Organic Thin-Film Transistor (OTFT)-Based Sensors
Abstract
:1. Introduction
Organic Thin Film Transistors
- -
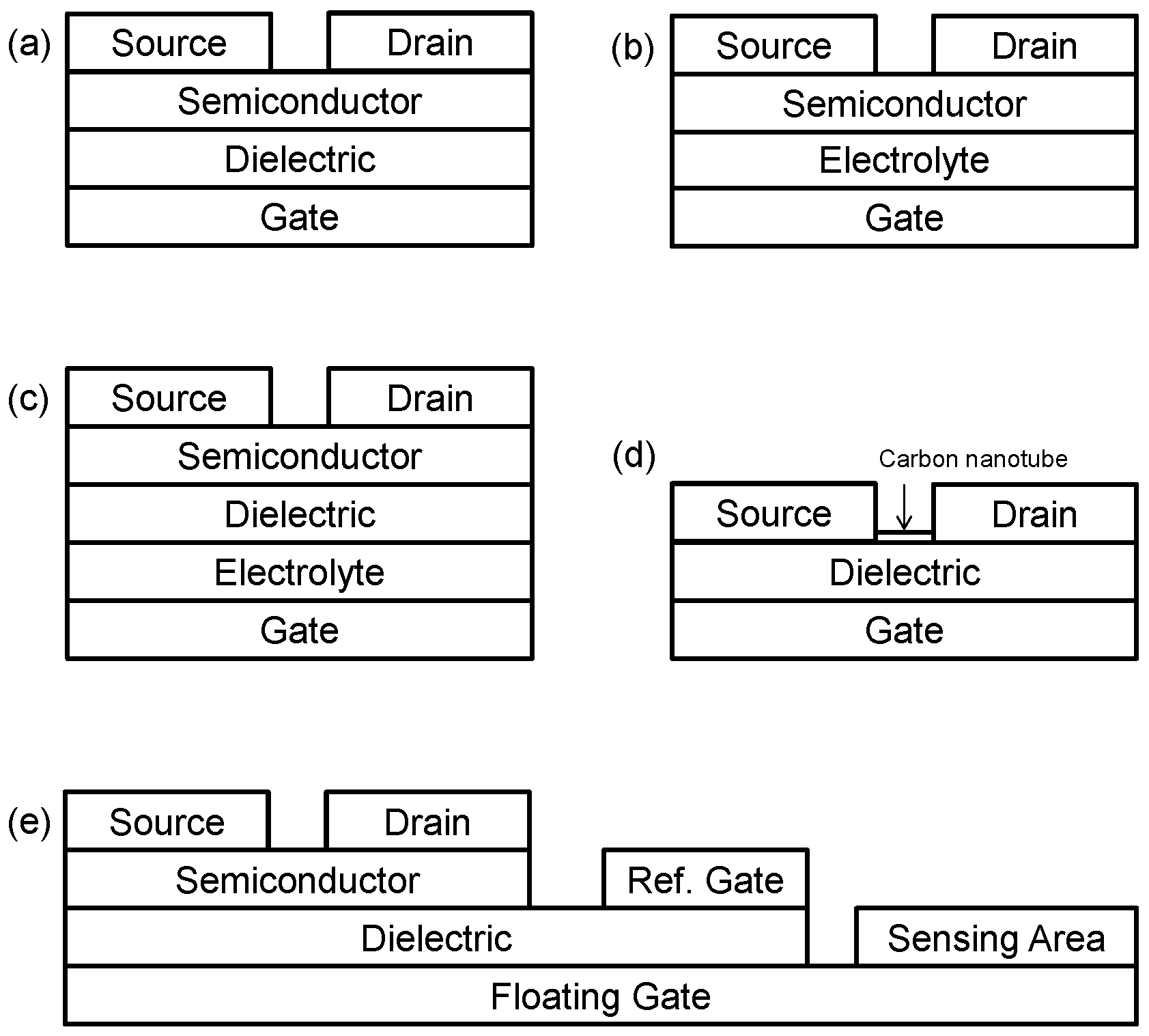
- Organic field effect-transistors (OFETs): OFETs operate in a similar way to conventional MOSFET (metal-oxide-semiconductor field effect transistor) or TFT (thin-film transistor) devices in which an electric field is established across a dielectric layer separating the gate electrode from the semiconductor layer (Figure 1a). This electric field can manipulate the size and shape of a region of high conductivity in, and hence, modulate the current flowing through, the semiconductor material, creating a relationship between gate voltage (VG) and drain current (ID).
- -
- Organic electrochemical transistors (OECTs): OECTs operate by inducing a reduction or oxidation reaction, which influences ID, due to a voltage at the gate electrode. Often, these devices can resemble conventional three-terminal electrochemical cells in which the source, drain and gate electrodes play the roles of the working, counter and reference electrodes. Other types of OECTs more closely resemble a standard OFET in which some electrochemical reaction is taking place at an interface of the semiconductor to facilitate current modulation. Many of the other categories of devices listed below could be thought of as sub-categories of OECTs.
- -
- Electrolyte-gated organic field effect transistors (EGOFETs): EGOFETs employ an electrolyte layer (in either a solid or liquid form) to separate the gate electrode from the semiconductor layer (Figure 1b). This electrolyte layer allows for ionic movement within it, and this leads to the build-up of charge at its interfaces and subsequent electrochemical reactions. One advantage of EGOFETs is their low operating voltage; however, they can suffer from poor switching speeds, due to their reliance on electrochemical activity [6]. EGOFETs featuring ion-selective membranes, which could improve their ability to be applied in sensing applications, have also been successfully demonstrated recently [7].
- -
- Ion-sensitive OFETs (ISOFETs): an analogue to the ion-sensitive FET (ISFET) family of silicon-based devices, ISOFETs are similar to EGOFETs in that they have an electrolyte layer adjacent to the gate electrode (Figure 1c). However, unlike EGOFETs, ISOFETs also have a dielectric layer, which isolates the electrolyte from the semiconductor [8].
