A Review on Opportunities To Assess Hydration in Wireless Body Area Networks
Abstract
:1. Introduction
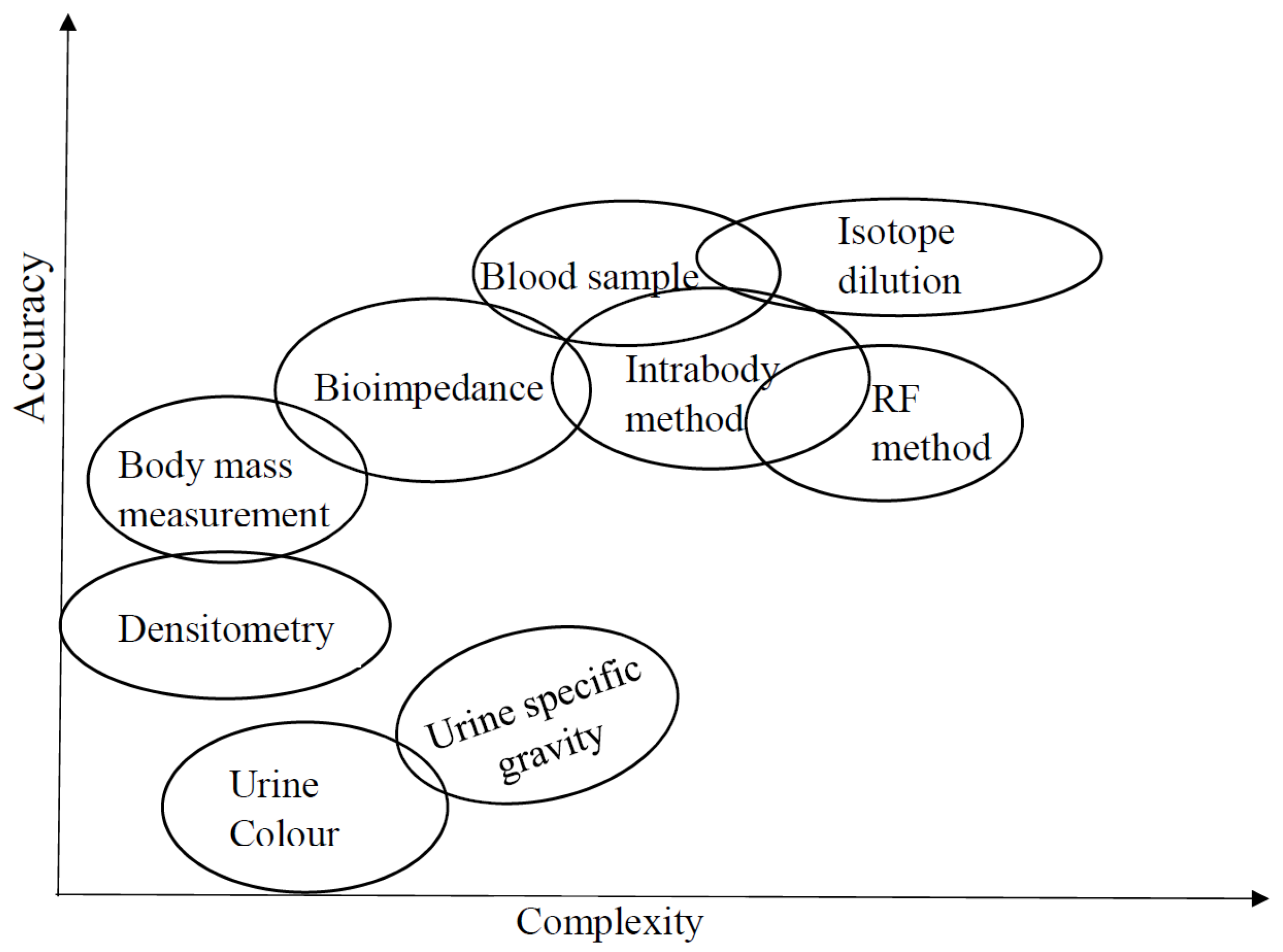
2. Classical Methods for Assessing Hydration
2.1. Urine Color and Urine Specific Gravity
2.2. Body Mass Changes
2.3. Analysis of Blood Sample
2.4. Isotope Dilution Method
3. Electrical Property of Human Tissues
- The low frequency region called dispersion is the region associated with ionic diffusion on cellular membrane and occurs between 1 Hz to 100 kHz.
- dispersion or mid-frequency region is associated with the polarization of the cellular structure of membranes between the intracellular and the extracellular tissue and lies between 100 kHz to 10 MHz.
- dispersion operates primarily at frequencies in the gigarhertz range which is associated with higher radiation.
