Development of a Multisensory Wearable System for Monitoring Cigarette Smoking Behavior in Free-Living Conditions
Abstract
:1. Introduction
2. Methods
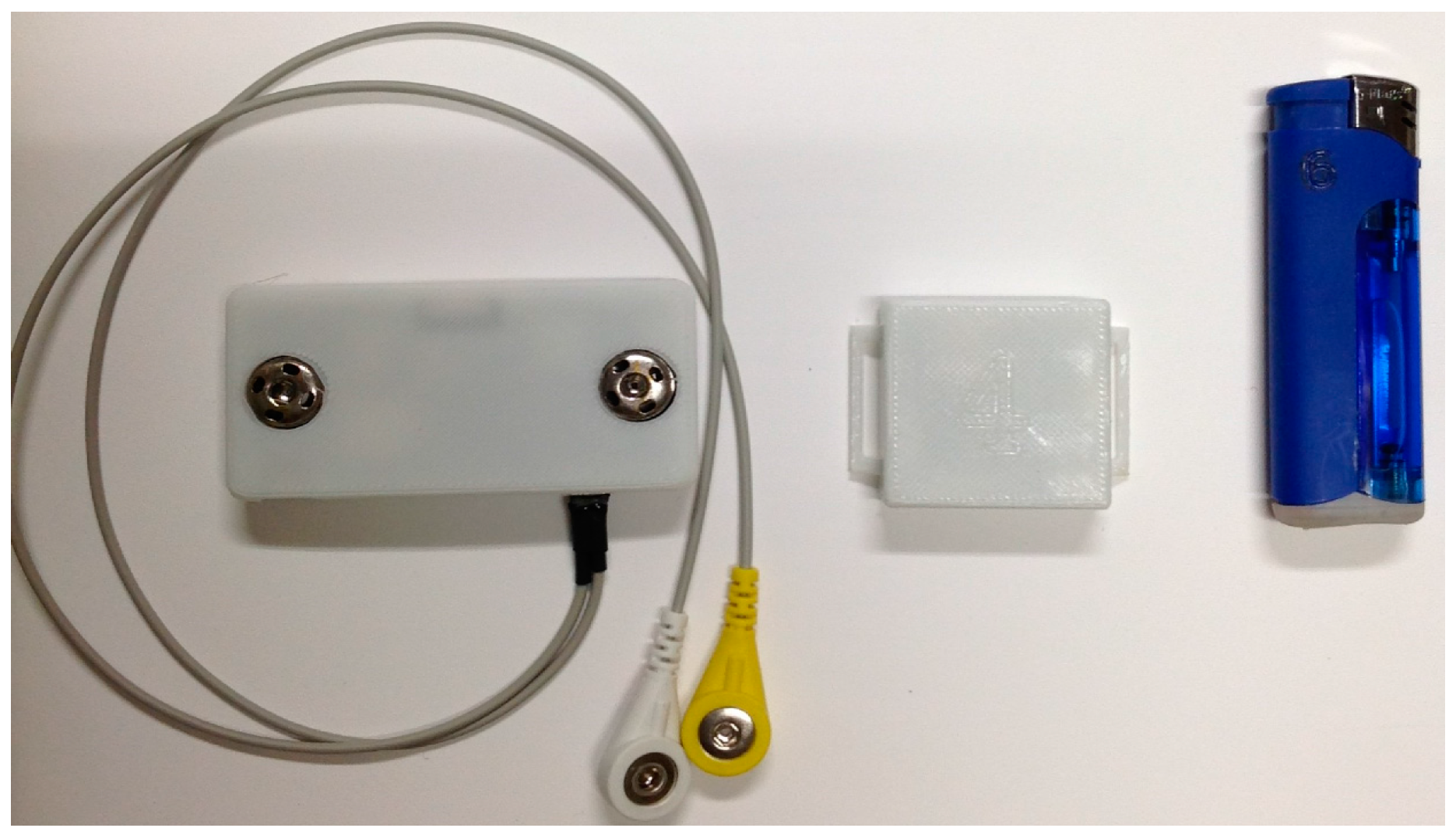
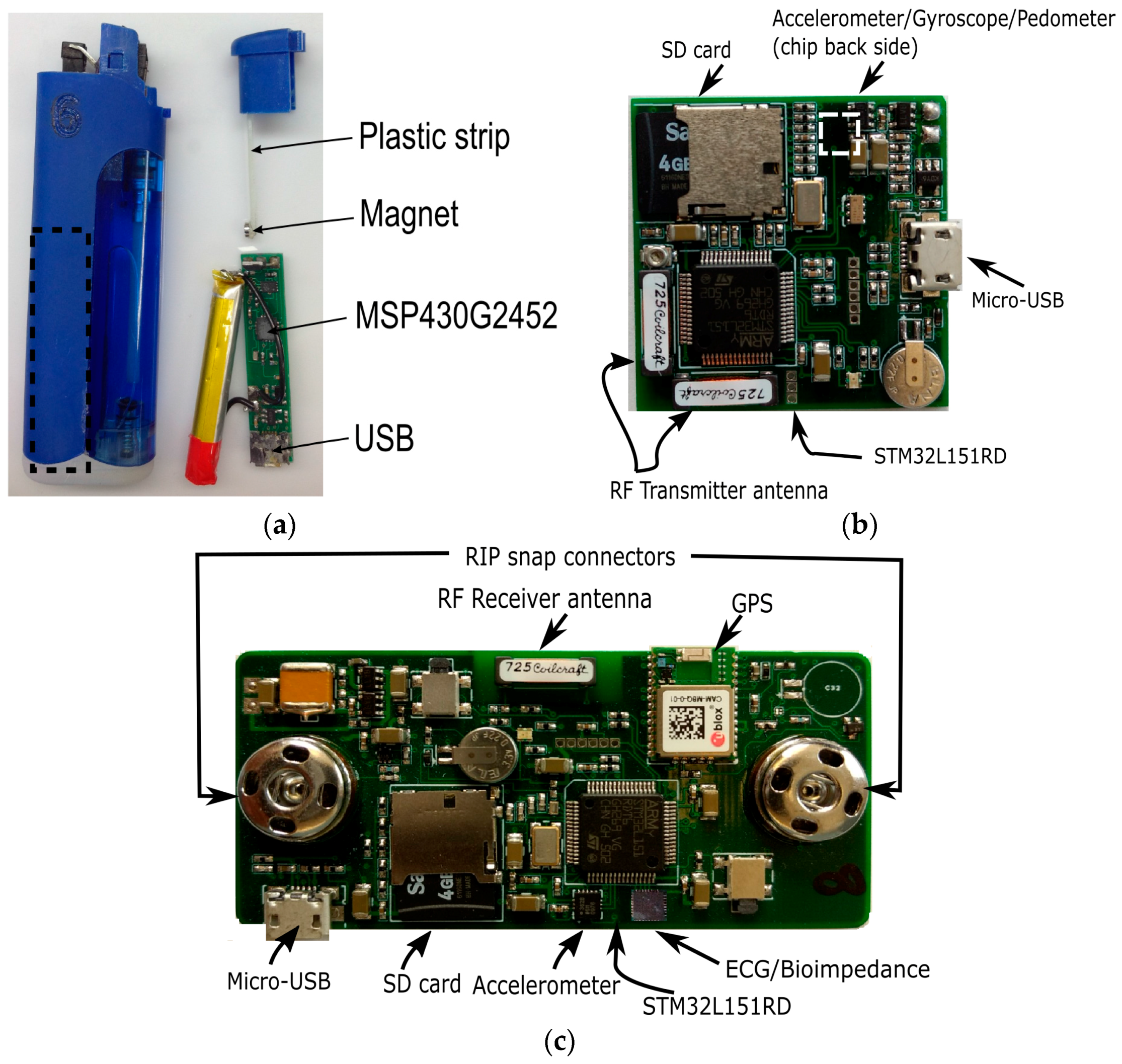
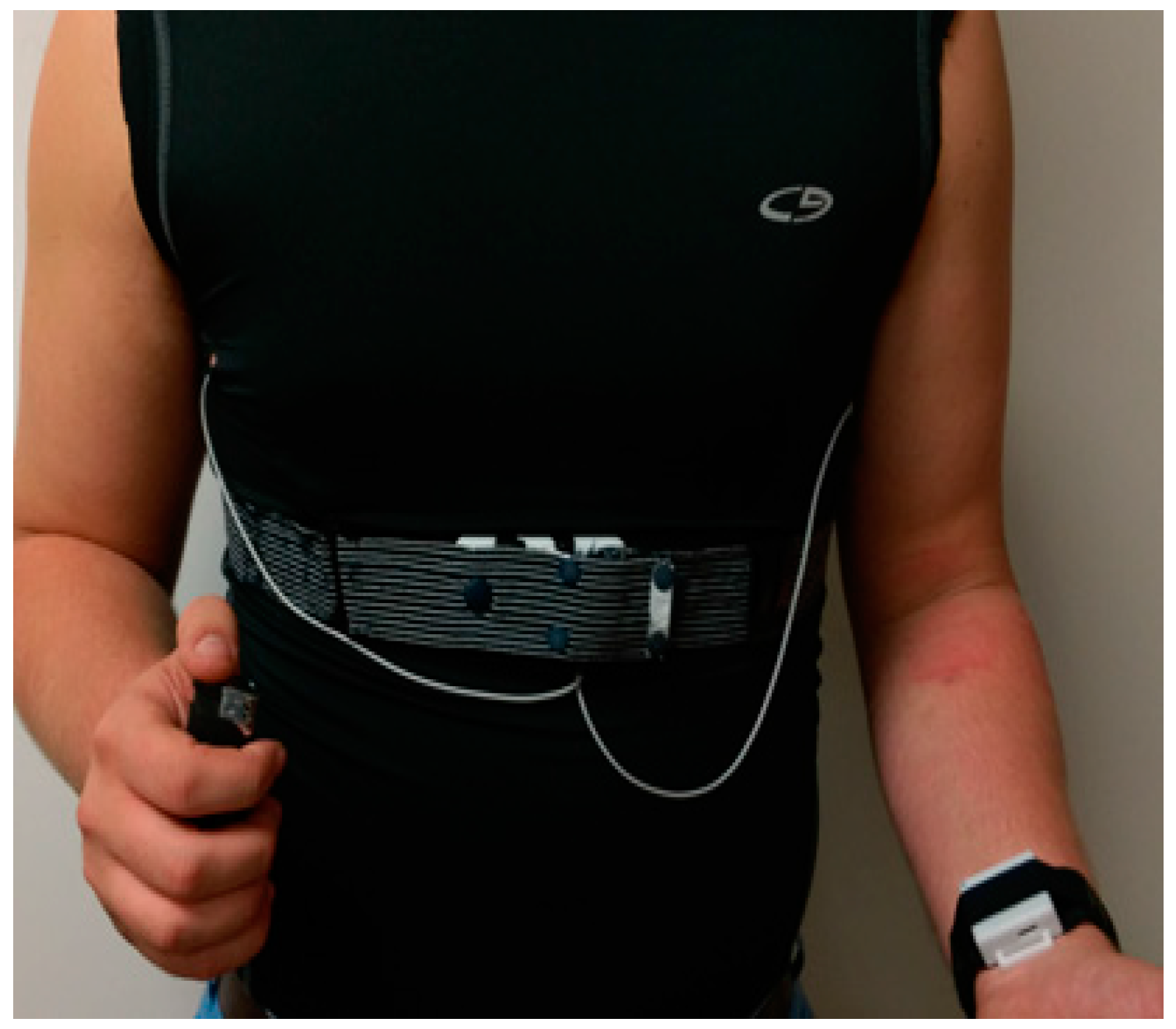
2.1. Wearable System
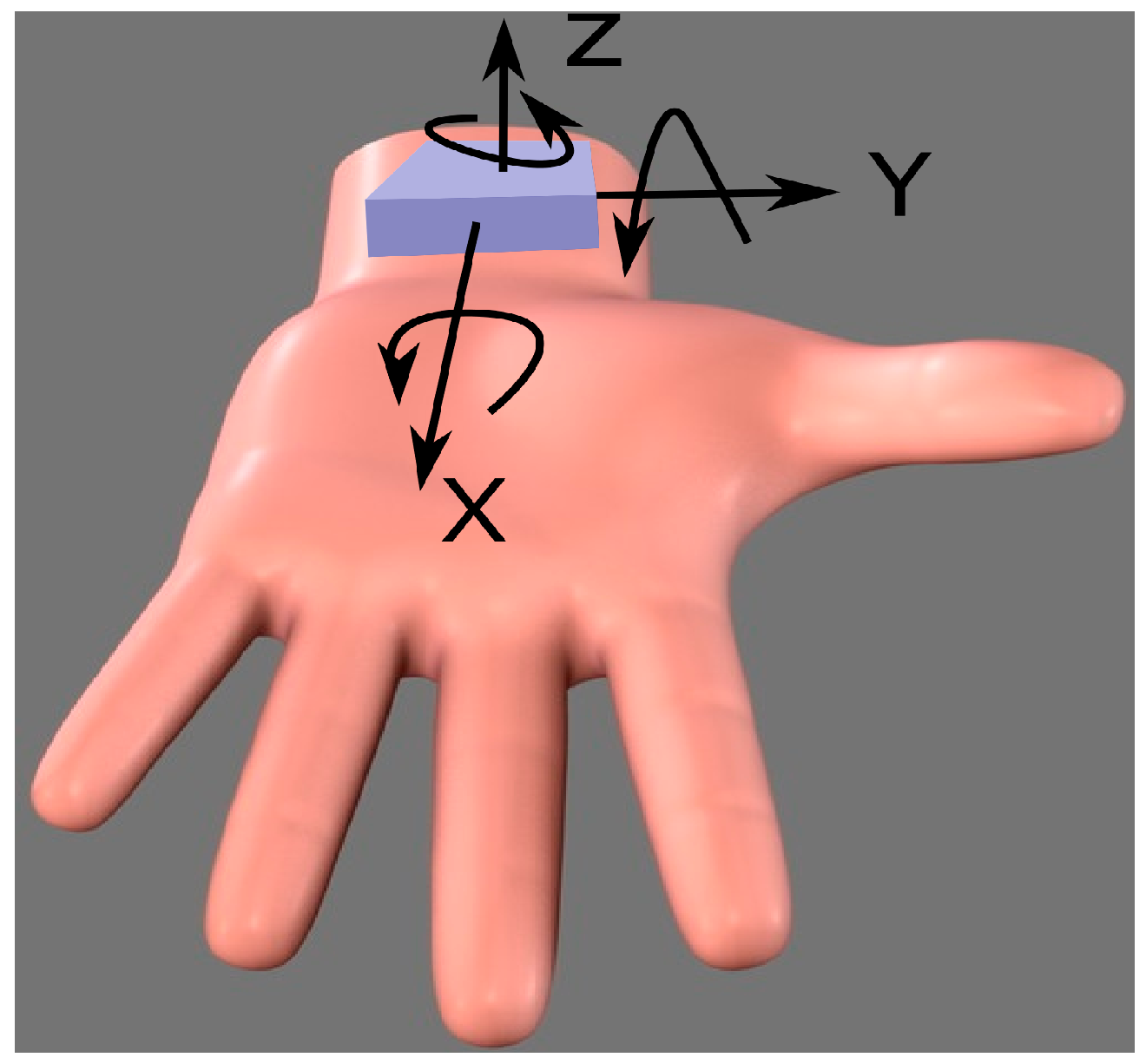
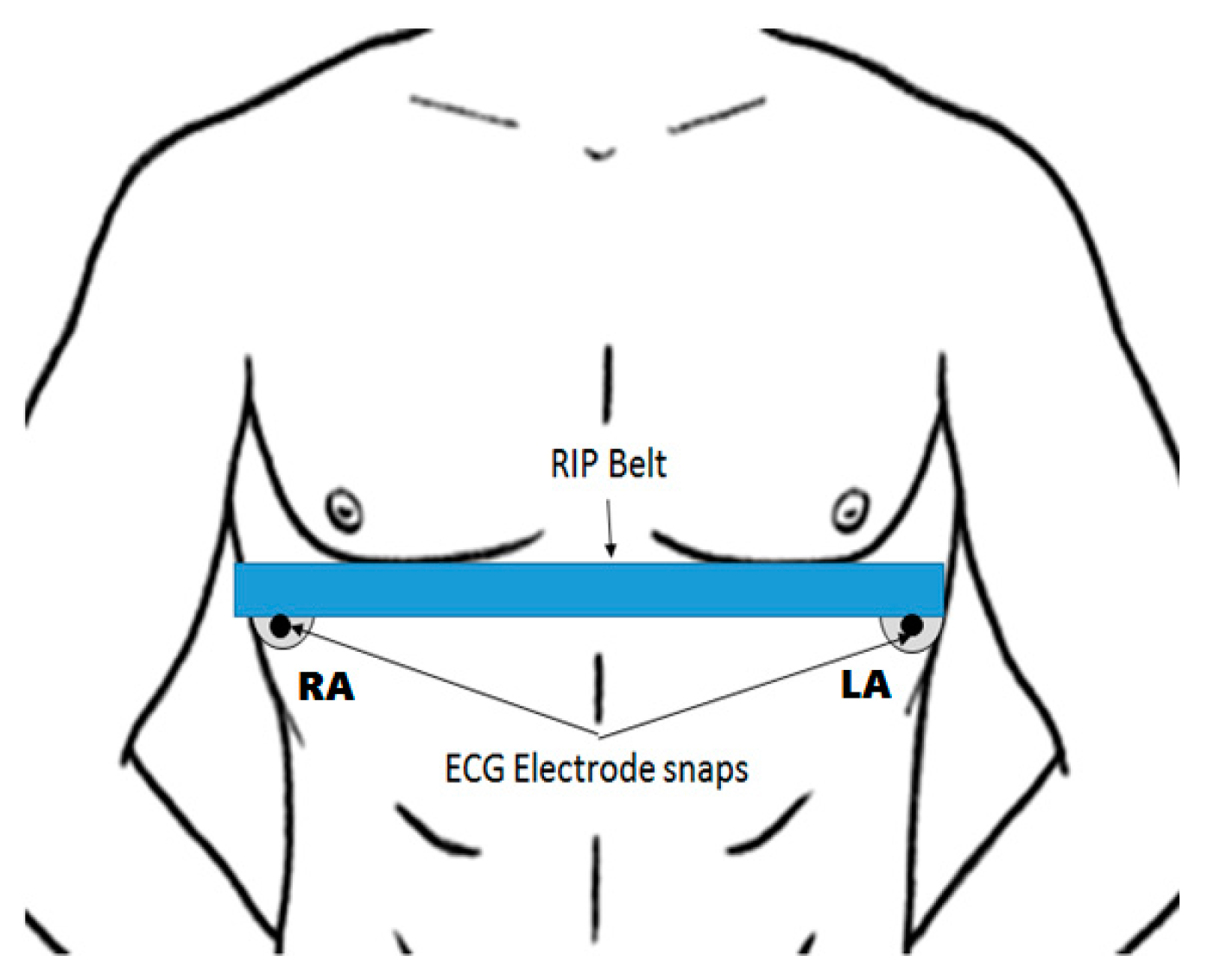
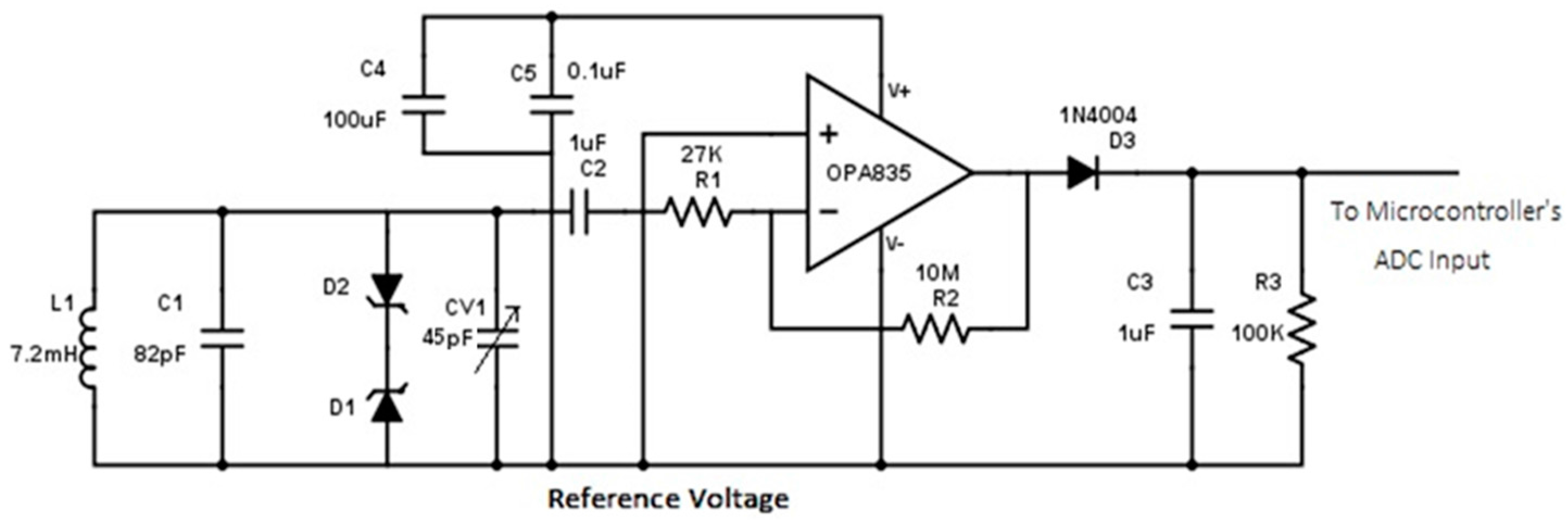
2.2. Sensing Elements
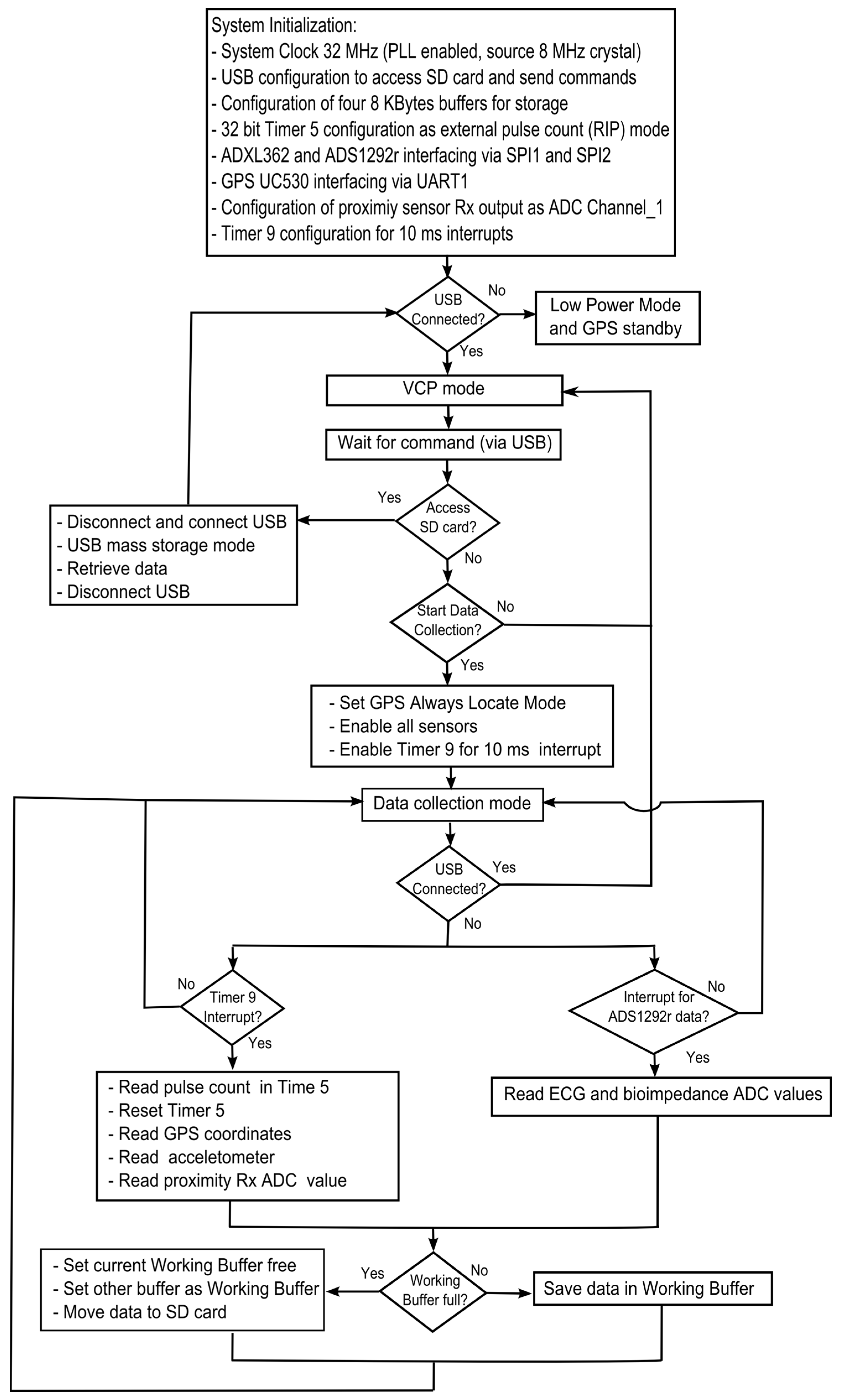
2.3. Embedded Software
2.4. Time Synchronization and Drift Compensation
2.5. System Validation
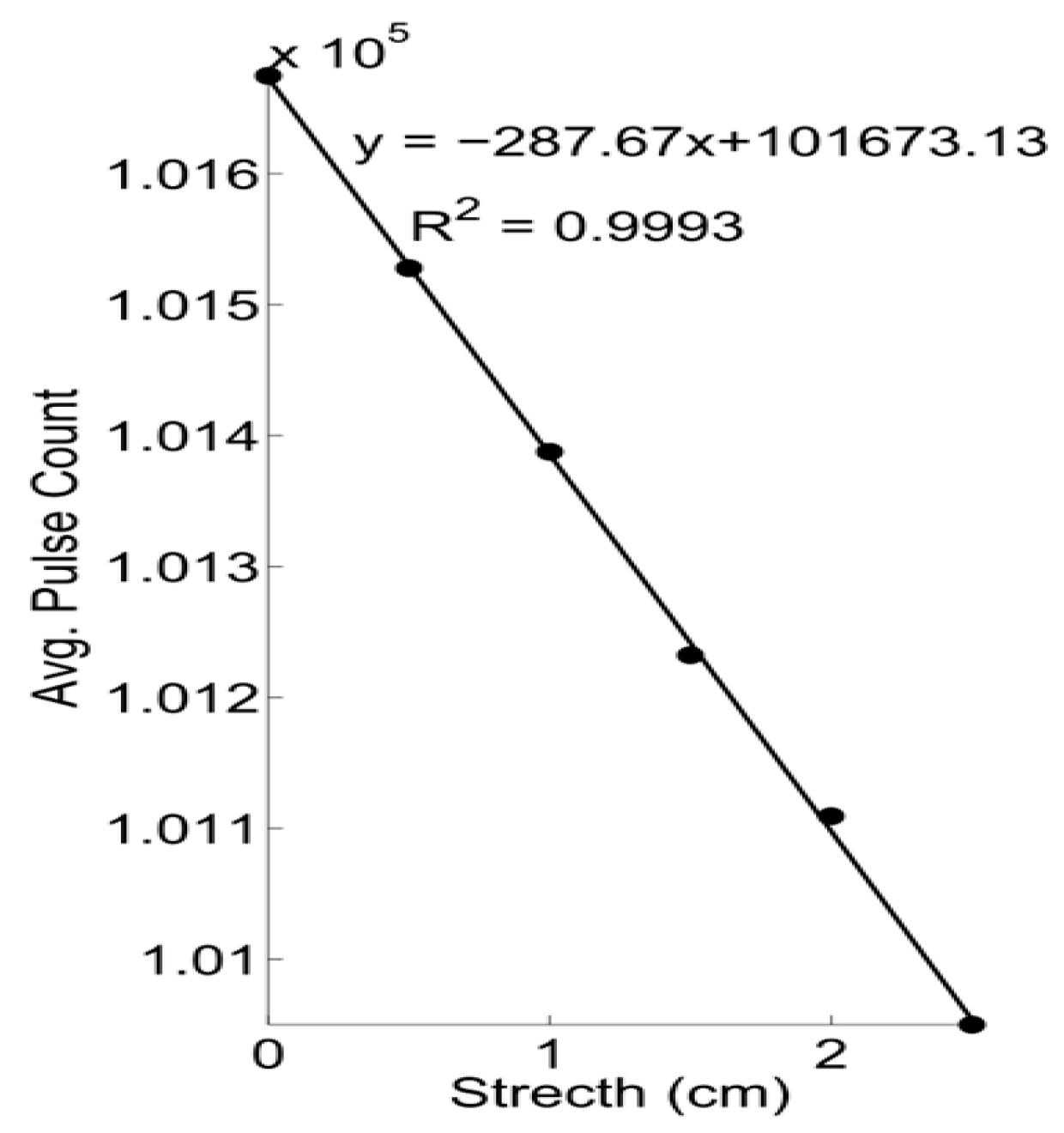
