A Viscoelastic Model to Evidence Reduced Upper-Limb-Swing Capabilities during Gait for Parkinson’s Disease-Affected Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
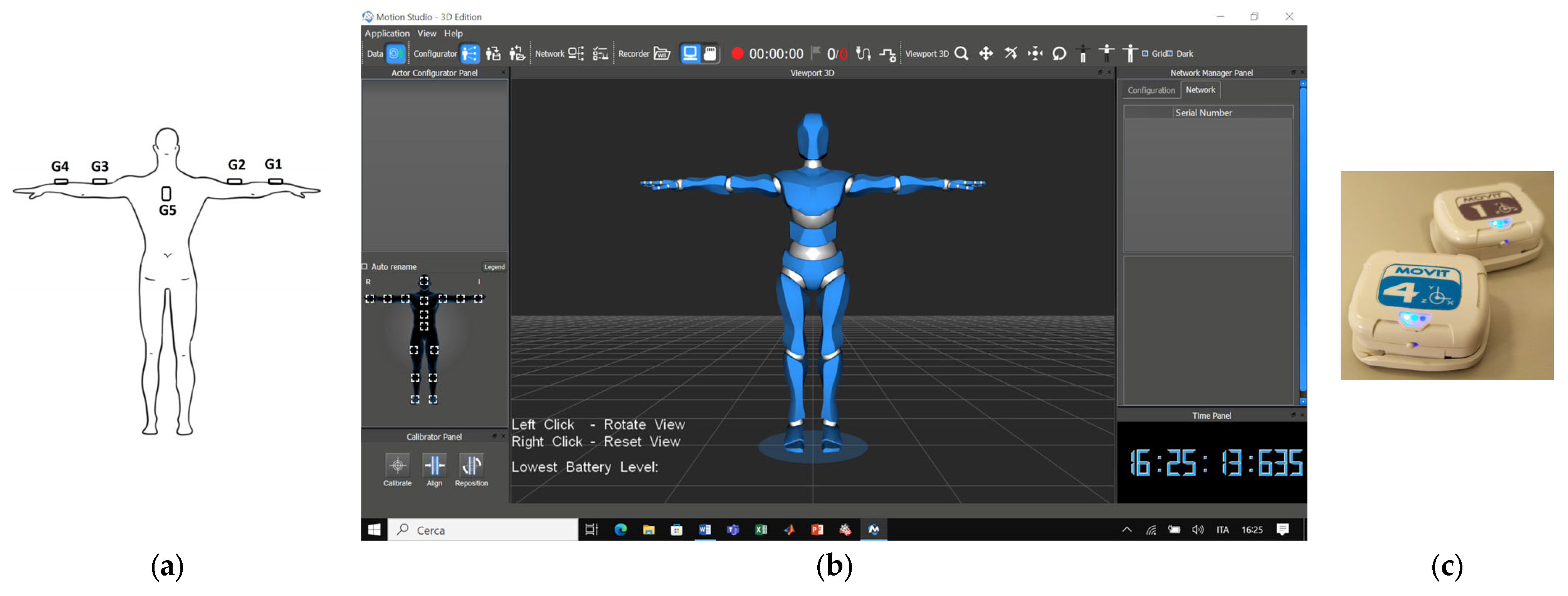
2.2. Wearable Electronics
2.3. Test Protocol
- Sit to Walk: Starting from sitting, the participant gets up from the chair without the aid of the arms;
- First linear walking: The subject walks (away from the chair) at a comfortable speed along a straight line for six meters;
- Turning: The participant turns 180° around a pin to go back;
- Second linear walking: Same as point 2, but in the opposite direction (toward the chair);
- Stand To Sit: The participant turns themself around 180° and sits down.