- -
- Hygroscopic-insulator field effect transistors (HIFETs): HIFETs could be considered a sub-category of both OECTs and EGOFETs. Initially proposed by the group of Österbacka et al. [9], HIFETs rely on the hygroscopic nature of their dielectric layer to create a moist environment for the free movement of ions within it. These ions then can interact with the semiconductor at the dielectric/semiconductor interface electrochemically and/or electrostatically to modulate current through the semiconductor (i.e., ID) by varying VG.
- -
- Carbon nanotube FETs (CNTFET or NTFETs): these devices use one or many carbon nanotubes as the material (semiconductor) connecting the source and drain electrodes (Figure 1d). CNTFETs are seen as promising devices for use in all types of electronics, due to the excellent electronic properties of carbon nanotubes (CNTs) [10]. In terms of biosensors, encapsulation by CNTs has been shown as an effective way to immobilise bio-recognition elements [11].
- -
- Organic charge-modulated FETs (OCMFETs): OCMFETs are a relatively new category of organic transistor, which have been proposed by the group of Bonfiglio et al. specifically for the purpose of sensing, and are somewhat similar in structure to ISOFETs [12,13]. However, OCMFETs have two gate electrodes: one, a “reference gate”, which is held at a fixed electrical potential (biasing the device), as well as a “floating gate”, which is coupled to the other gate electrode and to the rest of the device through a common dielectric layer (Figure 1e). The floating gate is electrically connected to the “sensing area” upon which charge is accumulated, depending on the quantity of analyte present. Two of the main claimed advantages of OCMFETs is that they can be miniaturised relative to similar devices implementing a reference electrode and also that the semiconductor is not directly exposed to analyte solution.
2. Biosensors
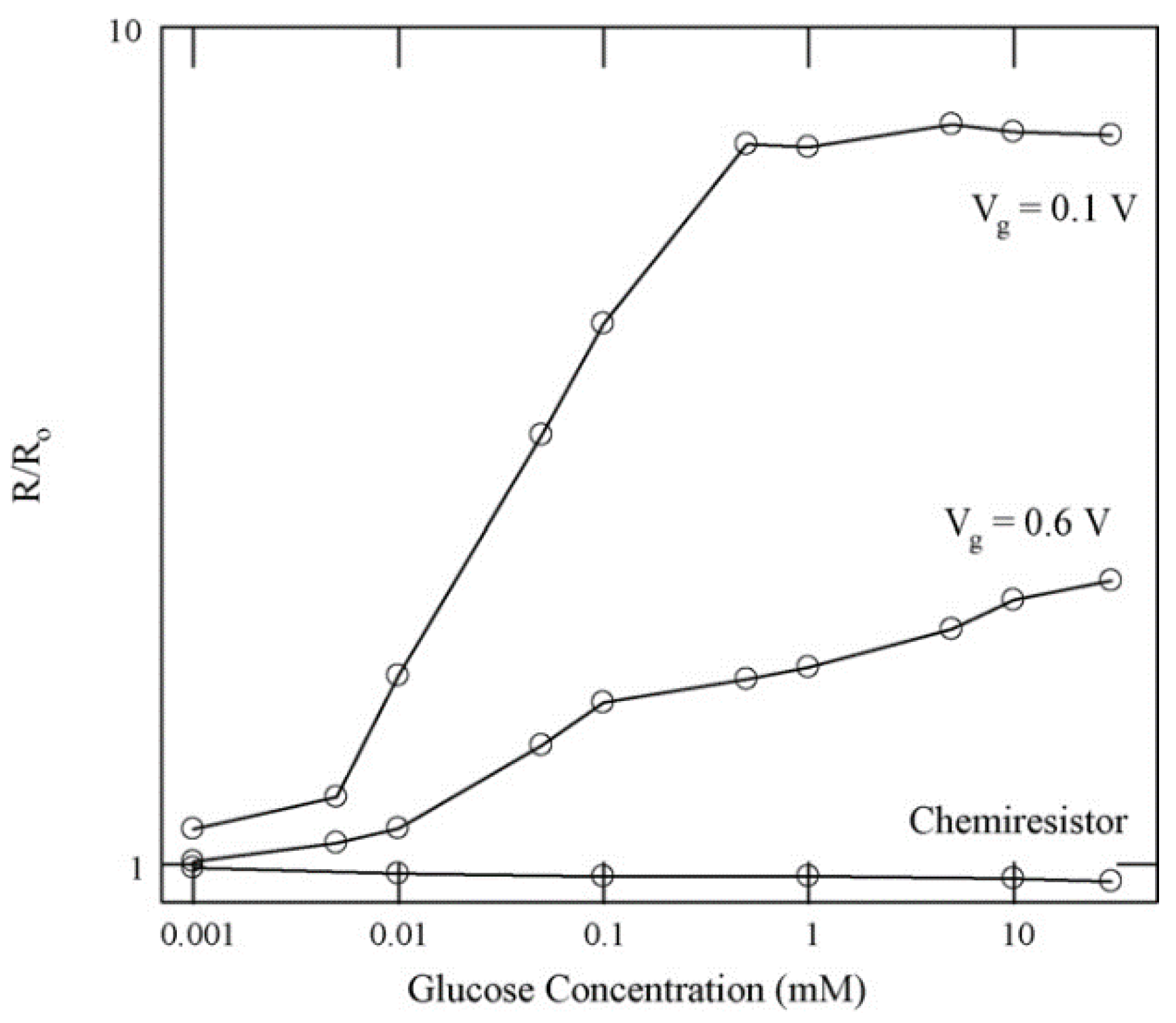
2.1. Enzymatic Glucose Sensing


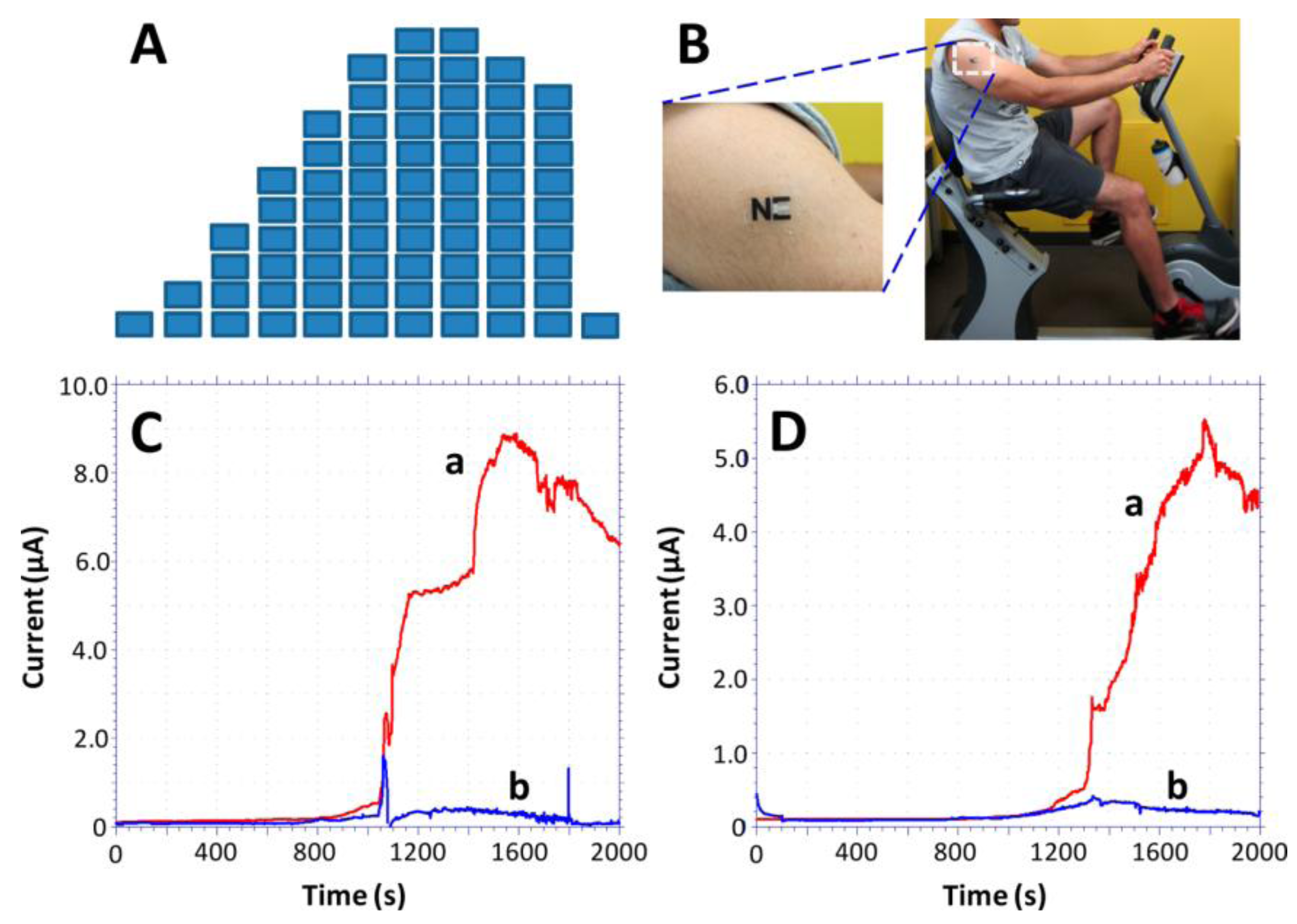
2.2. Other Enzymatic Sensors