2.5.1. Bench Test
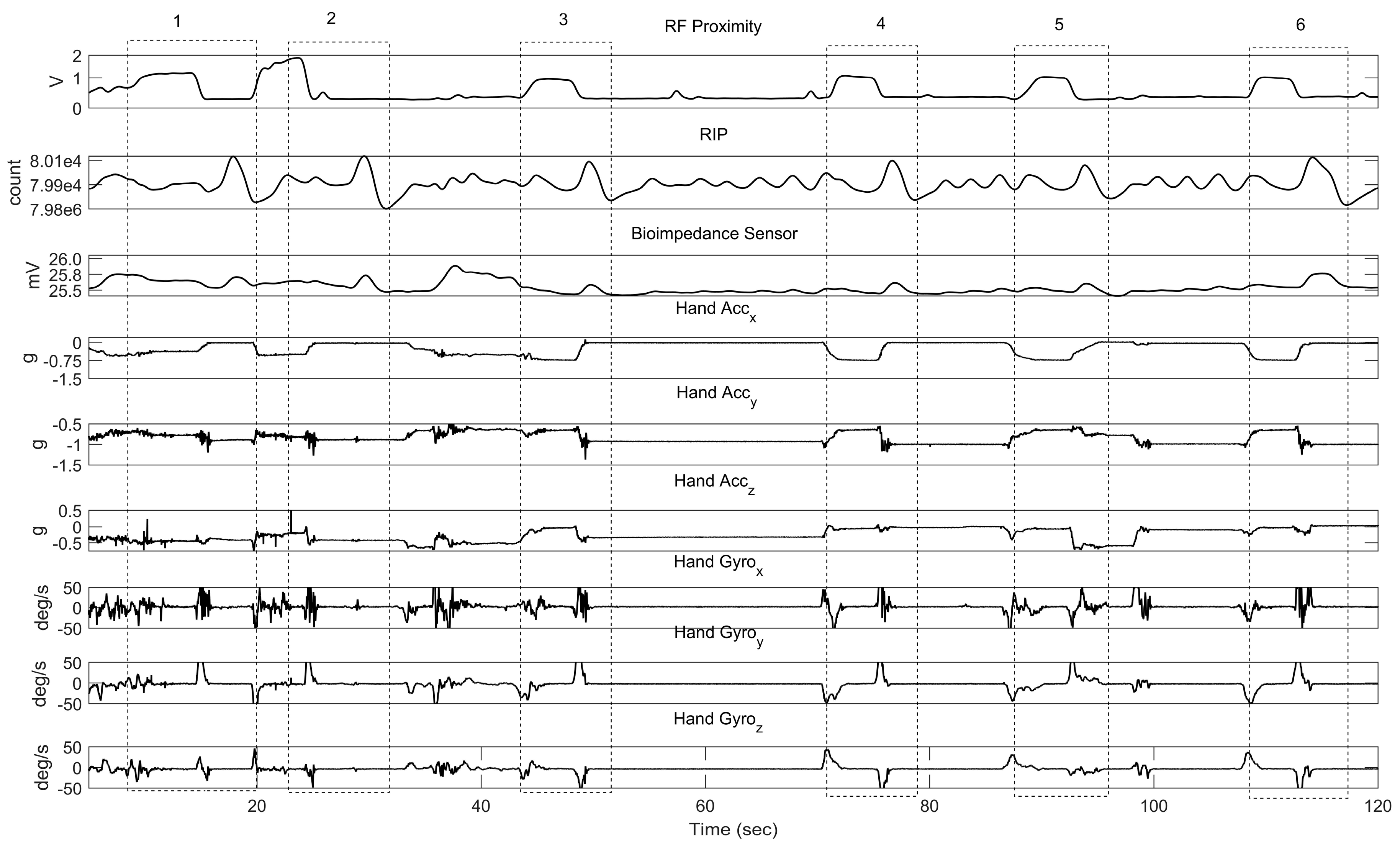
2.5.2. Human Study
- the total number of lighter press–releases performed by all subjects;
- the total number of consumed cigarettes estimated from the lighter data. For this, the start and end times of the smoking session were extracted from the smartphone log. Using these timestamps, multiple (consecutive) lighter presses prior to lighting the cigarette were considered as one and the unexpected lighter presses during the non-smoking events were discarded;
- the total number of consumed cigarettes from the subjects’ self-registration on their phone;