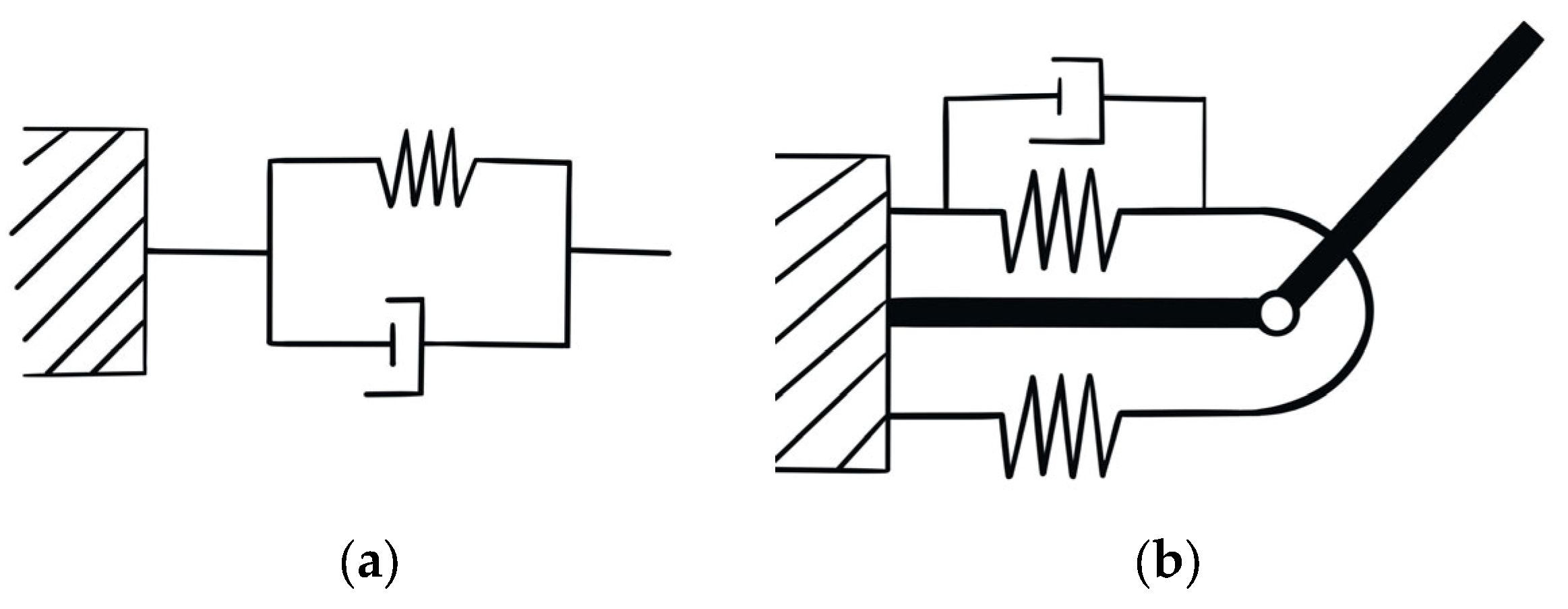
2.4. Double Pendulum Model
2.5. Dynamic of the Forearm
2.6. Data Processing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricci, M.; Giannini, F.; Saggio, G.; Cenci, C.; Di Lazzaro, G.; Pisani, A. A Novel Analytical Approach to Assess Dyskinesia in Patients with Parkinson Disease. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 975–979. [Google Scholar] [CrossRef]
- Zampogna, A.; Cavallieri, F.; Bove, F.; Suppa, A.; Castrioto, A.; Meoni, S.; Pélissier, P.; Schmitt, E.; Bichon, A.; Lhommée, E.; et al. Axial Impairment and Falls in Parkinson’s Disease: 15 Years of Subthalamic Deep Brain Stimulation. NPJ Park. Dis. 2022, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Saggio, G.; Sbernini, L. New Scenarios in Human Trunk Posture Measurements for Clinical Applications. In Proceedings of the 2011 IEEE International Symposium on Medical Measurements and Applications, Bari, Italy, 30–31 May 2011; pp. 13–17. [Google Scholar] [CrossRef] [Green Version]
- Poewe, W. Non-Motor Symptoms in Parkinson’s Disease. Eur. J. Neurol. 2008, 15 (Suppl. 1), 14–20. [Google Scholar] [CrossRef] [PubMed]
- Suppa, A.; Asci, F.; Saggio, G.; Marsili, L.; Casali, D.; Zarezadeh, Z.; Ruoppolo, G.; Berardelli, A.; Costantini, G. Voice Analysis in Adductor Spasmodic Dysphonia: Objective Diagnosis and Response to Botulinum Toxin. Park. Relat. Disord. 2020, 73, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Suppa, A.; Costantini, G.; Asci, F.; Di Leo, P.; Al-Wardat, M.S.; Di Lazzaro, G.; Scalise, S.; Pisani, A.; Saggio, G. Voice in Parkinson’s Disease: A Machine Learning Study. Front. Neurol. 2022, 13, 831428. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Fahn, S.; Marsden, C.; Goldstein, M.; Calne, D. State of the Art Review—The Unified Parkinson’s Disease Rating Scale (UPDRS):Status and Recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar]
- Siderowf, A.; McDermott, M.; Kieburtz, K.; Blindauer, K.; Plumb, S.; Shoulson, I. Test-Retest Reliability of the Unified Parkinson’s Disease Rating Scale in Patients with Early Parkinson’s Disease: Results from a Multicenter Clinical Trial. Mov. Disord. 2002, 17, 758–763. [Google Scholar] [CrossRef]
- Lonini, L.; Dai, A.; Shawen, N.; Simuni, T.; Poon, C.; Shimanovich, L.; Daeschler, M.; Ghaffari, R.; Rogers, J.A.; Jayaraman, A. Wearable Sensors for Parkinson’s Disease: Which Data Are Worth Collecting for Training Symptom Detection Models. NPJ Digit. Med. 2018, 1, 64. [Google Scholar] [CrossRef] [Green Version]
