Use of a Wireless Network of Accelerometers for Improved Measurement of Human Energy Expenditure
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Equipment
2.2.1. Wireless Accelerometer Network
2.2.2. Hip-Mounted Accelerometer
2.2.3. Portable Metabolic Analyzer
2.3. Description of Activities Performing during the Protocol
| Activity Category | Activity | Description of Activity |
|---|---|---|
| Sedentary Activities | (1) Lying down | Participants lay still on a mat, with arms at sides and feet straight out and not crossed. Participants were not allowed to sleep. |
| (2) Sitting reclined | Participants leaned back in their chair, extending their legs in front of them (while still resting them on the floor) and keeping their hands in their laps. | |
| (3) Sitting straight | Participants sat still in a chair with arms resting in their lap and feet flat on the floor. | |
| Ambulatory Activities | (6) Walking slow | Participants walked at 2.0 miles/hour on a treadmill without holding handrails. |
| (9) Walking fast | Participants walked at 4.0 miles/hour on a treadmill without holding handrails. | |
| (14) Jogging | Participants jogged at 6.0 miles/hour on a treadmill without holding handrails. | |
| Lifestyle Activities | (4) Standing | Participants stood still, keeping feet together and arms at their sides. |
| (7) Sweeping | Participants swept confetti back and forth between two cones eight feet apart. Participants swept at a self-selected pace. | |
| (12) Stair climbing | Participants climbed stairs on a stepmill exercise machine at a rate of 60 steps/min without holding handrails. | |
| Exercise Activities | (5) Bicep curls | Participants performed biceps flexion and extension at a self-selected pace while holding an unweighted broom handle and standing still. |
| (8) Cycling slow | Participants cycled on a cycle ergometer at 50 W (50 rpm and 1 kilopond resistance). | |