- the total number of hand-to-mouth gestures from the hand IMU data using the algorithm described in [25];
- the total number of breaths from the de-noised RIP and bioimpedance sensor using MATLAB’s findpeaks algorithm (by finding local maxima);
- the total number of hand-to-mouth proximity movements from the RF sensor. The signal qualifies as a valid hand-to-mouth proximity movement if the amplitude > the threshold 70 mV (ten times the mean noise amplitude of 7 mV).
- the average heart rate during a silent smoking session (in a sitting posture) with the comparison of pre- and post-smoking heart rates. For this, a similar findpeaks algorithm was applied on the de-noised ECG signal to find the characteristic R-waves. The instantaneous heart rate (number of R-peaks per minute) was computed to average the heart rates during the sitting–smoking session, 5 min before smoking, and 15 min after smoking.
3. Results
3.1. Bench Test
3.2. Human Study
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 2000–2004. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a3.htm (accessed on 16 November 2016).
- Hoffmann, D.; Djordjevic, M.V.; Hoffmann, I. The Changing Cigarette; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010.
- Results from the 2011 NSDUH: Summary of National Findings, SAMHSA, CBHSQ. Available online: http://www.samhsa.gov/data/sites/default/files/Revised2k11NSDUHSummNatFindings/Revised2k11NSDUHSummNatFindings/NSDUHresults2011.htm (accessed on 16 November 2016).
- Khati, I.; Menvielle, G.; Chollet, A.; Younès, N.; Metadieu, B.; Melchior, M. What distinguishes successful from unsuccessful tobacco smoking cessation? Data from a study of young adults (TEMPO). Prev. Med. Rep. 2015, 2, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.A.; Hammer, D.; Sanders, S.H. The physiological effects of cigarette smoking: Implications for psychophysiological research. Biofeedback Self-Regul. 1984, 9, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Gwaltney, C.J.; Balabanis, M.H.; Liu, K.S.; Paty, J.A.; Kassel, J.D.; Hickcox, M.; Gnys, M. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. J. Abnorm. Psychol. 2002, 111, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Ecological Momentary Assessment: Real-World, Real-Time Measurement of Patient Experience. Available online: https://www.researchgate.net/publication/232497943_Ecological_Momentary_Assessment_Real-world_real-time_measurement_of_patient_experience (accessed on 16 November 2016).