- Di Biase, L.; Summa, S.; Tosi, J.; Taffoni, F.; Marano, M.; Rizzo, A.C.; Vecchio, F.; Formica, D.; Di Lazzaro, V.; Di Pino, G.; et al. Quantitative Analysis of Bradykinesia and Rigidity in Parkinson’s Disease. Front. Neurol. 2018, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Cesarelli, G.; Donisi, L.; Amato, F.; Romano, M.; Cesarelli, M.; D’Addio, G.; Ponsiglione, A.M.; Ricciardi, C. Using Features Extracted from Upper Limb Reaching Tasks to Detect Parkinson’s Disease by Means of Machine Learning Models. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1056–1063. [Google Scholar] [CrossRef]
- Ricci, M.; Di Lazzaro, G.; Pisani, A.; Mercuri, N.B.; Giannini, F.; Saggio, G. Assessment of Motor Impairments in Early Untreated Parkinson’s Disease Patients: The Wearable Electronics Impact. IEEE J. Biomed. Health Inform. 2020, 24, 120–130. [Google Scholar] [CrossRef]
- Salarian, A.; Russmann, H.; Vingerhoets, F.J.G.; Dehollain, C.; Blanc, Y.; Burkhard, P.R.; Aminian, K. Gait Assessment in Parkinson’s Disease: Toward an Ambulatory System for Long-Term Monitoring. IEEE Trans. Biomed. Eng. 2004, 51, 1434–1443. [Google Scholar] [CrossRef]
- Di Lazzaro, G.; Ricci, M.; Al-Wardat, M.; Schirinzi, T.; Scalise, S.; Giannini, F.; Mercuri, N.B.; Saggio, G.; Pisani, A. Technology-Based Objective Measures Detect Subclinical Axial Signs in Untreated, de Novo Parkinson’s Disease. J. Park. Dis. 2020, 10, 113–122. [Google Scholar] [CrossRef]
- Asci, F.; Falletti, M.; Zampogna, A.; Patera, M.; Hallett, M.; Rothwell, J.; Suppa, A. Rigidity in Parkinson’s Disease: Evidence from Biomechanical and Neurophysiological Measures. Brain 2023, awad114. [Google Scholar] [CrossRef]
- Pietrosanti, L.; Calado, A.; Verrelli, C.M.; Pisani, A.; Suppa, A.; Fattapposta, F.; Zampogna, A.; Patera, M.; Rosati, V.; Giannini, F.; et al. Harmonic Distortion Aspects in Upper Limb Swings during Gait in Parkinson’s Disease. Electronics 2023, 12, 625. [Google Scholar] [CrossRef]
- Mirelman, A.; Ben Or Frank, M.; Melamed, M.; Granovsky, L.; Nieuwboer, A.; Rochester, L.; Del Din, S.; Avanzino, L.; Pelosin, E.; Bloem, B.R.; et al. Detecting Sensitive Mobility Features for Parkinson’s Disease Stages Via Machine Learning. Mov. Disord. 2021, 36, 2144–2155. [Google Scholar] [CrossRef]
- Navarro-López, V.; Fernández-Vázquez, D.; Molina-Rueda, F.; Cuesta-Gómez, A.; García-Prados, P.; del-Valle-Gratacós, M.; Carratalá-Tejada, M. Arm-Swing Kinematics in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Gait Posture 2022, 98, 85–95. [Google Scholar] [CrossRef]