| (10) Squatting | Participants started with an unweighted broom handle behind the head with feet shoulder width apart. Then, participants bent at the knee until 90° flexion before returning to an upright position. Squats were performed at a self-selected pace. | |
| (11) Cycling fast | Participants cycled on a cycle ergometer at 75 W (75 rpm and 1 kilopond resistance). | |
| (13) Jumping jacks | Participants started in a standing position with feet together and hands at their sides. Then, they jumped, spreading their feet to shoulder width and extending arms upward, clapping hands together above their head before jumping back to the original position. This was performed at a self-selected pace. |
2.4. Data Reduction
2.4.1. Artificial Neural Networks
 (which is a linear function) and H is the number of nodes in the hidden layer.
(which is a linear function) and H is the number of nodes in the hidden layer.
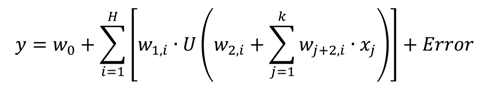
2.4.2. Oxycon and Accelerometer Data Collection and Processing
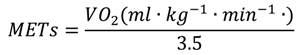
2.5. Statistical Analyses

3. Results
| a. Participants Included in Final Analysis | b. Participants Excluded from Final Analysis | |||||
|---|---|---|---|---|---|---|
| Total Sample (n = 23) | Females (n = 16) | Males (n = 7) | Total Sample (n = 7) | Females (n = 4) | Males (n = 3) | |
| Age (years) | 20.8 (1.4) | 20.5 (1.4) | 21.4 (1.4) | 21.0 (0.8) | 20.8 (0.5) | 21.3 (1.2) |
| Height (cm) | 168.5 (10.0) | 163.0 (5.1) | 181.1 (5.9) | 173.1 (6.6) | 169.3 (5.9) | 178.1 (3.5) |
| Weight (kg) | 66.0 (13.9) | 58.3 (5.1) | 83.4 (11.3) | 77.4 (8.9) * | 76.0 (11.3) * | 79.3 (5.8) |
| BMI (kg/m2) | 23.0 (2.6) | 21.9 (1.5) | 25.4 (3.0) | 25.9 (3.3) | 26.6 (4.4) | 25.0 (1.4) |
| Percent fat (%) | 23.9 (4.3) | 26.1 (2.9) | (2.2) | 21.5 (6.9) | 25.9 (3.9) | 15.7 (5.7) |
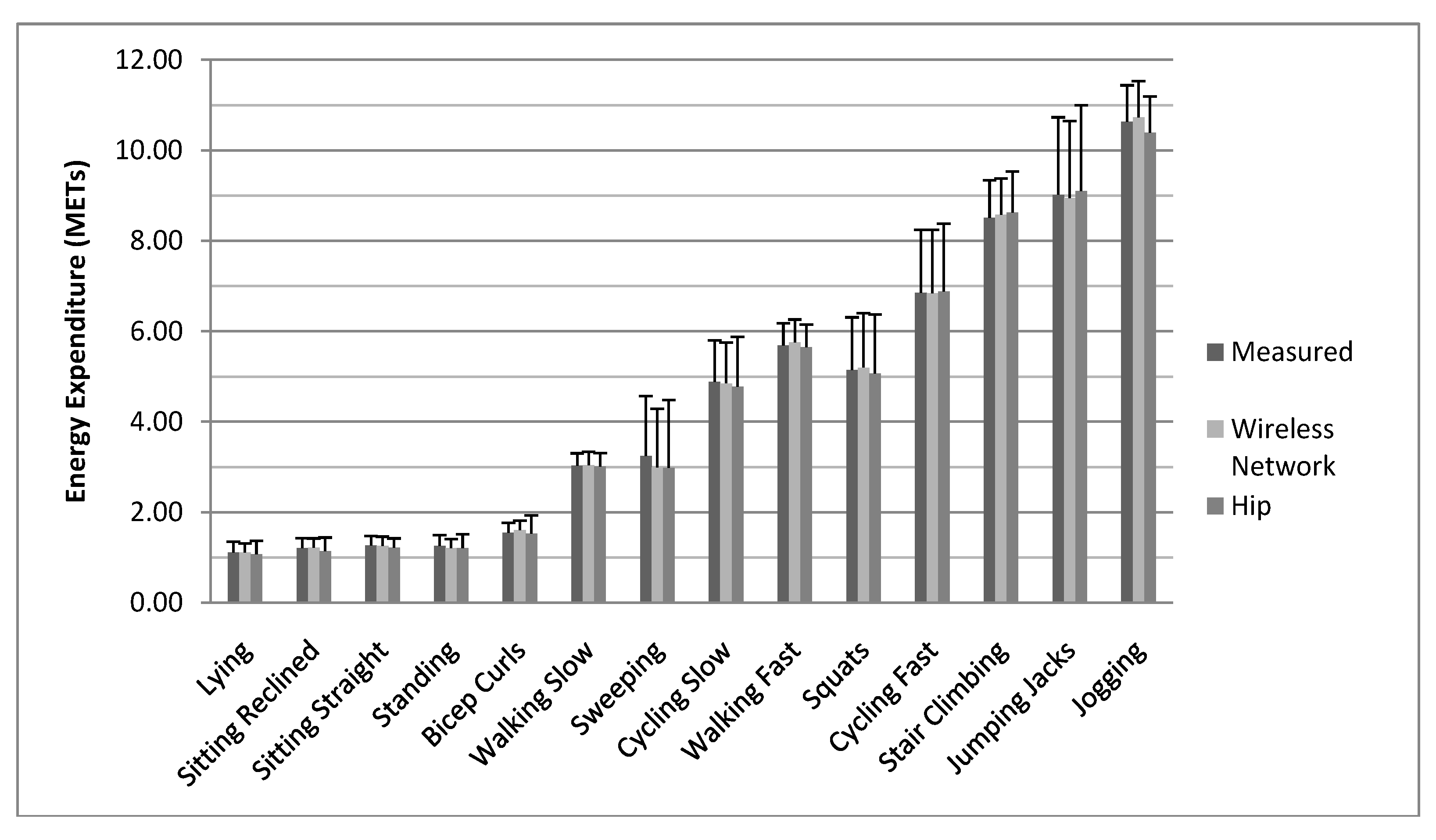
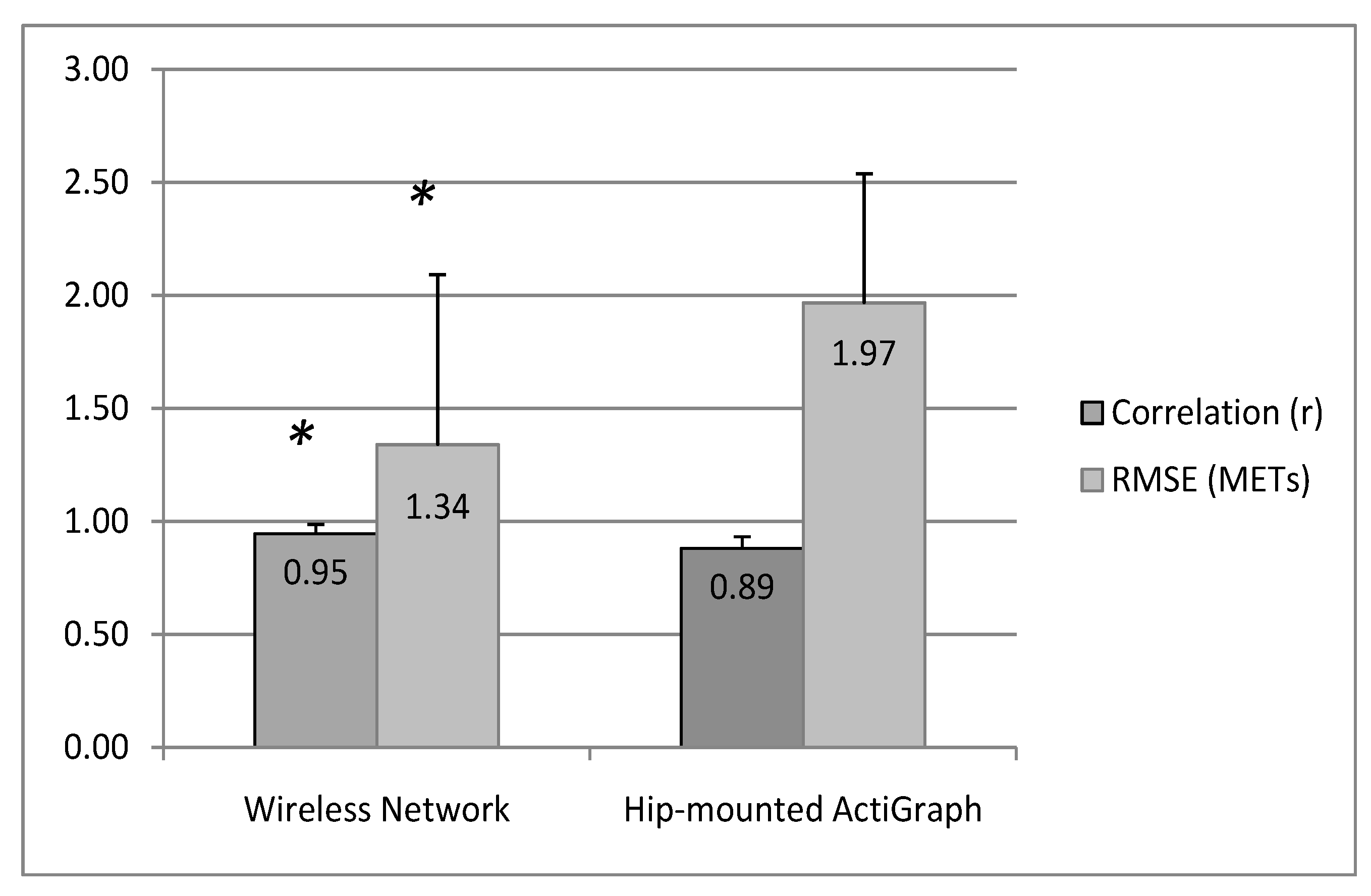