- Scholl, P.M.; Kücükyildiz, N.; Laerhoven, K.V. When Do You Light a Fire? Capturing Tobacco Use with Situated Wearable Sensors. In Proceedings of the 2013 ACM Conference on Pervasive and Ubiquitous Computing Adjunct Publication, Zurich, Switzerland, 8–12 September 2013; pp. 1309–1310. [Google Scholar]
- Quitbit|Smart Lighters to Help Track, Reduce & Quit Smoking. Available online: http://www.quitbitlighter.com/ (accessed on 12 December 2017).
- Scholl, P.M.; van Laerhoven, K. A Feasibility Study of Wrist-Worn Accelerometer Based Detection of Smoking Habits. In Proceedings of the 2012 Sixth International Conference on Innovative Mobile and Internet Services in Ubiquitous Computing (IMIS), Palermo, Italy, 4–6 July 2012; pp. 886–891. [Google Scholar]
- Parate, A.; Chiu, M.-C.; Chadowitz, C.; Ganesan, D.; Kalogerakis, E. RisQ: Recognizing Smoking Gestures with Inertial Sensors on a Wristband. In Proceedings of the 12th Annual International Conference on Mobile Systems, Applications, and Services, Bretton Woods, NH, USA, 16–19 June 2014; pp. 149–161. [Google Scholar]
- Raiff, B.R.; Karataş, Ç.; McClure, E.A.; Pompili, D.; Walls, T.A. Laboratory Validation of Inertial Body Sensors to Detect Cigarette Smoking Arm Movements. Electronics 2014, 3, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Varkey, J.P.; Pompili, D.; Walls, T.A. Human motion recognition using a wireless sensor-based wearable system. Pers. Ubiquitous Comput. 2012, 16, 897–910. [Google Scholar] [CrossRef]
- Tang, Q.; Vidrine, D.; Crowder, E.; Intille, S. Automated Detection of Puffing and Smoking with Wrist Accelerometers. In Proceedings of the 8th International Conference on Pervasive Computing Technologies for Healthcare, Oldenburg, Germany, 20–23 May 2014. [Google Scholar]
- Shoaib, M.; Scholten, H.; Havinga, P.J.M.; Incel, O.D. A hierarchical lazy smoking detection algorithm using smartwatch sensors. In Proceedings of the 2016 IEEE 18th International Conference on e-Health Networking, Applications and Services (Healthcom), Munich, Germany, 14–16 September 2016; pp. 1–6. [Google Scholar]
- Sazonov, E.; Metcalfe, K.; Lopez-Meyer, P.; Tiffany, S. RF hand gesture sensor for monitoring of cigarette smoking. In Proceedings of the 2011 Fifth International Conference on Sensing Technology (ICST), Palmerston North, New Zealand, 28 November–1 December 2011; pp. 426–430. [Google Scholar]
- Rodenstein, D.O.; Stănescu, D.C. Pattern of inhalation of tobacco smoke in pipe, cigarette, and never smokers. Am. Rev. Respir. Dis. 1985, 132, 628–632. [Google Scholar] [PubMed]
- Cohn, M.A.; Rao, A.S.; Broudy, M.; Birch, S.; Watson, H.; Atkins, N.; Davis, B.; Stott, F.D.; Sackner, M.A. The respiratory inductive plethysmograph: A new non-invasive monitor of respiration. Bull. Eur. Physiopathol. Respir. 1982, 18, 643–658. [Google Scholar] [PubMed]
- Subjective and Objective Measurement of Cigarette Smoke Inhalation—PubMed—NCBI. Available online: https://www.ncbi.nlm.nih.gov/pubmed/7140396 (accessed on 11 October 2017).
- Taylor, D.R.; Reid, W.D.; Paré, P.D.; Fleetham, J.A. Cigarette smoke inhalation patterns and bronchial reactivity. Thorax 1988, 43, 65–70. [Google Scholar] [CrossRef] [PubMed]
- St. Charles, F.K.; Krautter, G.R.; Mariner, D.C. Post-puff respiration measures on smokers of different tar yield cigarettes. Inhal. Toxicol. 2010, 21, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Meyer, P.; Sazonov, E. Automatic breathing segmentation from wearable respiration sensors. In Proceedings of the 2011 Fifth International Conference on Sensing Technology, Palmerston North, New Zealand, 28 November–1 December 2011; pp. 156–160. [Google Scholar]
- Ramos-Garcia, R.I.; Tiffany, S.; Sazonov, E. Using respiratory signals for the recognition of human activities. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 173–176. [Google Scholar]
- mPuff: Automated Detection of Cigarette Smoking Puffs from Respiration Measurements—IEEE Conference Publication. Available online: http://ieeexplore.ieee.org/document/6920942/ (accessed on 11 October 2017).
- puffMarker: A Multi-Sensor Approach for Pinpointing the Timing of First Lapse in Smoking Cessation (PDF Download Available). Available online: https://www.researchgate.net/publication/283543603_puffMarker_A_Multi-Sensor_Approach_for_Pinpointing_the_Timing_of_First_Lapse_in_Smoking_Cessation (accessed on 24 June 2017).
- Sazonov, E.; Lopez-Meyer, P.; Tiffany, S. A Wearable Sensor System for Monitoring Cigarette Smoking. J. Stud. Alcohol Drugs 2013, 74, 956–964. [Google Scholar] [CrossRef] [PubMed]
- AutoSense: A Wireless Sensor System to Quantify Personal Exposures to Psychosocial Stress and Addictive Substances in Natural Environments. Available online: https://sites.google.com/site/autosenseproject/ (accessed on 24 June 2017).
- Lopez-Meyer, P.; Tiffany, S.; Patil, Y.; Sazonov, E. Monitoring of Cigarette Smoking Using Wearable Sensors and Support Vector Machines. IEEE Trans. Biomed. Eng. 2013, 60, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Meyer, P.; Tiffany, S.; Sazonov, E. Identification of cigarette smoke inhalations from wearable sensor data using a Support Vector Machine classifier. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 4050–4053. [Google Scholar]
- Berry, D.; Bell, J.; Sazonov, E. Detection of cigarette smoke inhalations from respiratory signals using decision tree ensembles. In Proceedings of the SoutheastCon, Fort Lauderdale, FL, USA, 9–12 April 2015; pp. 1–4. [Google Scholar]
- Patil, Y.; Tiffany, S.; Sazonov, E. Understanding smoking behavior using wearable sensors: Relative importance of various sensor modalities. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 6899–6902. [Google Scholar]
- A Wearable Bioimpedance Device for Respiratory Monitoring—Semantic Scholar. Available online: https://www.semanticscholar.org/paper/A-Wearable-Bioimpedance-Device-for-Respiratory-Mon-Canni%C3%A8re-Smeets/48f04a2b7cdd7e94c0d4b081b89ebf38a4e1f291 (accessed on 24 June 2017).