- Mackay, W.A.; Crammond, D.J.; Kwan, H.C.; Murphy, J.T. Measurements of Human Forearm Viscoelasticity. J. Biomech. 1986, 19, 231–238. [Google Scholar] [CrossRef]
- Lacquaniti, F.; Carrozzo, M.; Borghese, N.A. Time-Varying Mechanical Behavior of Multijointed Arm in Man. J. Neurophysiol. 1993, 69, 1443–1464. [Google Scholar] [CrossRef]
- Katayama, M.; Kawato, M. Virtual Trajectory and Stiffness Ellipse during Multijoint Arm Movement Predicted by Neural Inverse Models; Springer: Berlin/Heidelberg, Germany, 1993; Volume 69. [Google Scholar]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Sun, J.; Threlkeld, A.J. Analysis of Interactive Effect of Stretch Reflex and Shortening Reaction on Rigidity in Parkinson’s Disease. Clin. Neurophysiol. 2009, 120, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Lewek, M.D.; Poole, R.; Johnson, J.; Halawa, O.; Huang, X. Arm Swing Magnitude and Asymmetry during Gait in the Early Stages of Parkinson’s Disease. Gait Posture 2010, 31, 256–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plagenhoef, S.; Gaynor Evans, F.; Abdelnour, T. Anatomical Data for Analyzing Human Motion. Res. Q. Exerc. Sport 1983, 54, 169–178. [Google Scholar] [CrossRef]
- Saggio, G.; Quitadamo, L.R.; Albero, L. Development and Evaluation of a Novel Low-Cost Sensor-Based Knee Flexion Angle Measurement System. Knee 2014, 21, 896–901. [Google Scholar] [CrossRef]
- Saggio, G.; Bocchetti, S.; Pinto, C.A.; Orengo, G.; Giannini, F. A Novel Application Method for Wearable Bend Sensors. In Proceedings of the 2nd International Symposium on Applied Sciences in Biomedical and Communication Technologies, Bratislava, Slovakia, 24–27 November 2009. [Google Scholar]
- Ricci, M.; Terribili, M.; Giannini, F.; Errico, V.; Pallotti, A.; Galasso, C.; Tomasello, L.; Sias, S.; Saggio, G. Wearable-Based Electronics to Objectively Support Diagnosis of Motor Impairments in School-Aged Children. J. Biomech. 2019, 83, 243–252. [Google Scholar] [CrossRef]
- Alessandrini, M.; Micarelli, A.; Viziano, A.; Pavone, I.; Costantini, G.; Casali, D.; Paolizzo, F.; Saggio, G. Impiego Degli Accelerometri Triassiali Nel Deficit Vestibolare Unilaterale: Affidabilità Rispetto Alla Posturografia Statica. Acta Otorhinolaryngol. Ital. 2017, 37, 231–236. [Google Scholar] [CrossRef]
