Characterization of Sn-xIn Solders and Thermomigration-Induced Interfacial IMC Growth of Cu/Sn-xIn/Cu Micro Solder Joints
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
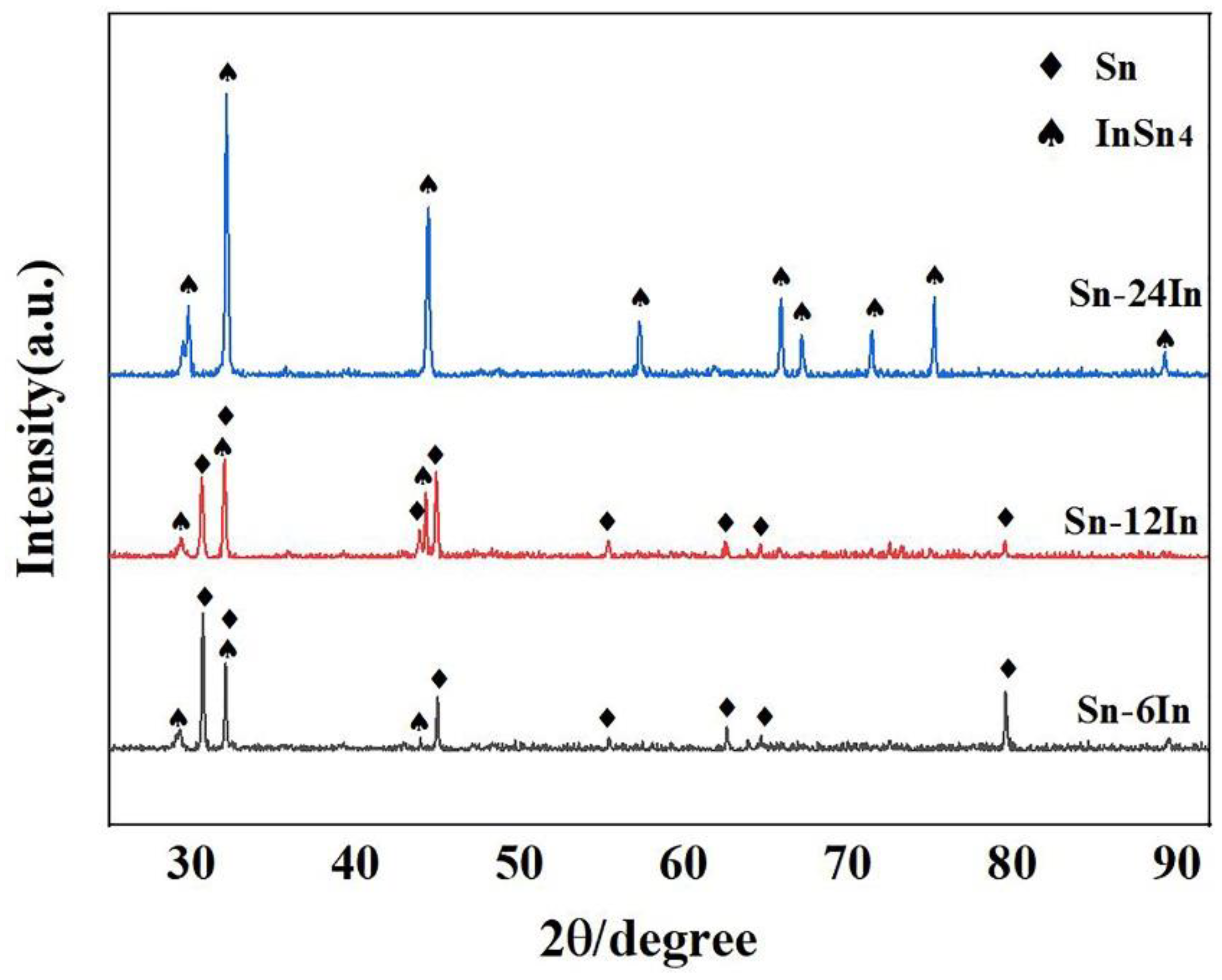
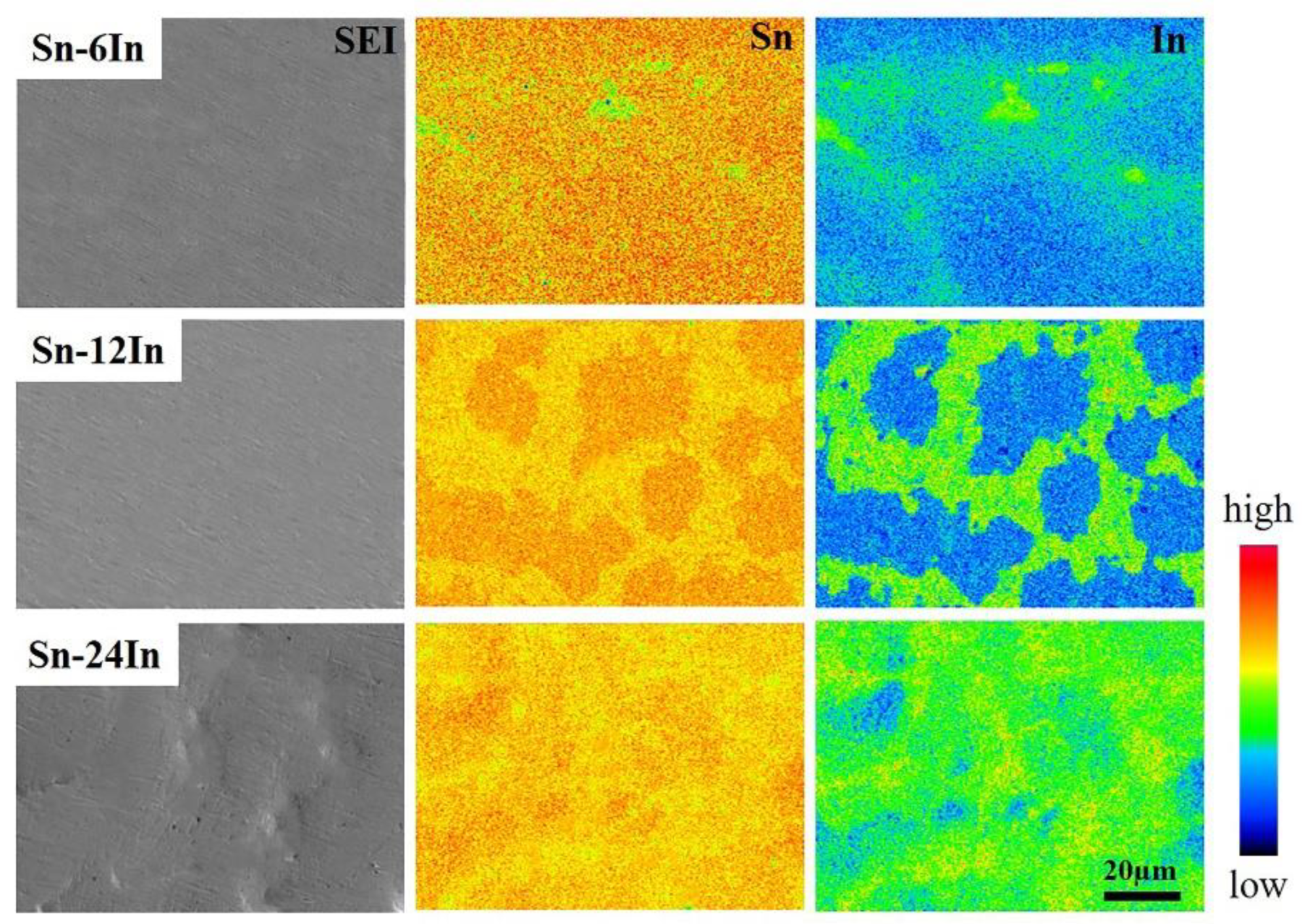
3.1. Characterization of the Sn-xIn Solders
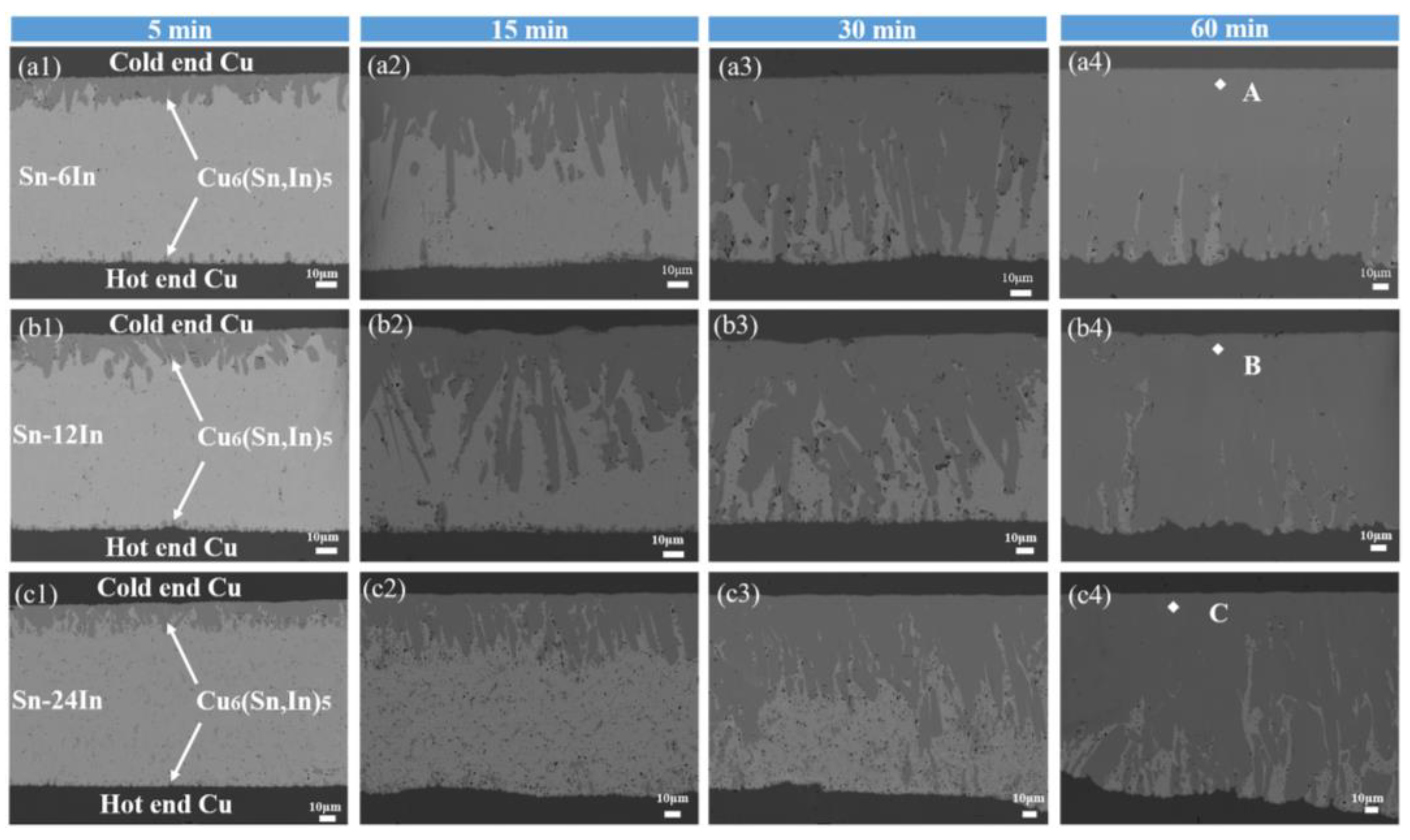
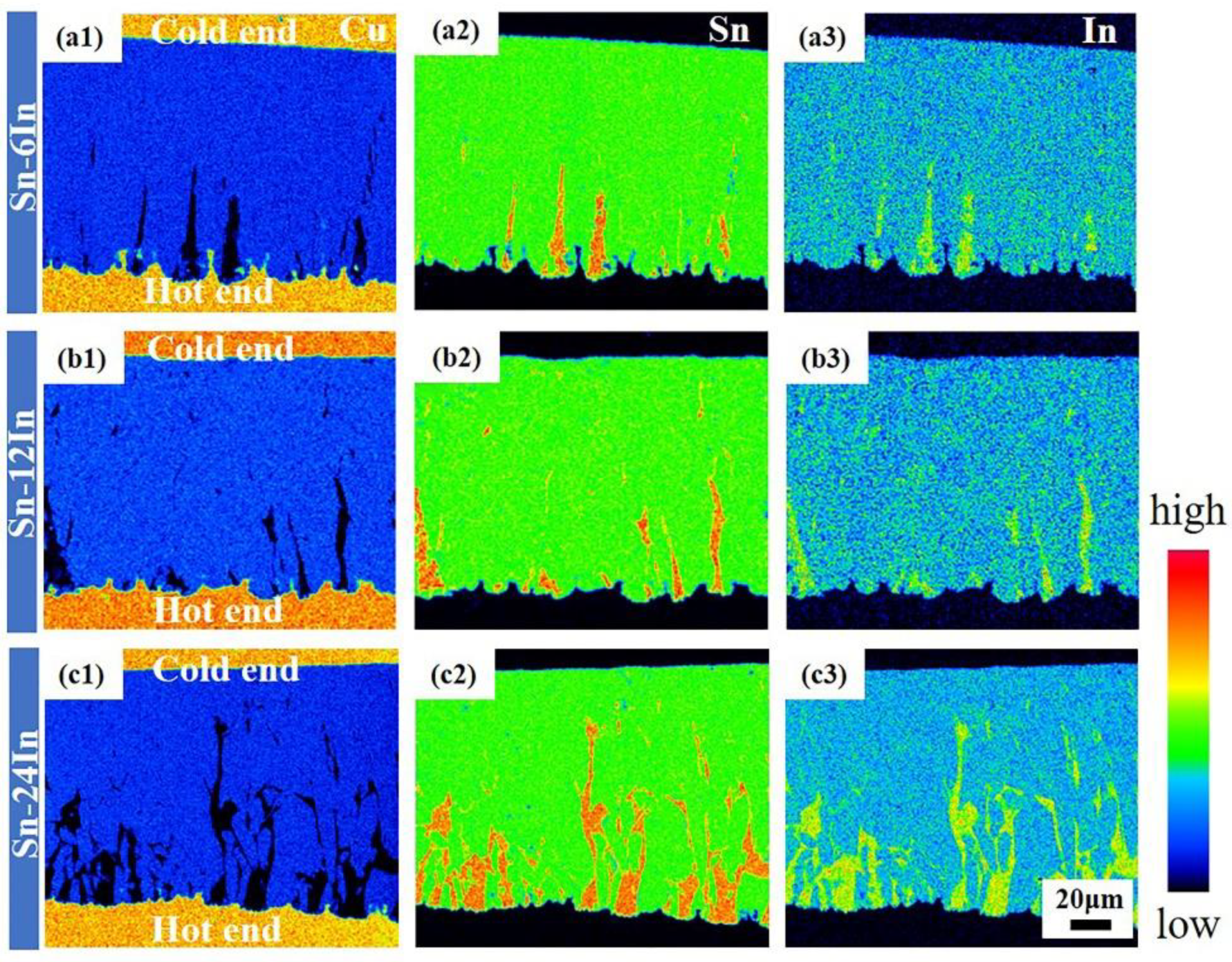
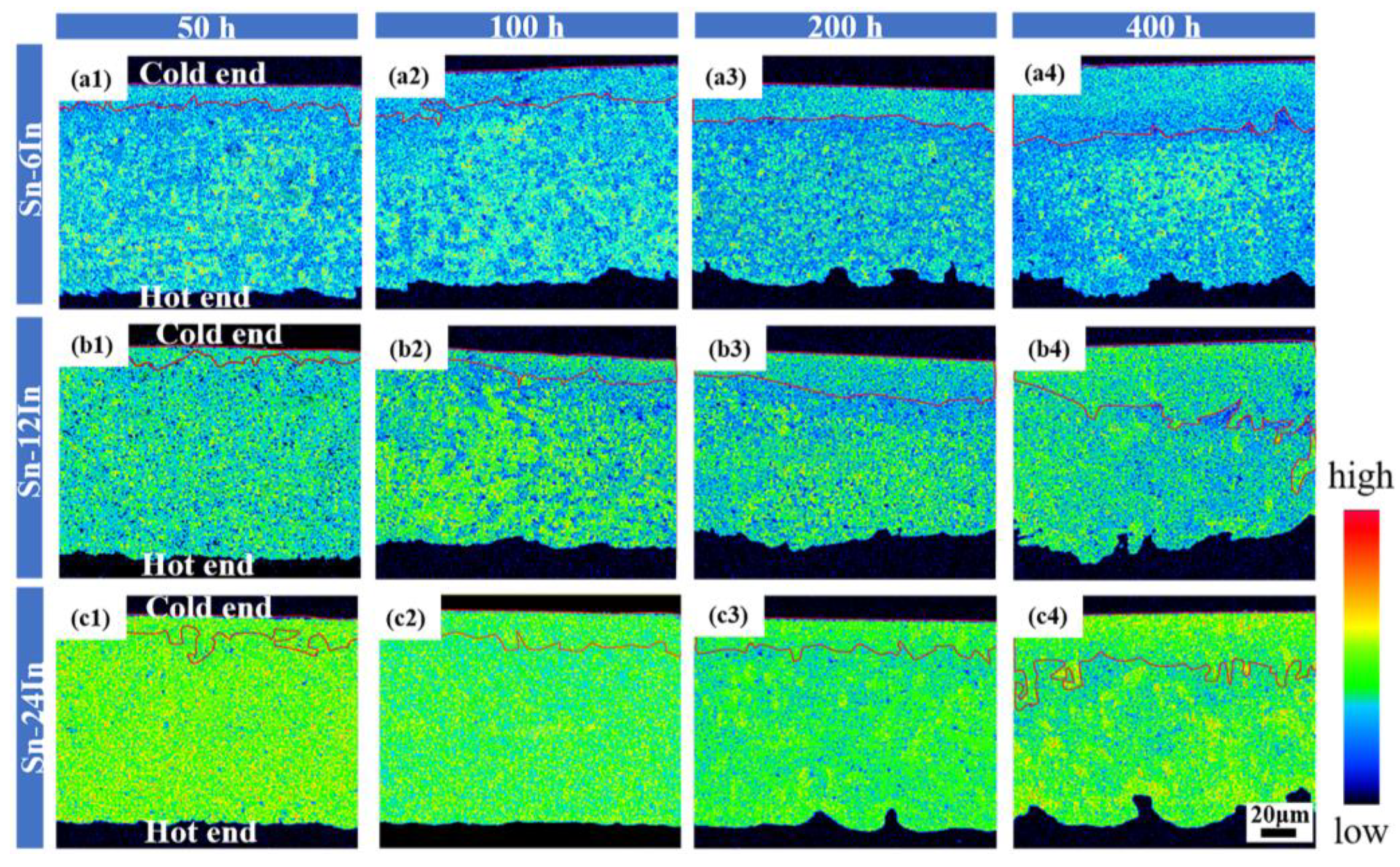
3.2. Microstructure Evolution of the Cu/Sn-xIn/Cu Micro Solder Joints under TG
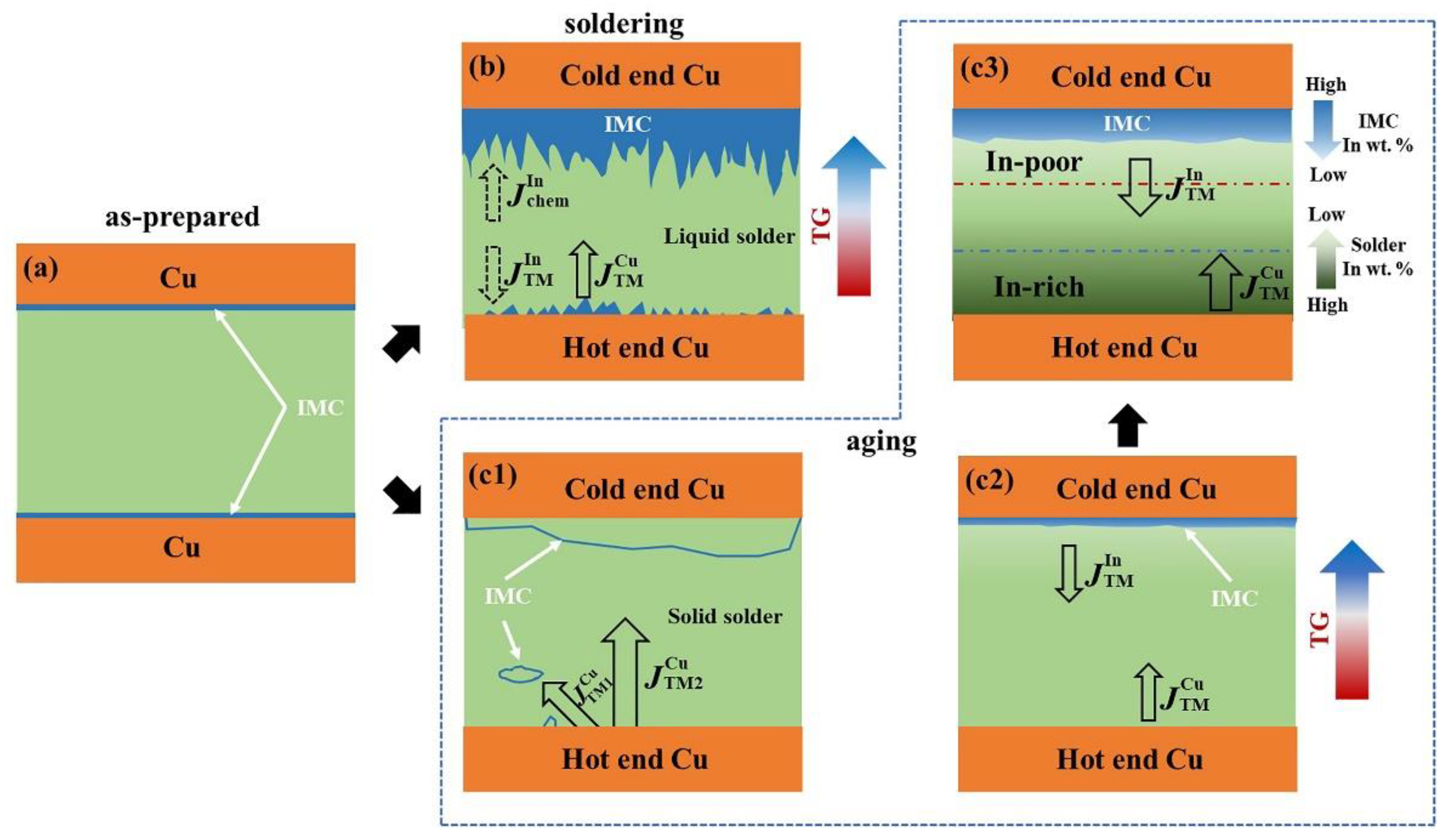
3.3. Mechanism of the Microstructure Evolution in the Micro Solder Joints
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fazal, M.A.; Liyana, N.K.; Rubaiee, S. A critical review on performance, microstructure and corrosion resistance of Pb-free solders. Measurement 2019, 134, 897–907. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.X.; Liu, Z.Y.; Jiu, Y.T.; Zhang, S.Y.; Geng, J.F.; Chen, X.M.; Wu, S.J.; He, P.; Long, W.M. Corrosion behavior of Sn-based lead-free solder alloys: A review. J. Mater. Sci.-Mater. Electron. 2020, 31, 9076–9090. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.Q. Inhibition of intermetallic compounds growth at Sn-58Bi/Cu interface bearing CuZnAl memory particles (2~6μm). J. Mater. Sci.-Mater. Electron. 2020, 31, 2466–2480. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, K.N. Low melting point solders based on Sn, Bi, and In elements. Mater. Today Adv. 2020, 8, 100115. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.Q.; Zhang, J.; Xiao, Y. Effect of Ni foam addition on the microstructure and mechanical properties of In-48Sn eutectic alloy. J. Mater. Sci.-Mater. Electron. 2022, 33, 12594–12603. [Google Scholar] [CrossRef]
- Tian, S.; Li, S.P.; Zhou, J.; Xue, F. Thermodynamic characteristics, microstructure and mechanical properties of Sn-0.7Cu-xIn lead-free solder alloy. J. Alloys Compd. 2018, 742, 835–843. [Google Scholar] [CrossRef]
- Sungkhaphaitoon, P.; Chantaramanee, S. Effects of Indium Content on Microstructural, Mechanical Properties and Melting Temperature of SAC305 Solder Alloys. Russ. J. Non-Ferrous Met. 2018, 59, 385–392. [Google Scholar] [CrossRef]
- Tian, F.; Li, C.F.; Zhou, M.; Liu, Z.Q. The interfacial reaction between In-48Sn solder and polycrystalline Cu substrate during solid state aging. J. Alloys Compd. 2018, 740, 500–509. [Google Scholar] [CrossRef]
- Gao, P.F.; Ma, X.; Chen, X.G.; Li, L.F.; Deng, Y. The microstructure, melting properties and wettability of Sn3.5Ag0.7CuxIn lead-free solder alloys. Philos. Mag. 2022, 102, 321–339. [Google Scholar]
- Wang, Z.; Zhang, Q.K.; Chen, Y.X.; Song, Z.L. Influences of Ag and In alloying on Sn-Bi eutectic solder and SnBi/Cu solder joints. J. Mater. Sci.-Mater. Electron. 2019, 30, 18524–18538. [Google Scholar] [CrossRef]
- Yang, A.C.; Xiao, K.X.; Duan, Y.H.; Li, C.J.; Yi, J.H.; Peng, M.J.; Shen, L. Effect of indium on microstructure, mechanical properties, phase stability and atomic diffusion of Sn-0.7Cu solder: Experiments and first-principles calculations. Mater. Sci. Eng. R-Rep. 2022, 855, 143938. [Google Scholar] [CrossRef]
- Hu, X.W.; Lai, Y.Q.; Jiang, X.X.; Li, Y.L. Effects of In addition on the wettability, interfacial characterization and properties of ternary Sn–Cu–Ni solders. J. Mater. Sci.-Mater. Electron. 2018, 29, 18840–18851. [Google Scholar] [CrossRef]