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ward, D.S.; Evenson, K.R.; Vaughn, A.; Rodgers, A.B.; Troiano, R.P. Accelerometer use in physical activity: Best practices and research recommendations. Med. Sci. Sports. Exerc. 2005, 37, S582–S588. [Google Scholar]
- Celis-Morales, C.A.; Perez-Bravo, F.; Ibanez, L.; Salas, C.; Bailey, M.E.; Gill, J.M. Objective vs. self-reported physical activity and sedentary time: Effects of measurement method on relationships with risk biomarkers. PLoS One 2012, 7, e36345. [Google Scholar]
- Freedson, P.S.; Miller, K. Objective monitoring of physical activity using motion sensors and heart rate. Res. Q. Exerc. Sport 2000, 71, S21–S29. [Google Scholar]
- John, D.; Freedson, P. ActiGraph and Actical physical activity monitors: A peek under the hood. Med. Sci. Sports. Exerc. 2012, 44, S86–S89. [Google Scholar] [CrossRef]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Welk, G.J. Use of Accelerometry-Based Activity Monitors to Assess Physical Activity. In Physical Activity Assessments for Health-Related Research; Welk, G.J., Ed.; Human Kinetics, Inc.: Champaign, IL, USA, 2002; pp. 125–142. [Google Scholar]
- Staudenmayer, J.; Pober, D.; Crouter, S.; Bassett, D.; Freedson, P. An artificial neural network to estimate physical activity energy expenditure and identify physical activity type from an accelerometer. J. Appl. Physiol. 2009, 107, 1300–1307. [Google Scholar] [CrossRef]
- Swartz, A.M.; Strath, S.J.; Bassett, D.R., Jr.; O’Brien, W.L.; King, G.A.; Ainsworth, B.E. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med. Sci. Sports Exerc. 2000, 32, S450–S456. [Google Scholar] [CrossRef]
- Preece, S.J.; Goulermas, J.Y.; Kenney, L.P.; Howard, D.; Meijer, K.; Crompton, R. Activity identification using body-mounted sensors--a review of classification techniques. Physiol. Meas. 2009, 30, R1–R33. [Google Scholar] [CrossRef]