- Saggio, G.; Tombolini, F.; Ruggiero, A. Technology-Based Complex Motor Tasks Assessment: A 6-DOF Inertial-Based System Versus a Gold-Standard Optoelectronic-Based One. IEEE Sens. J. 2021, 21, 1616–1624. [Google Scholar] [CrossRef]
- Ricci, M.; Di Lazzaro, G.; Pisani, A.; Scalise, S.; Alwardat, M.; Salimei, C.; Giannini, F.; Saggio, G. Wearable Electronics Assess the Effectiveness of Transcranial Direct Current Stimulation on Balance and Gait in Parkinson’s Disease Patients. Sensors 2019, 19, 5465. [Google Scholar] [CrossRef] [Green Version]
- Saggio, G.; Cavallo, P.; Ricci, M.; Errico, V.; Zea, J.; Benalcázar, M.E. Sign Language Recognition Using Wearable Electronics: Implementing K-Nearest Neighbors with Dynamic Time Warping and Convolutional Neural Network Algorithms. Sensors 2020, 20, 3879. [Google Scholar] [CrossRef]
- Ricci, M.; Lazzaro, G.D.; Errico, V.; Pisani, A.; Giannini, F.; Saggio, G. The Impact of Wearable Electronics in Assessing the Effectiveness of Levodopa Treatment in Parkinson’s Disease. IEEE J. Biomed. Health Inform. 2022, 26, 2920–2928. [Google Scholar] [CrossRef]
- Kuhtz-Buschbeck, J.P.; Jing, B. Activity of Upper Limb Muscles during Human Walking. J. Electromyogr. Kinesiol. 2012, 22, 199–206. [Google Scholar] [CrossRef]
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor Patterns in Human Walking and Running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, A.D.; Tempelman, J.R.; Petrushenko, D.; Khasawneh, F.A. Low-Cost Double Pendulum for High-Quality Data Collection with Open-Source Video Tracking and Analysis. HardwareX 2020, 8, e00138. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.M.; Joseph, J.; Wyard, S.J. A Mathematical Model of Arm Swing during Human Locomotion. J. Biomech. 1978, 11, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, N.; Goldsheyder, D.; Nordin, M.; Leger, D. Fundamentals of Biomechanics: Equilibrium, Motion, and Deformation, Fourth Edition. In Fundamentals of Biomechanics, 4th ed.; Springer: Cham, Switzerland, 2016; pp. 1–454. [Google Scholar] [CrossRef] [Green Version]
- Takada, Y.; Nakamura, S.; Morioka, S.; Iwase, M. Development of Myoelectric Prostheses with Elbow Joint. In Proceedings of the International Conference on Advanced Mechatronic Systems, ICAMechS, Hanoi, Vietnam, 10–13 December 2020; pp. 60–64. [Google Scholar]