- Pandey, P.; Tiwary, C.S.; Chattopadhyay, K. Effects of Cu and In Trace Elements on Microstructure and Thermal and Mechanical Properties of Sn-Zn Eutectic Alloy. J. Electron. Mater. 2019, 48, 2660–2669. [Google Scholar] [CrossRef]
- Wang, D.L.; Yuan, Y.; Luo, L. Indium Addition on Intermetallic Compound Evolution in Tin-Silver Solder Bump. Mater. Trans. 2011, 52, 1522–1524. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, E.L.; Chu, Q.L.; Xu, H.R.; Luo, H.L. Influence of Indium on Microstructure and Properties of Sn-Pb Binary Alloy Applied in Photovoltaic Ribbon. J. Mater. Eng. Perform. 2019, 28, 3714–3723. [Google Scholar] [CrossRef]
- Nabihah, A.; Nurulakmal, M.S. Effect of In Addition on Microstructure, Wettability and Strength of Sn-Cu Solder. Mater. Today Proc. 2019, 17, 803–809. [Google Scholar] [CrossRef]
- Wang, D.L.; Yuan, Y.; Luo, L. Liquid-solid interfacial reactions of Sn-Ag and Sn-Ag-In solders with Cu under bump metallization. J. Mater. Sci.-Mater. Electron. 2012, 23, 61–67. [Google Scholar] [CrossRef]
- Tian, S.; Li, S.P.; Zhou, J.; Xue, F.; Cao, R.H. Effect of indium addition on interfacial IMC growth and bending properties of eutectic Sn-0.7Cu solder joints. J. Mater. Sci.-Mater. Electron. 2017, 28, 16120–16132. [Google Scholar] [CrossRef]
