.49 | ||
| 50 | Chlorphenesin | 0 | 193 | 50 | 202 | 4 | 2 | 3 | 5 | 1.10 | −1.50 | ||
| 56 | 3-Iodo-2-propynylbutylcarbamate | 0 | 218 | 38 | 281 | 6 | 1 | 3 | 4 | 2.54 | −3.14 | - | A |
| 23 | Triclocarban | 0 | 231 | 41 | 316 | 2 | 2 | 3 | 5 | 4.93 | −5.48 | Intracell. enzyme inhibitors [22,23,24,25] | A |
| 25 | Triclosan | 0 | 224 | 30 | 290 | 2 | 1 | 2 | 3 | 4.97 | −5.19 | ||
| 32 | Climbazol | 0 | 263 | 42 | 293 | 5 | 0 | 4 | 4 | 3.07 | −3.96 | ||
| 35 | Piroctone and its monoethanolamine salt | −1 | 246 | 43 | 236 | 4 | 0 | 2 | 2 | 3.13 | −3.62 | ||
| 19 | Hexetidine | 1 | 456 | 37 | 341 | 12 | 3 | 2 | 5 | 2.75 | −4.67 | B | |
| 15 | Dibromohexamidine and its salts | 2 | 412 | 122 | 514 | 11 | 8 | 4 | 12 | −0.53 | −8.08 | Interaction with membrane component destroying membrane integrity [21] | B |
| 28 | Polyaminopropyl biguanide (here: n = 22) | 22 | 4932 | 1881 | 4000 | 176 | 132 | 88 | 220 | - | −2.82 | ||
| 42 | Chlorhexidin and its salts | 2 | 488 | 181 | 507 | 13 | 12 | 8 | 20 | −3.90 | −7.52 | ||
| 44 | Alkyl (C12-C22) trimethyl ammonium salts 6 (here: C12) | 1 | 320 | 0 | 257 | 13 | 0 | 0 | 0 | 0.91 | −3.25 | ||
| 44 | Alkyl (C12-C22) trimethyl ammonium salts 6 (here: C16) | 1 | 394 | 0 | 285 | 15 | 0 | 0 | 0 | 2.69 | −4.61 | ||
| 44 | Alkyl (C12-C22) trimethyl ammonium salts 6 (here: C22) | 1 | 507 | 0 | 369 | 21 | 0 | 0 | 0 | 5.35 | −6.64 | ||
| 47 | Hexamidine and its salts | 2 | 371 | 122 | 356 | 11 | 8 | 4 | 12 | −2.06 | −6.50 | ||
| 53 | Benzethonium chloride | 1 | 396 | 19 | 448 | 12 | 0 | 2 | 2 | 1.78 | −5.32 | ||
| 54 | Benzalkonium salts 7 (here: C12) | 1 | 379 | 0 | 304 | 13 | 0 | 0 | 0 | 3.63 | −5.83 | ||
| 58 | Ethyl Lauroyl Arginate Hydrochloride | 1 | 476 | 121 | 386 | 19 | 6 | 6 | 12 | 1.12 | −5.48 |
3.3.3. Hydrogen Bonding
3.4. Physicochemical Properties: Lipophilicity and Solubility
3.4.1. Lipophilicity
3.4.2. Solubility
4. Discussion
4.1. Calculation and Analysis of Molecular Desciptors
4.2. Dependencies of Molecular Descriptors
4.3. Mechanism-of-Action and Rules for Privileged Chemical Space
4.4. Comparison of Cosmetic Preservatives with Marketed Drugs and Antibiotics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becks, V.E.; Lorenzoni, N.M. Pseudomonas aeruginosa Outbreak in a Neonatal Intensive Care Unit: A Possible Link to Contaminated Hand Lotion. Am. J. Infect. Control 1995, 23, 396–398. [Google Scholar] [CrossRef]
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products. Available online: https://ec.europa.eu/safety-gate-alerts/screen/webReportusingsearchforalertoption (accessed on 22 April 2021).
- The European Parliament and the Council of the European Union. Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products–Consolidated Version of 25/12/2017. Off. J. Eur. Union Lex 2009, 342, 59.
- McLaughlin, J.K. Formaldehyde and cancer: A critical review. Int. Arch. Occup. Environ. Heath 1994, 66, 295–301. [Google Scholar] [CrossRef]
- De Groot, A.C.; Flyvholm, M.-A.; Lensen, G.; Menné, T.; Coenraads, P.-J. Formaldehyde-releasers: Relationship to formal-dehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde-releasers. Contact Dermat. 2009, 61, 63–85. [Google Scholar] [CrossRef] [Green Version]
- Denyer, S.P. Mechanisms of Action of Antibacterial Biocides. Int. Biodeterior. 1995, 36, 221–245. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Moeller, B.C.; Lu, K.; Rager, J.E.; Fry, R.C.; Starr, T.B. Formaldehyde Carcinogenicity Research: 30 Years and Counting for Mode of Action, Epidemiology, and Cancer Risk Assessment. Toxicol. Pathol. 2013, 41, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, T.M. The Mechanism of Action of Isothiazolone Biocides. Power Plant Chem. 2007, 9, 14–22. [Google Scholar]
- Irwin, S.V.; Fisher, P.; Graham, E.; Malek, A.; Robidoux, A. Sulfites Inhibit the Growth of Four Species of Beneficial Gut Bacteria at Concentrations Regarded as Safe for Food. PLoS ONE 2017, 12, e0186629. [Google Scholar] [CrossRef] [Green Version]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, J.A.; Waigh, R.D.; Gilbert, P. Antibacterial Action of 2-Bromo-2-Nitropropane-1,3-Diol (Bronopol). Antimicrob. Agents Chemother. 1988, 32, 1693–1698. [Google Scholar] [CrossRef] [Green Version]
- Ghannoum, M.; Thomson, M.; Bowman, W.; Al-Khalil, S. Mode of Action of the Antimicrobial Compound 5-Bromo-5-nitro-1,3-dioxane (Bronidox). Folia Microbiol. 1986, 31, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Yin, E.X.; Zhang, J.; Zhao, E.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y. Factors Affecting the Activities of Microbicides. In Hugo and Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization, 5th ed.; Fraise, A.P., Maillard, J.-Y., Sattar, S.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 71–86. [Google Scholar]
- Tschierske, N.; Emeis, D.; Biel, S.S. Efficacy Loss of Antimicrobial Actives in Emulsions. SOFW-J. 2012, 4, 1–4. [Google Scholar]
- Denyer, S.P. Mechanism of Action of Biocides. Int. Biodeterior. 1990, 26, 89–100. [Google Scholar] [CrossRef]
- Available online: https://disco.chemaxon.com/calculators/demo/plugins/logd/ (accessed on 15 April 2021).