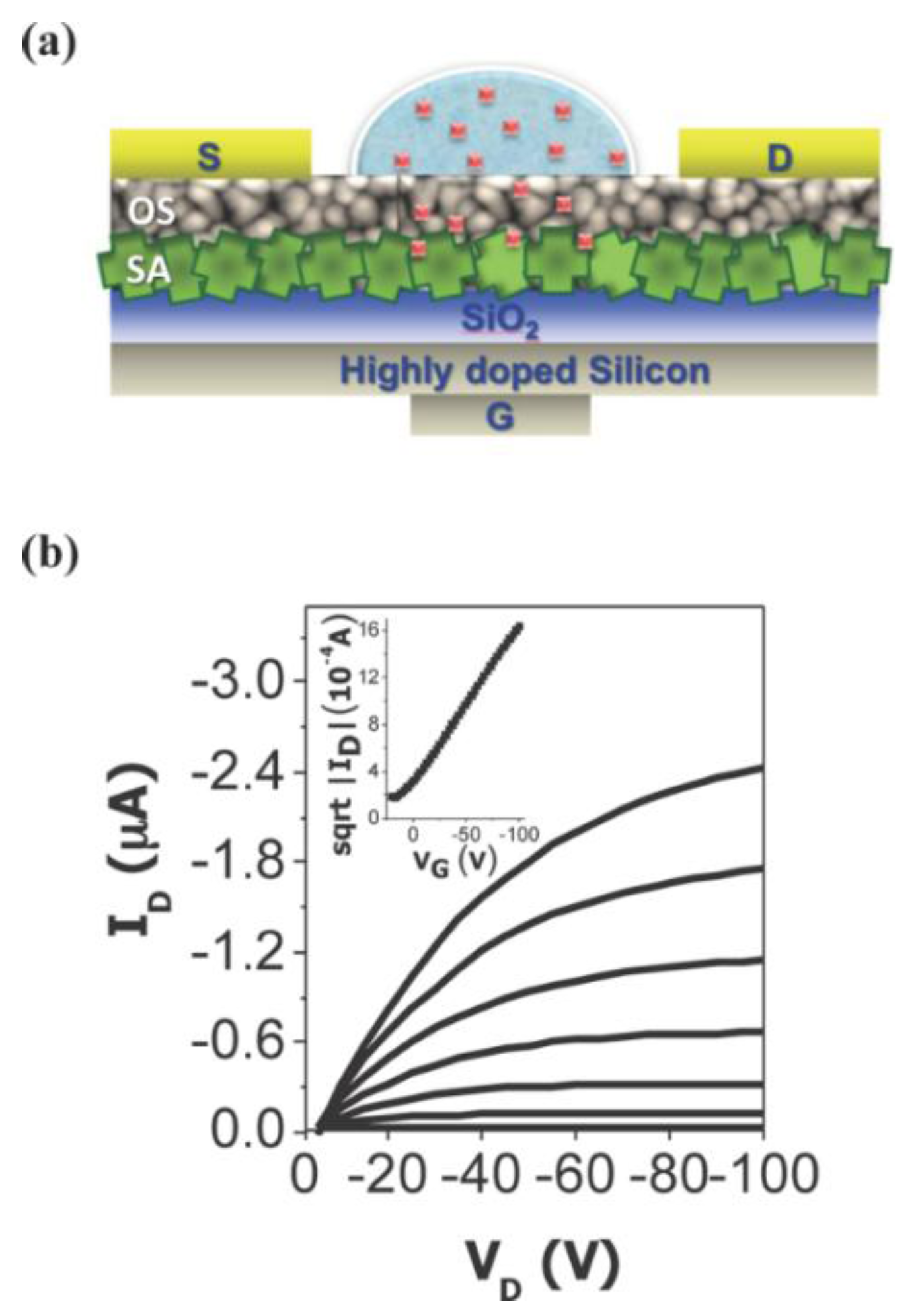
2.3. Non-Enzymatic Biosensors
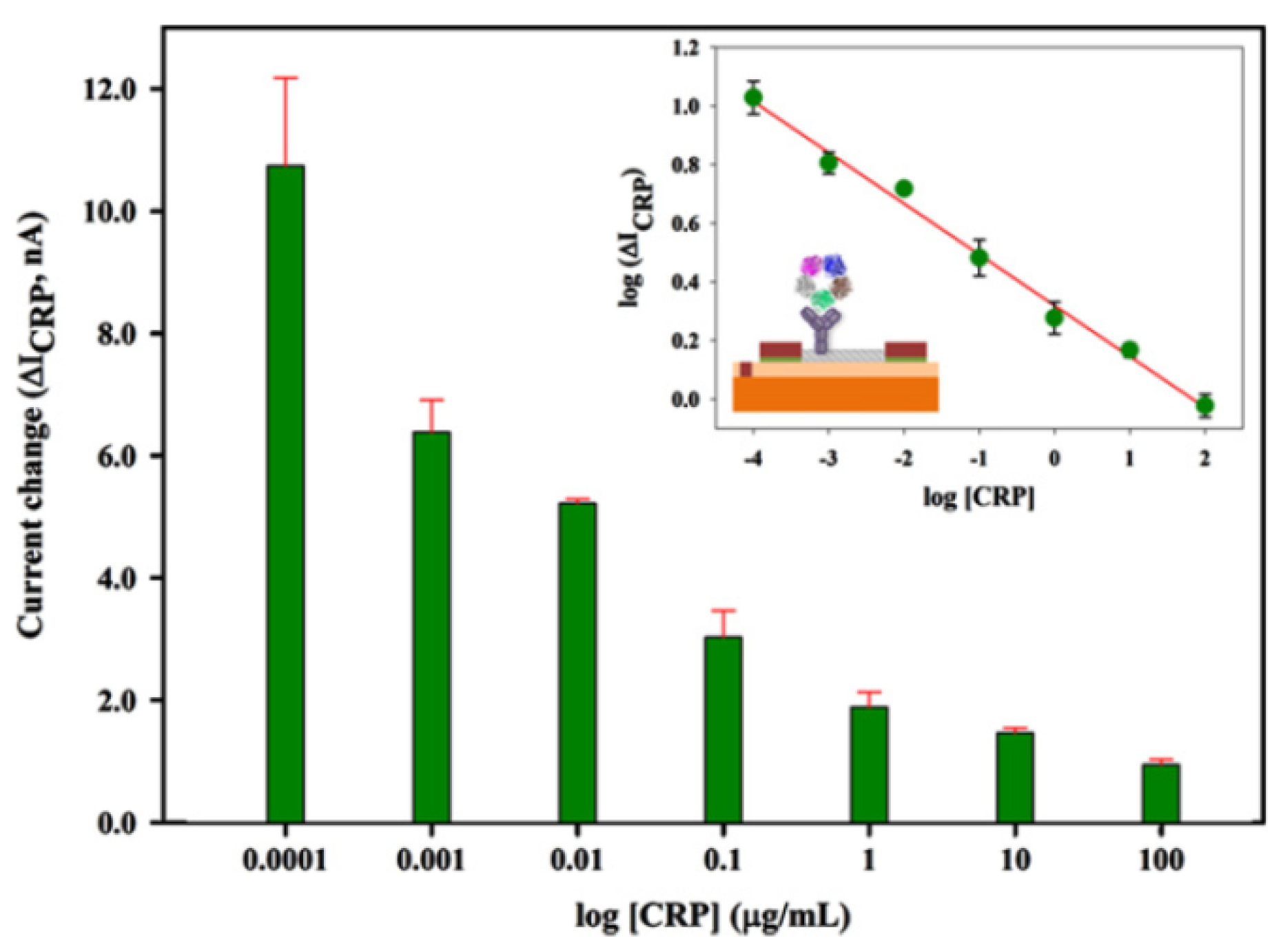
2.4. Biosensor Summary
| Analyte(s) | Recognition Element | Device Type | Claimed Lower and Upper Detection Limit | Year | Ref. | |
|---|---|---|---|---|---|---|
| Glucose | Glucose oxidase | OECT | 0.1 mM | 1 mM | 2004 | [23] |
| OECT | 1 μM | 30 mM | 2007 | [20] | ||
| OECT | 1 μM | 1 mM | 2007 | [24] | ||
| OTFT | 1.1 mM | 16.5 mM | 2008 | [25] | ||
| OECT (device and analyte in solution) | 5 nM | >1 mM | 2011 | [28] | ||
| OECT | 10 nM | 1 μM | 2013 | [26] | ||
| Lactic acid | CuPc * | OFET | 10 uM | 2 mM | 2002 | [35] |
| Urea | Urease | BioFET | 0.1 μM | 1 mM | 2010 | [29] |
| SEGFET (device and analyte in solution) | 50 μM | 10 mM | 2013 | [34] | ||
| Penicillin | Penicillinase | SGFET | 10 μM (approx.) | 600 μM | 2012 | [30] |
| Lactate | Lactose Oxidase | OECT | 10 mM | 100 mM | 2013 | [31] |
| Non-transistor electrochemical device | 1 mM | 30 mM | 2013 | [33] | ||
| Liposome | PEDOT:PSS * | OECT | 10 μg∙mL−1 | 0.1 mg∙mL−1 | 2013 | [45] |
| Biotin | Streptavidin | FBI-OFET | 15 pM | 500 nM | 2013 | [36] |
| Streptavidin | Biotin | FBI-OFET | 10 nM | 1 μM | 2013 | [37] |
| C-reactive protein (CRP) | CRP antibodies | NTFET | 0.1 ng∙mL−1 | 100 μg∙mL−1 | 2013 | [11] |
| Cucumber mosaic virus (CMV) | CMV antibodies | Chemiresistor | 1 ng∙mL−1 | 100 μg∙mL−1 | 2013 | [38] |
| Triglyceride | Lipase, glycerol kinase and glycerol-3-phosphate oxidase | Non-transistor electrochemistry | 7.88 mg∙dL−1 | 531 mg∙dL−1 | 2013 | [32] |
| Dopamine | Metal electrode | OECT | 5 nM | 2011 | [47] | |
| Glial fibrillary acidic protein | GFAP antibodies | OTFT | 20 pM | 20 nM | 2014 | [46] |
| BSA | Anti-BSA | OTFT | 1 μM | 10 μM | 2011 | [48] |
| Anti-BSA | BSA | OTFT | 10 nM | 2 μM | 2011 | [49] |
| pH | n/a | Dual-gate OTFT | pH 2 | pH 10 | 2010 | [50] |
| DNA | Single-stranded DNA | OFET | 100 nM | 50 μM | 2010 | [39] |
| PNA | OTFT | 1 nM | 100 nM | 2010 | [40] | |
| Single-stranded DNA | OECT | 10 pM | >1 μM | 2011 | [43] | |