- Kelly, M.B.; Antoniswamy, A.; Mahajan, R.; Chawla, N. Effect of Trace Addition of In on Sn-Cu Solder Joint Microstructure under Electromigration. J. Electron. Mater. 2021, 50, 893–902. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, C.; Tu, K.N. Failure induced by thermomigration of interstitial Cu in Pb-free flip chip solder joints. Appl. Phys. Lett. 2008, 93, 122103. [Google Scholar] [CrossRef]
- Qiao, Y.Y.; Ma, H.T.; Yu, F.Y.; Zhao, N. Quasi-in-situ observation on diffusion anisotropy dominated asymmetrical growth of Cu-Sn IMCs under temperature gradient. Acta Mater. 2021, 217, 117168. [Google Scholar] [CrossRef]
- Zhao, N.; Zhong, Y.; Huang, M.L.; Ma, H.T.; Dong, W. Growth kinetics of Cu6Sn5 intermetallic compound at liquid-solid interfaces in Cu/Sn/Cu interconnects under temperature gradient. Sci. Rep. 2015, 5, 13491. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhao, N.; Ma, H.T.; Dong, W.; Huang, M.L. Retardation of thermomigration-induced Cu substrate consumption in Pb-free solder joints by Zn addition. J. Alloys Compd. 2017, 695, 1436–1443. [Google Scholar] [CrossRef]