- Freedson, P.S.; Lyden, K.; Kozey-Keadle, S.; Staudenmayer, J. Evaluation of artificial neural network algorithms for predicting METs and activity type from accelerometer data: validation on an independent sample. J. Appl. Physiol. 2011, 111, 1804–1812. [Google Scholar] [CrossRef]
- Rothney, M.P.; Neumann, M.; Beziat, A.; Chen, K.Y. An artificial neural network model of energy expenditure using nonintegrated acceleration signals. J. Appl. Physiol. 2007, 103, 1419–1427. [Google Scholar] [CrossRef]
- Parkka, J.; Ermes, M.; Korpipaa, P.; Mantyjarvi, J.; Peltola, J.; Korhonen, I. Activity classification using realistic data from wearable sensors. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 119–128. [Google Scholar] [CrossRef]
- Zhang, K.; Werner, P.; Sun, M.; Pi-Sunyer, F.X.; Boozer, C.N. Measurement of human daily physical activity. Obes. Res. 2003, 11, 33–40. [Google Scholar] [CrossRef]
- Zhang, K.; Pi-Sunyer, F.X.; Boozer, C.N. Improving energy expenditure estimation for physical activity. Med. Sci. Sports Exerc. 2004, 36, 883–889. [Google Scholar]
- Welk, G.J.; McClain, J.J.; Eisenmann, J.C.; Wickel, E.E. Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity (Silver Spring) 2007, 15, 918–928. [Google Scholar] [CrossRef]
- Heil, D.P.; Bennett, G.G.; Bond, K.S.; Webster, M.D.; Wolin, K.Y. Influence of activity monitor location and bout duration on free-living physical activity. Res. Q. Exerc. Sport. 2009, 80, 424–433. [Google Scholar] [CrossRef]
- Whybrow, S.; Ritz, P.; Horgan, G.W.; Stubbs, R.J. An evaluation of the IDEEA activity monitor for estimating energy expenditure. Br. J. Nutr. 2013, 109, 173–183. [Google Scholar] [CrossRef]
- Tapia, E.M.; Intillie, S.S.; Haskell, W.; Larson, K.; Wright, J.; King, A.; Friedman, R. Real-time recognition of physical activities and their intensities using wireless accelerometers and a heart rate monitor. In Proceedings of the 2007 11th IEEE International Symposium on Wearable Computers, Boston, MA, USA, 11–13 October 2007; pp. 37–40.