| PNA | OTFT | ~1 nM | ~100 nM | 2012 | [42] | |
| DNA | Water-gated OFET | 100 nM | n/a | 2012 | [41] | |
3. Pressure Sensors

| Device Materials/Structure | Sensitivity | Response Time | Current Modulation Mechanism | Year | Ref. |
|---|---|---|---|---|---|
| Polyimide/pentacene/conductive rubber network | 30 kPa−1 | n/a | Pressure- and thermal-sensitive materials | 2004 | [51] |
| Graphite containing rubber/pentacene | 30 kPa−1 | Hundreds of milliseconds | Change in transconductance | 2005 | [52] |
| Single-walled carbon nanotubes as a conducting dopant in a rubber, FET matrix | n/a | n/a | Pressure-sensitive rubber conductor | 2010 | [53] |
| Ionic liquid/conductive rubber/sensor array | 30 kPa−1 | n/a | Tension-dependent conductive rubber | 2010 | [54] |
| Pressure-sensitive rubber | n/a | 22 ms | Space charge limited, low voltage operation at ~3 V | 2009 | [55] |
| Plastic foil as dielectric layer (Mylar) | n/a | Tens to hundreds of seconds | Mobility or interface effects | 2007 | [56] |
| Plastic foil as dielectric layer (Mylar) | n/a | Tens to hundreds of seconds | Interface effects | 2006 | [57] |
| PDMS mould on gold | 0.14~0.3 (N/mm2)−1 or 140~300 kPa−1 | n/a | Pressure dependent channel length | 2010 | [58] |
| Microstructured PDMS dielectric layer, rubrene semiconducting layer | n/a | Millisecond range | Capacitive effect | 2010 | [59] |
| Nano-needle dielectric layer | 1.76 kPa−1 | n/a | The sharpness of the nano-needles | 2012 | [60] |
| PVP/Pentacene | n/a | 20 s | Trapped charges | 2005 | [61] |
| Devices with P3HT and pentacene semiconducting layers | n/a | 100 ms | P3HT layer is less crystalline than pentacene | 2012 | [62] |
| Microstructured PDMS dielectric and polyisoindigobithiophene-siloxane semiconductor | 8.4 kPa−1 | Less than 10 ms | Operating device in the sub-threshold regime | 2013 | [63] |
| PDMS dielectric layer with a floating gate | n/a | n/a | Ultra-low voltage operation, variation in the PDMS capacitance | 2013 | [64] |
4. Vapour Sensing
5. Conclusions
Conflicts of Interest
References