- Sharif, A.; Chan, Y.C. Effect of indium addition in Sn-rich solder on the dissolution of Cu metallization. J. Alloys Compd. 2005, 390, 67–73. [Google Scholar] [CrossRef]
- Sasangka, W.A.; Gan, C.L.; Thompson, C.V.; Choi, W.K.; Wei, J. Influence of Bonding Parameters on the Interaction between Cu and Noneutectic Sn-In Solder Thin Films. J. Electron. Mater. 2011, 40, 2329–2336. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic Radii in Crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Kanlayasiri, K.; Sukpimai, K. Effects of indium on the intermetallic layer between low-Ag SAC0307-xIn lead-free solders and Cu substrate. J. Alloys Compd. 2016, 668, 169–175. [Google Scholar] [CrossRef]
- Huang, W.; Pan, K.L.; Zhang, J.; Gong, y.b. Effect of In-Doping on Mechanical Properties of Cu6Sn5-Based Intermetallic Compounds: A First-Principles Study. J. Electron. Mater. 2021, 50, 4164–4171. [Google Scholar] [CrossRef]
- Ouyang, F.Y.; Kao, C.L. In situ observation of thermomigration of Sn atoms to the hot end of 96.5Sn-3Ag-0.5Cu flip chip solder joints. J. Appl. Phys. 2011, 110, 022110. [Google Scholar] [CrossRef]