- Ramos-Garcia, R.; Imtiaz, M.; Farooq, M.; Tiffany, S.; Sazonov, E. Evaluation of RIP Sensor Calibration Stability for Daily Estimation of Lung Volume. In Proceedings of the International Symposium on Medical Measurements and Applications, Rochester, MN, USA, 7–10 May 2017. [Google Scholar]
- Quectel Wireless Solutions—Dedicated M2M Wireless Module Supplier. Available online: http://www.top-electronicsusa.com/quectel-wireless-solutions-productcode0-p-1-mf-38.html (accessed on 10 October 2017).
- Coordinate Distance Calculator. Available online: http://boulter.com/gps/distance/ (accessed on 30 October 2016).
- Haines, V.; Elton, E.; Hussey, M. Revision of Body Size Criteria in Standards—Protecting People Who Work at Height; RR342; Ergonomics and Safety Research Institute: Loughborough, UK, 2005. [Google Scholar]
- How to Smoke a Cigarette: 13 Steps (with Pictures)—Wikihow. Available online: https://www.wikihow.com/Smoke-a-Cigarette (accessed on 10 October 2017).
- Vitalograph—BreathCO. Available online: https://vitalograph.com/product/162449/breathco (accessed on 16 November 2016).
- Chhabra, S.K. Forced vital capacity, slow vital capacity, or inspiratory vital capacity: Which is the best measure of vital capacity? J. Asthma Off. J. Assoc. Care Asthma 1998, 35, 361–365. [Google Scholar] [CrossRef]
- Botanic Gardens Conservation International (BGCI). aTimeLogger—Time Tracker; BGCI: Surrey, UK, 2017. [Google Scholar]
- Errors Due to sampling Frequency of the Electrocardiogram in spectral Analysis of Heart Rate Signals with Low Variability—IEEE Xplore Document. Available online: http://ieeexplore.ieee.org/document/482685/?reload=true (accessed on 17 August 2017).
- Portable Health Monitoring Device for Electrocardiogram and Impedance Cardiography Based on Bluetooth Low Energy (PDF Download Available). Available online: https://www.researchgate.net/publication/286621781_Portable_Health_Monitoring_Device_for_Electrocardiogram_and_Impedance_Cardiography_Based_on_Bluetooth_Low_Energy (accessed on 24 June 2017).
- Ramakrishnan, S.; Bhatt, K.; Dubey, A.K.; Roy, A.; Singh, S.; Naik, N.; Seth, S.; Bhargava, B. Acute electrocardiographic changes during smoking: An observational study. BMJ Open 2013, 3, e002486. [Google Scholar] [CrossRef] [PubMed]







| Module | Sensing Element | Noise Mean Value | Noise Standard Deviation |
|---|---|---|---|
| Hand | Hand Accelerometer | 0.19 g | 0.00324 g |
| Hand Gyroscope | 10 dps | 1.57 dps | |
| Pedometer Step-Counter | 0 count | 0 count | |
| Chest | Chest Accelerometer | 0.04 g | 0.00416 g |
| respiratory inductive plethysmograph (RIP) Sensor | 5.23 count | 1.29 count | |
| Bioimpedance Sensor | 0 mV | 0 mV | |
| ECG (Electrocardiogram) Sensor | 0.007 mV | 0.003 mV | |
| Proximity Sensor | 7.31 mV | 1.21 mV | |
| GPS (coordinate displacement) | 15.11 m | 2.639 m |
| Simulated Chest Circumference | Average Mean Pulse Count | Average Pulse Count Range | Average frequency (f0) Measured (MHz) |
|---|---|---|---|
| 60 cm | 102,376.76 ± 1.55 | 6.33 ± 1.15 | 10.23 |
| 80 cm | 96,199.76 ± 0.74 | 5.67 ± 4.73 | 9.61 |
| 100 cm | 92,954.09 ± 1.75 | 7.67 ± 4.16 | 9.29 |
| 120 cm | 89,088.46 ± 1.31 | 5.67 ± 2.89 | 8.90 |
| Recorded Events | Controlled Portion | Free Living Portion | Total |
|---|---|---|---|
| Lighter press–release | 193 | 356 | 549 |
| Cigarette consumption estimated from lighter data | 185 | 337 | 522 |
| Cigarette consumption from self-report | 185 | 319 | 504 |
| Hand-to-mouth gestures from IMUs | 2519 | 17,639 | 20,158 |
| Breaths from RIP sensor | 14,232 | 99,985 | 114,217 |
| Breaths from bio-impedance | 13,629 | 98,546 | 112,175 |
| Hand-to-mouth proximity | 2819 | 19,388 | 22,207 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imtiaz, M.H.; Ramos-Garcia, R.I.; Senyurek, V.Y.; Tiffany, S.; Sazonov, E. Development of a Multisensory Wearable System for Monitoring Cigarette Smoking Behavior in Free-Living Conditions. Electronics 2017, 6, 104. https://doi.org/10.3390/electronics6040104
Imtiaz MH, Ramos-Garcia RI, Senyurek VY, Tiffany S, Sazonov E. Development of a Multisensory Wearable System for Monitoring Cigarette Smoking Behavior in Free-Living Conditions. Electronics. 2017; 6(4):104. https://doi.org/10.3390/electronics6040104
Chicago/Turabian StyleImtiaz, Masudul Haider, Raul I. Ramos-Garcia, Volkan Yusuf Senyurek, Stephen Tiffany, and Edward Sazonov. 2017. "Development of a Multisensory Wearable System for Monitoring Cigarette Smoking Behavior in Free-Living Conditions" Electronics 6, no. 4: 104. https://doi.org/10.3390/electronics6040104