- Dimitrakopoulos, C.D.; Mascaro, D.J. Organic thin-film transistors: A review of recent advances. IBM J. Res. Dev. 2001, 45, 11–27. [Google Scholar] [CrossRef]
- Katz, H.E. Recent advances in semiconductor performance and printing processes for organic transistor-based electronics. Chem. Mater. 2004, 16, 4748–4756. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F. Organic thin-film transistors for chemical and biological sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef]
- Liao, C.; Yan, F. Organic semiconductors in organic thin-film transistor-based chemical and biological sensors. Polym. Rev. 2013, 53, 352–406. [Google Scholar] [CrossRef]
- Vieira, N.C.S.; Fernandes, E.G.R.; de Queiroz, A.A.A.; Guimarães, F.E.G.; Zucolotto, V. Indium tin oxide synthesized by a low cost route as segfet ph sensor. Mater. Res. 2013, 16, 1156–1160. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Berggren, M.; Horowitz, G.; Pham, M.-C. Advances in organic transistor-based biosensors: From organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem. 2012, 402, 1813–1826. [Google Scholar] [CrossRef]
- Schmoltner, K.; Kofler, J.; Klug, A.; List-Kratochvil, E.J.W. Electrolyte-gated organic field-effect transistor for selective reversible ion detection. Adv. Mater. 2013, 25, 6895–6899. [Google Scholar] [CrossRef]
- Loi, A.; Manunza, I.; Bonfiglio, A. Flexible, organic, ion-sensitive field-effect transistor. Appl. Phys. Lett. 2005, 86, 103512. [Google Scholar] [CrossRef]
- Sandberg, H.G.O.; Bäcklund, T.G.; Österbacka, R.; Stubb, H. High-performance all-polymer transistor utilizing a hygroscopic insulator. Adv. Mater. 2004, 16, 1112–1115. [Google Scholar] [CrossRef]
- Deng, J.; Patil, N.; Ryu, K.; Badmaev, A.; Zhou, C.; Mitra, S.; Wong, H.-S. Carbon nanotube Transistor Circuits: Circuit-Level Performance Benchmarking and Design Options for Living with Imperfections. In Proceedings of the IEEE International Solid-State Circuits Conference, 2007, ISSCC 2007, Digest of Technical Papers. San Francisco, CA, USA, 11–15 Febuary 2007; pp. 70–588.