- Intille, S.S.; Albinali, F.; Mota, S.; Kuris, B.; Botana, P.; Haskell, W.L. Design of a wearable physical activity monitoring system using mobile phones and accelerometers. In Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Boston, MA, USA, 30 August–3 September 2011; pp. 3636–3639.
- Khan, J.Y.; Yuce, M.R.; Karami, F. Performance evaluation of a Wireless Body Area sensor network for remote patient monitoring. In Proccedings of the 30th Annual International Conference of the IEEE, Engineering in Medicine and Biology Society (EMBC), Vancouver, BC, Canada, 20–25 August 2008; pp. 1266–1269.
- Lin, B.S.; Chou, N.K.; Chong, F.C.; Chen, S.J. RTWPMS: A real-time wireless physiological monitoring system. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 647–656. [Google Scholar] [CrossRef]
- Bonato, P. Wearable sensors and systems. From enabling technology to clinical applications. IEEE Eng. Med. Biol. Mag. 2010, 29, 25–36. [Google Scholar] [CrossRef]
- Dong, B.; Montoye, A.; Moore, R.; Pfeiffer, K.; Biswas, S. Energy-aware activity classification using wearable sensor networks. 2013, 8723, 87230Y. [Google Scholar]
- Mo, L.; Liu, S.; Gao, R.X.; John, D.; Staudenmayer, J.W.; Freedson, P.S. Wireless design of a multisensor system for physical activity monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 3230–3237. [Google Scholar] [CrossRef]
- Malina, R.M. Anthropometry. In Physiological Assessment of Human Fitness; Maud, P., Foster, C., Eds.; Human Kinetics: Champaign, IL, USA, 1995; pp. 205–219. [Google Scholar]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Chinapaw, M.J.; Mokkink, L.B.; van Poppel, M.N.; van Mechelen, W.; Terwee, C.B. Physical activity questionnaires for youth: A systematic review of measurement properties. Sports Med. 2010, 40, 539–563. [Google Scholar] [CrossRef]
- Rosdahl, H.; Gullstrand, L.; Salier-Eriksson, J.; Johansson, P.; Schantz, P. Evaluation of the Oxycon Mobile metabolic system against the Douglas bag method. Eur. J. Appl. Physiol. 2010, 109, 159–171. [Google Scholar]
- McClave, S.A.; Spain, D.A.; Skolnick, J.L.; Lowen, C.C.; Kieber, M.J.; Wickerham, P.S.; Vogt, J.R.; Looney, S.W. Achievement of steady state optimizes results when performing indirect calorimetry. J. Parenter. Enteral. Nutr. 2003, 27, 16–20. [Google Scholar] [CrossRef]
- Tabachnick, B.; Fidell, L. Using Multivariate Statistics, 6th ed.; Pearson: Upper Saddle River: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Kozey-Keadle, S.; Libertine, A.; Lyden, K.; Staudenmayer, J.; Freedson, P.S. Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sports Exerc. 2011, 43, 1561–1567. [Google Scholar]
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting: The population health science of sedentary behavior. Exerc. Sport Sci. Rev. 2010, 38, 105–113. [Google Scholar] [CrossRef]
- Trost, S.G.; McIver, K.L.; Pate, R.R. Conducting accelerometer-based activity assessments in field-based research. Med. Sci. Sports Exerc. 2005, 37, S531–S543. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Montoye, A.H.; Dong, B.; Biswas, S.; Pfeiffer, K.A. Use of a Wireless Network of Accelerometers for Improved Measurement of Human Energy Expenditure. Electronics 2014, 3, 205-220. https://doi.org/10.3390/electronics3020205
Montoye AH, Dong B, Biswas S, Pfeiffer KA. Use of a Wireless Network of Accelerometers for Improved Measurement of Human Energy Expenditure. Electronics. 2014; 3(2):205-220. https://doi.org/10.3390/electronics3020205
Chicago/Turabian StyleMontoye, Alexander H., Bo Dong, Subir Biswas, and Karin A. Pfeiffer. 2014. "Use of a Wireless Network of Accelerometers for Improved Measurement of Human Energy Expenditure" Electronics 3, no. 2: 205-220. https://doi.org/10.3390/electronics3020205