- Roush, W.; Jaspal, J. Thermomigration in lead-indium solder. In Proceedings of the 32nd Electronic Components and Technology Conference, San Diego, CA, USA, 10–12 May 1982; pp. 342–345. [Google Scholar]
- Ouyang, F.Y.; Huang, A.T.; Tu, K.N. Thermomigration in SnPb composite solder joints and wires. In Proceedings of the 56th Electronic Components and Technology Conference, San Diego, CA, USA, 30 May–2 June 2006; pp. 1974–1978. [Google Scholar]
- Chen, C.; Hsiao, H.Y.; Chang, Y.W.; Ouyang, F.Y.; Tu, K.N. Thermomigration in solder joints. Mater. Sci. Eng. R-Rep. 2012, 73, 85–100. [Google Scholar] [CrossRef]
- Shen, Y.N.; Lin, Y.X.; Ouyang, F.Y.; Nishikawa, H.; Tsai, M.H. Thermomigration suppression in Sn3.5Ag solder joints by hot-end FeCoNiMn alloy. Intermetallics 2023, 154, 17821. [Google Scholar] [CrossRef]

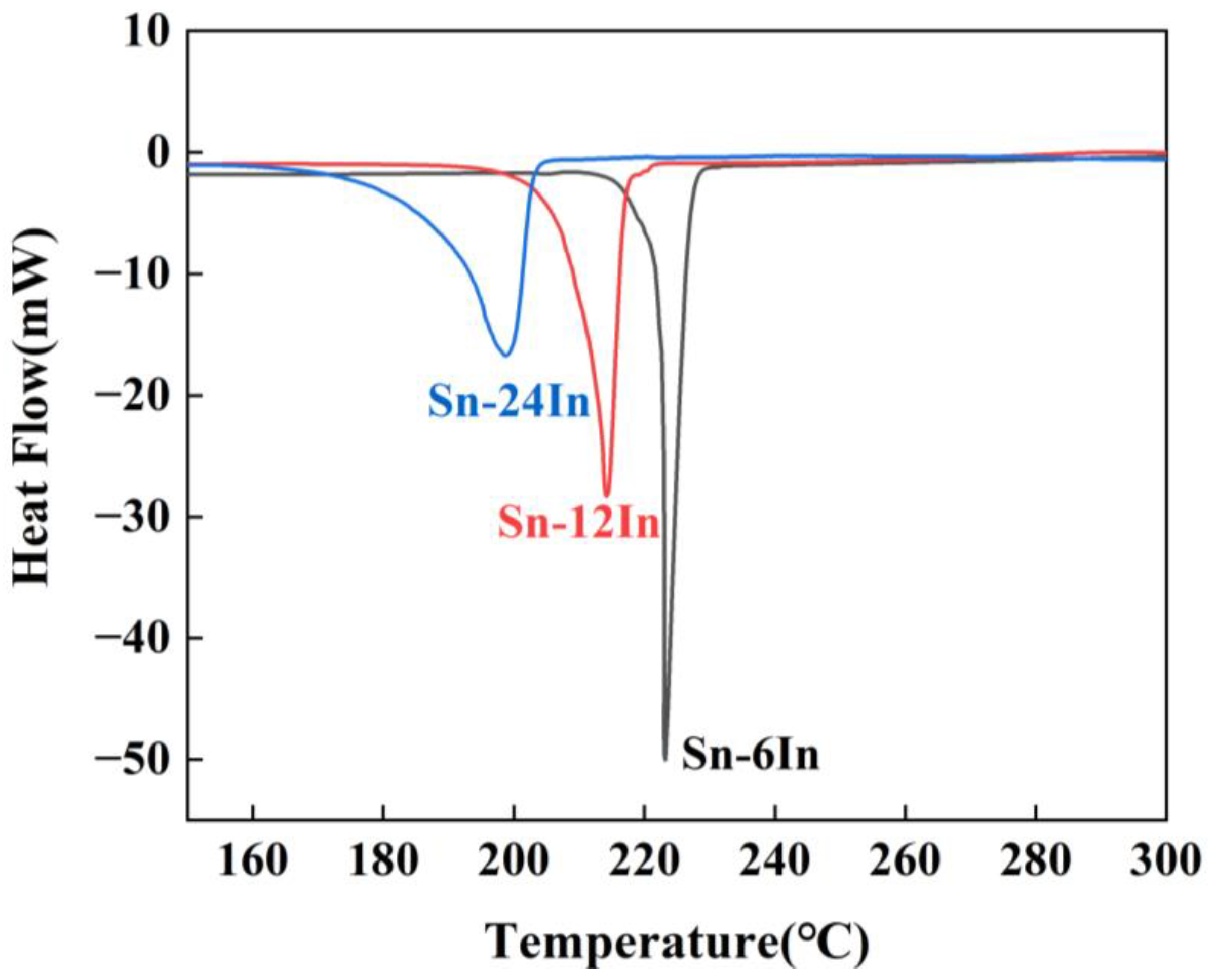


| Solder | T1 (°C) | Peak (°C) | T2 (°C) | ΔT (°C) |
|---|---|---|---|---|
| Sn-6In | 222.28 | 223.20 | 226.54 | 4.26 |
| Sn-12In | 210.21 | 214.16 | 216.86 | 6.65 |
| Sn-24In | 188.99 | 198.82 | 202.79 | 13.80 |