- Justino, C.I.; Freitas, A.C.; Amaral, J.P.; Rocha-Santos, T.A.; Cardoso, S.; Duarte, A.C. Disposable immunosensors for c-reactive protein based on carbon nanotubes field effect transistors. Talanta 2013, 108, 165–170. [Google Scholar] [CrossRef]
- Lai, S.; Demelas, M.; Casula, G.; Cosseddu, P.; Barbaro, M.; Bonfiglio, A. Ultralow voltage, otft-based sensor for label-free DNA detection. Adv. Mater. 2013, 25, 103–107. [Google Scholar] [CrossRef]
- Demelas, M.; Lai, S.; Spanu, A.; Martinoia, S.; Cosseddu, P.; Barbaro, M.; Bonfiglio, A. Charge sensing by organic charge-modulated field effect transistors: Application to the detection of bio-related effects. J. Mater. Chem. B 2013, 1, 3811–3819. [Google Scholar]
- Bartic, C.; Campitelli, A.; Borghs, S. Field-effect detection of chemical species with hybrid organic/inorganic transistors. Appl. Phys. Lett. 2003, 82, 475–477. [Google Scholar] [CrossRef]
- Magliulo, M.; Pistillo, B.R.; Mulla, M.Y.; Cotrone, S.; Ditaranto, N.; Cioffi, N.; Favia, P.; Torsi, L. PE-CVD of hydrophilic-COOH functionalized coatings on electrolyte gated field-effect transistor electronic layers. Plasma Process. Polym. 2013, 10, 102–109. [Google Scholar]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Heller, A. Implanted electrochemical glucose sensors for the management of diabetes. Annu. Rev. Biomed. Eng. 1999, 1, 153–175. [Google Scholar] [CrossRef]
- Wilson, R.; Turner, A.P.F. Glucose oxidase: An ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Macaya, D.J.; Nikolou, M.; Takamatsu, S.; Mabeck, J.T.; Owens, R.M.; Malliaras, G.G. Simple glucose sensors with micromolar sensitivity based on organic electrochemical transistors. Sens. Actuators B 2007, 123, 374–378. [Google Scholar] [CrossRef]
- Umana, M.; Waller, J. Protein-modified electrodes. The glucose oxidase/polypyrrole system. Anal. Chem. 1986, 58, 2979–2983. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Whitaker, R.G. Electrochemical immobilisation of enzymes: Part II. Glucose oxidase immobilised in poly-n-methylpyrrole. J. Electroanal. Chem. 1987, 224, 37–48. [Google Scholar] [CrossRef]
- Zhu, Z.-T.; Mabeck, J.T.; Zhu, C.; Cady, N.C.; Batt, C.A.; Malliaras, G.G. A simple poly(3,4-ethylene dioxythiophene)/poly(styrene sulfonic acid) transistor for glucose sensing at neutral pH. Chem. Commun. 2004, 1556–1557. [Google Scholar]
- Bernards, D.A.; Macaya, D.J.; Nikolou, M.; DeFranco, J.A.; Takamatsu, S.; Malliaras, G.G. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 2007, 18, 116–120. [Google Scholar]
- Liu, J.; Agarwal, M.; Varahramyan, K. Glucose sensor based on organic thin film transistor using glucose oxidase and conducting polymer. Sens. Actuators B: Chem. 2008, 135, 195–199. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Organic electrochemical transistors with graphene-modified gate electrodes for highly sensitive and selective dopamine sensors. J. Mater. Chem. B 2014, 2, 191–200. [Google Scholar] [CrossRef]
- Tang, H.; Yan, F.; Lin, P.; Xu, J.; Chan, H.L. Highly sensitive glucose biosensors based on organic electrochemical transistors using platinum gate electrodes modified with enzyme and nanomaterials. Adv. Funct. Mater. 2011, 21, 2264–2272. [Google Scholar]
- Wang, Z.; Gao, J. Research on the urease biosensor with ppy material as carrier and sensitive membrane. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics (BMEI), Yantai, China, 16–18 October 2010; pp. 1574–1576.