- Mirelman, A.; Bernad-Elazari, H.; Thaler, A.; Giladi-Yacobi, E.; Gurevich, T.; Gana-Weisz, M.; Saunders-Pullman, R.; Raymond, D.; Doan, N.; Bressman, S.B.; et al. Arm Swing as a Potential New Prodromal Marker of Parkinson’s Disease. Mov. Disord. 2016, 31, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Cole, M.H.; Silburn, P.A.; Wood, J.M.; Worringham, C.J.; Kerr, G.K. Falls in Parkinson’s Disease: Kinematic Evidence for Impaired Head and Trunk Control. Mov. Disord. 2010, 25, 2369–2378. [Google Scholar] [CrossRef] [Green Version]



| PD | HC | |
|---|---|---|
| Age (years) | 65 ± 8 | 69 ± 11.2 |
| Gender | 12 M, 6 F | 10 M, 22 F |
| Height (cm) | 170.5 ± 11.5 | 163.1 ± 10.7 |
| Weight (Kg) | 73 ± 16.0 | 67.5 ± 12.4 |
| MDS-UPDRS III | 29.8 ± 10.7 |
| PD (MDS-UPDRS Item 3.3 Score) | HC (Median ± Sd) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| PD (0) (Median ± Sd) | PD (1) (Median ± Sd) | PD (2) (Median ± Sd) | |||||
| PD 0 vs. HC | PD 1 vs. HC | PD 2 vs. HC | |||||
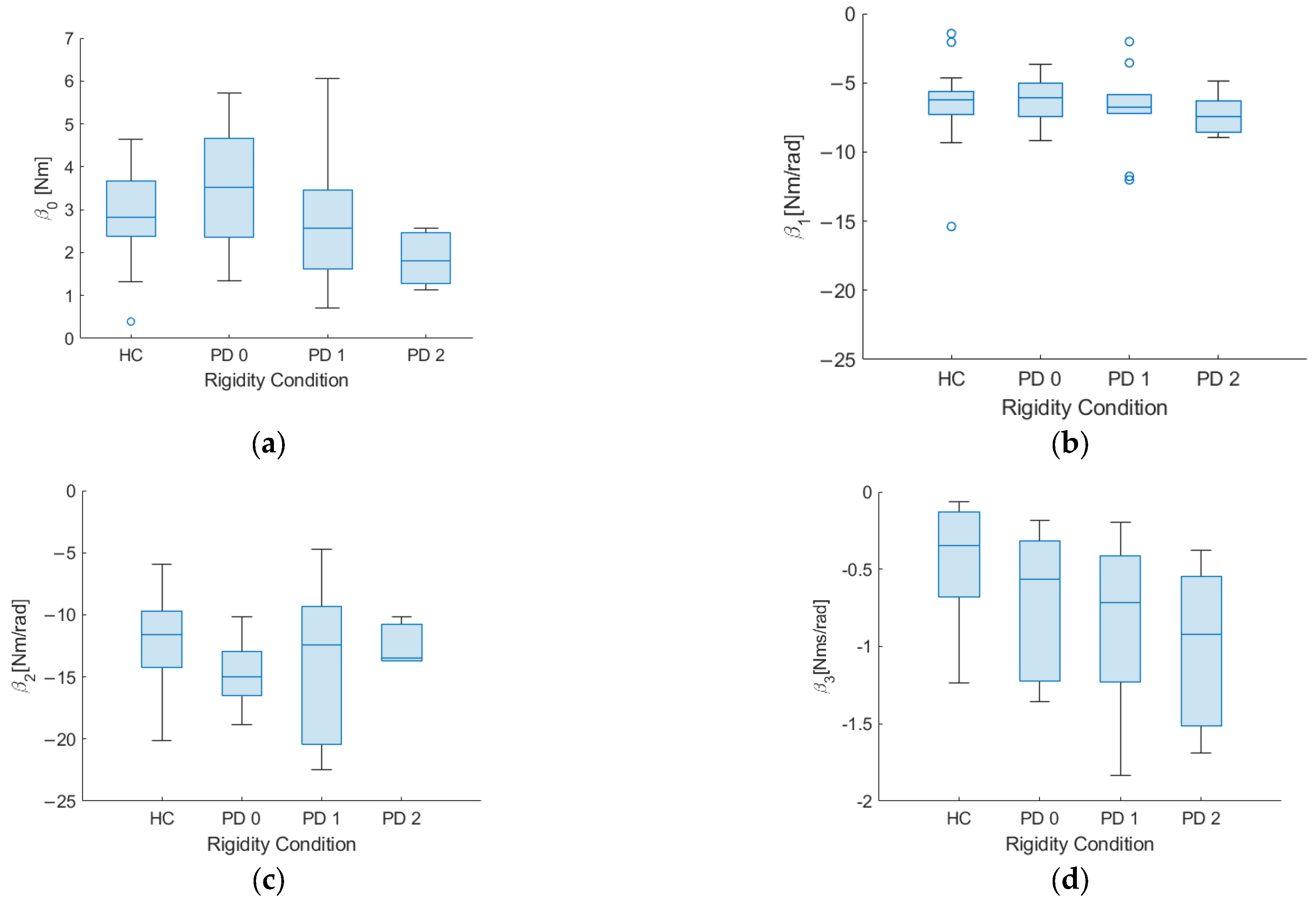
| β0 | 3.53 ± 1.81 | 2.56 ± 1.71 | 2.27 ± 0.70 | 2.69 ± 1.02 | 0.419 | 0.386 | 0.047 |
| β1 | −6.09 ± 2.01 | −6.49 ± 4.89 | −6.74 ± 7.21 | −6.23 ± 2.30 | 0.760 | 0.792 | 0.348 |
| β2 | −15.0 ± 5.31 | −12.3 ± 6.85 | −13.6 ± 9.63 | −11.6 ± 3.88 | 0.075 | 0.522 | 0.402 |
| β3 | −0.56 ± 0.44 | −0.47 ± 0.63 | −0.83 ± 0.63 | −0.26 ± 0.35 | 0.166 | 0.049 | 0.040 |
| Feature | Rigidity (Sub-Item 3.3) | |
|---|---|---|
| r | p-Value | |
| β0 | −0.378 | 0.041 |
| β1 | −0.155 | 0.245 |
| β2 | 0.116 | 0.697 |
| β3 | −0.262 | 0.119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrosanti, L.; Verrelli, C.M.; Giannini, F.; Suppa, A.; Fattapposta, F.; Zampogna, A.; Patera, M.; Rosati, V.; Saggio, G. A Viscoelastic Model to Evidence Reduced Upper-Limb-Swing Capabilities during Gait for Parkinson’s Disease-Affected Subjects. Electronics 2023, 12, 3347. https://doi.org/10.3390/electronics12153347
Pietrosanti L, Verrelli CM, Giannini F, Suppa A, Fattapposta F, Zampogna A, Patera M, Rosati V, Saggio G. A Viscoelastic Model to Evidence Reduced Upper-Limb-Swing Capabilities during Gait for Parkinson’s Disease-Affected Subjects. Electronics. 2023; 12(15):3347. https://doi.org/10.3390/electronics12153347
Chicago/Turabian StylePietrosanti, Luca, Cristiano Maria Verrelli, Franco Giannini, Antonio Suppa, Francesco Fattapposta, Alessandro Zampogna, Martina Patera, Viviana Rosati, and Giovanni Saggio. 2023. "A Viscoelastic Model to Evidence Reduced Upper-Limb-Swing Capabilities during Gait for Parkinson’s Disease-Affected Subjects" Electronics 12, no. 15: 3347. https://doi.org/10.3390/electronics12153347