| Point | Cu (at.%) | Sn (at.%) | In (at.%) | IMC |
|---|---|---|---|---|
| A | 53.1 | 44.1 | 2.8 | Cu6(Sn,In)5 |
| B | 54.4 | 42.8 | 2.8 | Cu6(Sn,In)5 |
| C | 54.2 | 39.4 | 6.4 | Cu6(Sn,In)5 |
| D | 52.6 | 42.6 | 4.8 | Cu6(Sn,In)5 |
| E | 52.2 | 42.9 | 4.9 | Cu6(Sn,In)5 |
| F | 54.5 | 33.7 | 11.8 | Cu6(Sn,In)5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Qiao, Y.; Ren, X.; Lai, Y.; Zhao, N. Characterization of Sn-xIn Solders and Thermomigration-Induced Interfacial IMC Growth of Cu/Sn-xIn/Cu Micro Solder Joints. Electronics 2023, 12, 1899. https://doi.org/10.3390/electronics12081899
Du Y, Qiao Y, Ren X, Lai Y, Zhao N. Characterization of Sn-xIn Solders and Thermomigration-Induced Interfacial IMC Growth of Cu/Sn-xIn/Cu Micro Solder Joints. Electronics. 2023; 12(8):1899. https://doi.org/10.3390/electronics12081899
Chicago/Turabian StyleDu, Yanfeng, Yuanyuan Qiao, Xiaolei Ren, Yanqing Lai, and Ning Zhao. 2023. "Characterization of Sn-xIn Solders and Thermomigration-Induced Interfacial IMC Growth of Cu/Sn-xIn/Cu Micro Solder Joints" Electronics 12, no. 8: 1899. https://doi.org/10.3390/electronics12081899