- Buth, F.; Donner, A.; Sachsenhauser, M.; Stutzmann, M.; Garrido, J.A. Biofunctional electrolyte-gated organic field-effect transistors. Adv. Mater. 2012, 24, 4511–4517. [Google Scholar] [CrossRef]
- Khodagholy, D.; Curto, V.F.; Fraser, K.J.; Gurfinkel, M.; Byrne, R.; Diamond, D.; Malliaras, G.G.; Benito-Lopez, F.; Owens, R.M. Organic electrochemical transistor incorporating an ionogel as a solid state electrolyte for lactate sensing. J. Mater. Chem. 2012, 22, 4440–4443. [Google Scholar] [CrossRef] [Green Version]
- Phongphut, A.; Sriprachuabwong, C.; Wisitsoraat, A.; Tuantranont, A.; Prichanont, S.; Sritongkham, P. A disposable amperometric biosensor based on inkjet-printed au/pedot-pss nanocomposite for triglyceride determination. Sens. Actuators B 2013, 178, 501–507. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdes-Ramirez, G.; Windmiller, J.R.; Yang, Z.; Ramirez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Vieira, N.C.S.; Figueiredo, A.; Fernandes, E.G.R.; Guimarães, F.E.G.; Zucolotto, V. Nanostructured polyaniline thin films as urea-sensing membranes in field-effect devices. Synth. Met. 2013, 175, 108–111. [Google Scholar] [CrossRef]
- Someya, T.; Dodabalapur, A.; Gelperin, A.; Katz, H.E.; Bao, Z. Integration and response of organic electronics with aqueous microfluidics. Langmuir 2002, 18, 5299–5302. [Google Scholar] [CrossRef]
- Magliulo, M.; Mallardi, A.; Gristina, R.; Ridi, F.; Sabbatini, L.; Cioffi, N.; Palazzo, G.; Torsi, L. Part per trillion label-free electronic bioanalytical detection. Anal. Chem. 2013, 85, 3849–3857. [Google Scholar] [CrossRef]
- Magliulo, M.; Mallardi, A.; Mulla, M.Y.; Cotrone, S.; Pistillo, B.R.; Favia, P.; Vikholm-Lundin, I.; Palazzo, G.; Torsi, L. Electrolyte-gated organic field-effect transistor sensors based on supported biotinylated phospholipid bilayer. Adv. Mater. 2013, 25, 2090–2094. [Google Scholar] [CrossRef]
- Chartuprayoon, N.; Rheem, Y.; Ng, J.C.; Nam, J.; Chen, W.; Myung, N.V. Polypyrrole nanoribbon based chemiresistive immunosensor for viral plant pathogen detection. Anal. Methods 2013, 5, 3497–3502. [Google Scholar] [CrossRef]
- Lin, T.-W.; Kekuda, D.; Chu, C.-W. Label-free detection of DNA using novel organic-based electrolyte-insulator-semiconductor. Biosens. Bioelectron. 2010, 25, 2706–2710. [Google Scholar] [CrossRef]
- Khan, H.U.; Roberts, M.E.; Johnson, O.; Förch, R.; Knoll, W.; Bao, Z. In situ, label-free DNA detection using organic transistor sensors. Adv. Mater. 2010, 22, 4452–4456. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Berggren, M.; Pham, M.-C.; Yassar, A.; Horowitz, G. DNA detection with a water-gated organic field-effect transistor. Org. Electron. 2012, 13, 1–6. [Google Scholar] [CrossRef]
- Khan, H.U.; Roberts, M.E.; Johnson, O.; Knoll, W.; Bao, Z. The effect of ph and DNA concentration on organic thin-film transistor biosensors. Org. Electron. 2012, 13, 519–524. [Google Scholar] [CrossRef]
- Lin, P.; Luo, X.; Hsing, I.; Yan, F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label-free DNA sensing. Adv. Mater. 2011, 23, 4035–4040. [Google Scholar] [CrossRef]
- Hammock, M.L.; Knopfmacher, O.; Naab, B.D.; Tok, J.B.-H.; Bao, Z. Investigation of protein detection parameters using nano-functionalized organic field-effect transistors. ACS Nano 2013, 7, 3970–3980. [Google Scholar] [CrossRef]
- Tarabella, G.; Balducci, A.G.; Coppedè, N.; Marasso, S.; D’Angelo, P.; Barbieri, S.; Cocuzza, M.; Colombo, P.; Sonvico, F.; Mosca, R. Liposome sensing and monitoring by organic electrochemical transistors integrated in microfluidics. Biochim. Biophys. Acta 2013, 1830, 4374–4380. [Google Scholar] [CrossRef]
- Huang, W.; Besar, K.; LeCover, R.; Dulloor, P.; Sinha, J.; Hardigree, J.F.M.; Pick, C.; Swavola, J.; Everett, A.D.; Frechette, J. Label-free brain injury biomarker detection based on highly sensitive large area organic thin film transistor with hybrid coupling layer. Chem. Sci. 2014, 5, 416–426. [Google Scholar] [CrossRef]
- Tang, H.; Lin, P.; Chan, H.L.; Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 2011, 26, 4559–4563. [Google Scholar] [CrossRef]
- Khan, H.U.; Jang, J.; Kim, J.-J.; Knoll, W. Effect of passivation on the sensitivity and stability of pentacene transistor sensors in aqueous media. Biosens. Bioelectron. 2011, 26, 4217–4221. [Google Scholar] [CrossRef]
- Khan, H.U.; Jang, J.; Kim, J.-J.; Knoll, W. In situ antibody detection and charge discrimination using aqueous stable pentacene transistor biosensors. J. Am. Chem. Soc. 2011, 133, 2170–2176. [Google Scholar]
- Spijkman, M.J.; Brondijk, J.J.; Geuns, T.C.; Smits, E.C.; Cramer, T.; Zerbetto, F.; Stoliar, P.; Biscarini, F.; Blom, P.W.; de Leeuw, D.M. Dual-gate organic field-effect transistors as potentiometric sensors in aqueous solution. Adv. Funct. Mater. 2010, 20, 898–905. [Google Scholar] [CrossRef]
- Someya, T.; Sekitani, T.; Iba, S.; Kato, Y.; Kawaguchi, H.; Sakurai, T. A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc. Natl. Acad. Sci. USA 2004, 101, 9966–9970. [Google Scholar]