- Bradley, C.D.; Kihlberg, J. Drug Discovery Beyond the Rule of 5-Opportunities and Challenges. Expert Opin. Drug Discov. 2017, 12, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Geis, P.A. Appendix: Common Cosmetic Preservatives. In Cosmetic Microbiology A Practical Guide, 2nd ed.; Geis, P.A., Ed.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 228–281. [Google Scholar]
- Macsics, R.; Hackl, M.W.; Fetzer, C.; Mostert, D.; Bender, J.; Layer, F.; Sieber, S.A. Comparative Target Analysis of Chlorinated Biphenyl Antimicrobials Highlights MenG as a Molecular Target of Triclocarban. Appl. Environ. Microbiol. 2020, 86, e00933-20. [Google Scholar] [CrossRef]
- Russell, D. Whither triclosan? J. Antimicrob. Chemother. 2004, 53, 693–695. [Google Scholar] [CrossRef] [Green Version]
- Richter, E.; Wick, A.; Ternes, T.A.; Coors, A. Ecotoxicity of Climbazole, a Fungicide Contained in Antidandruff Shampoo. Environ. Toxicol. Chem. 2013, 32, 2816–2825. [Google Scholar] [CrossRef]
- do Couto, F.M.; do Nascimento, S.C.; Júnior, S.F.; da Silva, V.K.; Leal, A.F.; Neves, R.P. Antifungal Activity of the Piroctone Olamine in Experimental Intra-Abdominal Candidiasis. SpringerPlus 2016, 5, 468. [Google Scholar] [CrossRef] [Green Version]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An update of Possible Mechanisms of Action and Implications in the Development of the Next-generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Valkova, N.; Lépine, F.; Valeanu, L.; Dupont, M.; Labrie, L.; Bisaillon, J.G.; Beaudet, R.; Shareck, F.; Villemur, R. Hydrolysis of 4-Hydroxybenzoic Acid Esters (Parabens) and Their Aerobic Transformation into Phenol by the Resistant Enterobacter cloacae Strain EM. Appl. Environ. Microbiol. 2001, 67, 2404–2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, B.M.P.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar]
- Constantinescu, T.; Lungu, C.N.; Lung, I. Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives. Molecules 2019, 24, 1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, G.; Ermondi, G. Molecular Descriptors for Polarity: The Need for Going Beyond Polar Surface Area. Future Med. Chem. 2016, 8, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, R.; Moser, H.E. Physicochemical Properties of Antibacterial Compounds: Implications for Drug Discovery. J. Med. Chem. 2008, 51, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Ebejer, J.-P.; Charlton, M.H.; Finn, P.W. Are the Physicochemical Properties of Antibacterial Compounds Really Different From Other Drugs? J. Cheminform. 2016, 8, 30. [Google Scholar] [CrossRef] [Green Version]


| Molecular Property | Annex V 1 (Q = −1; 0) | Annex V 1,2 (Q ≥ 1) | Antibiotics 3 (Gram-Positive) | Antibiotics 3 (Gram-Negative) | Non-Antibiotics 3 |
|---|---|---|---|---|---|
| MW | 184 | 377 | 813 | 414 | 338 |
| nrotb | 2.3 | 14.0 | 13.8 | 6.8 | 5.9 |
| tPSA | 36 | 60 | 243 | 165 | 70 |
| HBDnA | 2.9 | 6.3 | 23.4 | 14.5 | 6.5 |
| logD | 1.98 | 1.17 | −0.20 | −2.79 | 1.59 |
| logS | −2.33 | −5.79 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storz, M.P.; Holsten, L. Empirical Analysis Revealing Privileged Chemical Space of Cosmetic Preservatives. Cosmetics 2021, 8, 80. https://doi.org/10.3390/cosmetics8030080
Storz MP, Holsten L. Empirical Analysis Revealing Privileged Chemical Space of Cosmetic Preservatives. Cosmetics. 2021; 8(3):80. https://doi.org/10.3390/cosmetics8030080
Chicago/Turabian StyleStorz, Michael P., and Lea Holsten. 2021. "Empirical Analysis Revealing Privileged Chemical Space of Cosmetic Preservatives" Cosmetics 8, no. 3: 80. https://doi.org/10.3390/cosmetics8030080