- Someya, T.; Kato, Y.; Sekitani, T.; Iba, S.; Noguchi, Y.; Murase, Y.; Kawaguchi, H.; Sakurai, T. Conformable, flexible, large-area networks of pressure and thermal sensors with organic transistor active matrixes. Proc. Natl. Acad. Sci. USA 2005, 102, 12321–12325. [Google Scholar]
- Sekitani, T.; Someya, T. Stretchable, large-area organic electronics. Adv. Mater. 2010, 22, 2228–2246. [Google Scholar] [CrossRef]
- Someya, T.; Dodabalapur, A.; Huang, J.; See, K.C.; Katz, H.E. Chemical and physical sensing by organic field-effect transistors and related devices. Adv. Mater. 2010, 22, 3799–3811. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Lai, W.-J.; Chen, C.-Y.; Meng, H.-F.; Zan, H.-W.; Horng, S.-F. Low voltage active pressure sensor based on polymer space-charge-limited transistor. Appl. Phys. Lett. 2009, 95, 253306. [Google Scholar] [CrossRef]
- Manunza, I.; Bonfiglio, A. Pressure sensing using a completely flexible organic transistor. Biosens. Bioelectron. 2007, 22, 2775–2779. [Google Scholar] [CrossRef]
- Manunza, I.; Sulis, A.; Bonfiglio, A. Pressure sensing by flexible, organic, field effect transistors. Appl. Phys. Lett. 2006, 89, 143502. [Google Scholar] [CrossRef]
- Kim, J.-H.; Sun, Q.; Seo, S. Pressure dependent current-controllable devices based on organic thin film transistors by soft-contact lamination. Org. Electron. 2010, 11, 964–968. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.; Tee, B.C.; Stoltenberg, R.M.; Chen, C.V.H.; Barman, S.; Muir, B.V.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef]
- Kim, J.; Nga Ng, T.; Soo Kim, W. Highly sensitive tactile sensors integrated with organic transistors. Appl. Phys. Lett. 2012, 101, 103308. [Google Scholar] [CrossRef]
- Darlinski, G.; Bottger, U.; Waser, R.; Klauk, H.; Halik, M.; Zschieschang, U.; Schmid, G.; Dehm, C. Mechanical force sensors using organic thin-film transistors. J. App. Phys. 2005, 97, 093708. [Google Scholar] [CrossRef] [Green Version]
- Cosseddu, P.; Milita, S.; Bonfiglio, A. Strain sensitivity and transport properties in organic field-effect transistors. IEEE Electron Device Lett. 2012, 33, 113–115. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Lai, S.; Cosseddu, P.; Bonfiglio, A.; Barbaro, M. Ultralow voltage pressure sensors based on organic fets and compressible capacitors. IEEE Electron Device Lett. 2013, 34, 801–803. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Schaller, E.; Bosset, J.O.; Escher, F. ‘Electronic noses’ and their application to food. LWT—Food Sci. Technol. 1998, 31, 305–316. [Google Scholar]
- Liao, F.; Chen, C.; Subramanian, V. Organic tfts as gas sensors for electronic nose applications. Sens. Actuators B 2005, 107, 849–855. [Google Scholar] [CrossRef]
- Li, B.; Lambeth, D.N. Chemical sensing using nanostructured polythiophene transistors. Nano Lett. 2008, 8, 3563–3567. [Google Scholar] [CrossRef]
- Wedge, D.C.; Das, A.; Dost, R.; Kettle, J.; Madec, M.-B.; Morrison, J.J.; Grell, M.; Kell, D.B.; Richardson, T.H.; Yeates, S. Real-time vapour sensing using an OFET-based electronic nose and genetic programming. Sens. Actuators B 2009, 143, 365–372. [Google Scholar] [CrossRef]
- Liao, F.; Yin, S.; Toney, M.; Subramanian, V. Physical discrimination of amine vapor mixtures using polythiophene gas sensor arrays. Sens. Actuators B 2010, 150, 254–263. [Google Scholar] [CrossRef]
- Wang, L.; Swensen, J.S. Dual-transduction-mode sensing approach for chemical detection. Sens. Actuators B 2012, 174, 366–372. [Google Scholar] [CrossRef]
- Huang, J.; Dawidczyk, T.; Jung, B.; Sun, J.; Mason, A.; Katz, H. Response diversity and dual response mechanism of organic field-effect transistors with dinitrotoluene vapor. J. Mater. Chem. 2010, 20, 2644–2650. [Google Scholar] [CrossRef]
- Kong, H.; Jung, B.J.; Sinha, J.; Katz, H.E. Electrical “turn-on” response of poly (3,3‴-didodecylquaterthiophene) and electron donor blend transistors to 2,4,6-trinitrotoluene. Chem. Mater. 2012, 24, 2621–2623. [Google Scholar] [CrossRef]
- Kybert, N.J.; Lerner, M.B.; Yodh, J.S.; Preti, G.; Johnson, A.C. Differentiation of complex vapor mixtures using versatile DNA-carbon nanotube chemical sensor arrays. ACS Nano 2013, 7, 2800–2807. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Elkington, D.; Cooling, N.; Belcher, W.; Dastoor, P.C.; Zhou, X. Organic Thin-Film Transistor (OTFT)-Based Sensors. Electronics 2014, 3, 234-254. https://doi.org/10.3390/electronics3020234
Elkington D, Cooling N, Belcher W, Dastoor PC, Zhou X. Organic Thin-Film Transistor (OTFT)-Based Sensors. Electronics. 2014; 3(2):234-254. https://doi.org/10.3390/electronics3020234
Chicago/Turabian StyleElkington, Daniel, Nathan Cooling, Warwick Belcher, Paul C. Dastoor, and Xiaojing Zhou. 2014. "Organic Thin-Film Transistor (OTFT)-Based Sensors" Electronics 3, no. 2: 234-254. https://doi.org/10.3390/electronics3020234
APA StyleElkington, D., Cooling, N., Belcher, W., Dastoor, P. C., & Zhou, X. (2014). Organic Thin-Film Transistor (OTFT)-Based Sensors. Electronics, 3(2), 234-254. https://doi.org/10.3390/electronics3020234