4. Human Body as a Transmission Channel
5. Hydration Assessment by Electrical Methods
5.1. Bioelectrical Impedance Analysis
5.2. Bioelectrical Impedance Spectroscopy (BIS)
5.3. Galvanic Coupling Method
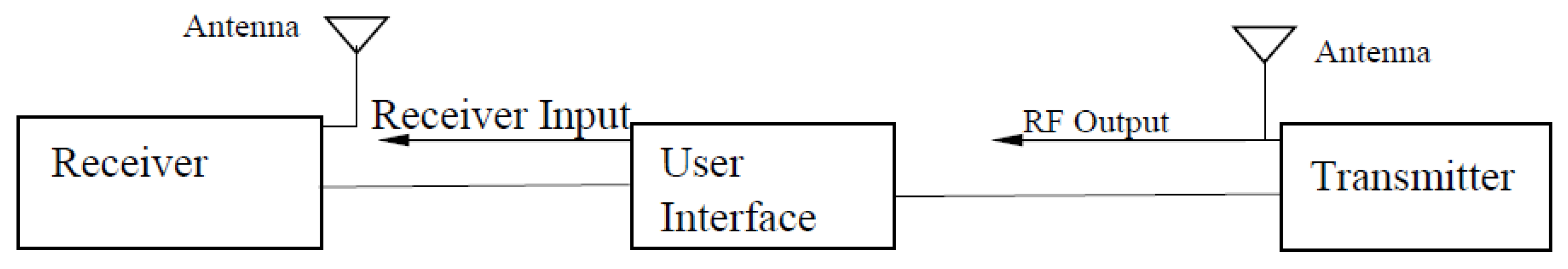
5.4. Radio Frequency Method
5.5. Remote Monitoring by Wireless Network
6. Research Challenges
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26, 575s–584s. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Amalia, T.; Carolynn, G. Methods of Assessment of Hydration Status and their Usefulness in Detecting Dehydration in the Elderly. Curr. Res. Nutr. Food Sci. J. 2017, 5. [Google Scholar] [CrossRef]
- Mitchell, H.H.; Hamilton, T.S.; Steggerda, F.R.; Bean, H.W. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J. Biol. Chem. 1945, 168, 625–637. [Google Scholar]
- Agostoni, C.V.; Bresson, J.L.; Fairweather-Tait, S. Scientific opinion on dietary reference values for water. EFSA J. 2010, 8, 3. [Google Scholar]
- Martinsen, O.G.; Grimnes, S. Bioimpedance and Bioelectricity Basics. In Bioimpedance and Bioelectricity Basics (Second Edition); Academic Press: London, UK, 2008; pp. 7–55. ISBN 978-0-12-374004-5. [Google Scholar]
- John, G.W. A role for water in cell structure. Biochem. J. 1987, 248, 615–617. [Google Scholar]
- Dieter, H. The role of cellular hydration in the regulation of cell function. Biochem. J. 1996, 313, 697–710. [Google Scholar]
- Peronnet, F.; Mignault, D.; Du Souich, P.; Vergne, S.; Le Bellego, L.; Jimenez, L.; Rabasa-Lhoret, R. Pharmacokinetic analysis of absorption, distribution and disappearance of ingested water labeled with d2o in humans. Eur. J. Appl. Physiol. 2012, 112, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Rothlingshofer, L.; Ulbrich, M.; Hahne, S.; Leonhardt, S. Monitoring change of body fluid during physical exercise using bioimpedance spectroscopy and finite element simulations. J. Electr. Bioimp. 2011, 2, 79–85. [Google Scholar] [CrossRef]
- Max, L.; David, H.; Hardinsyah, I.; Jean-Francois, D.; Simon, B.; Lawrence, E.A. Hydration for Health. Available online: http://www.h4hinitiative.com/ (accessed on 17 July 2017).
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [PubMed]
- Chamney, P.W.; Wabel, P.; Moissl, U.M.; Muller, M.J.; Bosy-Westphal, A.; Korth, O.; Fuller, N.J. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am. J. Clin. Nutr. 2007, 85, 80–89. [Google Scholar] [PubMed]
- Oppliger, R.A.; Bartok, C. Hydration testing of athletes. Sports Med. 2002, 32, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Shirrefs, S.M. Markers of hydration status. Eur. J. Clin. Nutr. 2003, 57, S6–S9. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E. Hydration Assessment Techniques. Nutr. Rev. 2005, 63, S40–S54. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.N. Thirst and hydration: Physiology and consequences of dysfunction. Physiol. Behav. 2010, 100, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pace, N.; Rathbun, E.N. Studies on body composition 3. The body water and chemically combined nitrogen content in relation to fat content. J. Biol. Chem. 1945, 158, 685–691. [Google Scholar]
- Behnke, A.R.; Feen, B.G.; Welham, W.C. The specific gravity of healthy men: Body weight and volume as an index of obesity. J. Am. Med. Assoc. 1942, 118, 495–498. [Google Scholar] [CrossRef]
- Deurenberg, P.; Weststrate, J.A.; Van Der Kooy, K. Is an adaptation of siri’s formula for the calculation of body fat percentage from body density in the elderly necessary? Eur. J. Clin. Nutr. 1989, 43, 559–567. [Google Scholar] [PubMed]
- Ridner, S.H.; Montgomery, L.D.; Hepworth, J.T.; Stewart, B.R.; Armer, J.M. Comparison of upper limb volume measurement techniques and arm symptoms between healthy volunteers and individuals with known lymphedema. Lymphology 2007, 40, 35–46. [Google Scholar] [PubMed]
- Wang, Z.; Pierson, R.N.; Heymsfield, S.B. The five-level model: A new approach to organizing body-composition research. Am. J. Clin. Nutr. 1992, 56, 19–28. [Google Scholar] [PubMed]
- Armstrong, L.E.; Maresh, C.M.; Castellani, J.W.; Bergeron, M.F.; Kenefick, R.W.; LaGasse, K.E.; Riebe, D. Urinary indices of hydration status. Int. J. Sport Nutr. 1994, 4, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Mentes, J.C.; Bonnie, W.; Kennith, C. Use of a urine color chart to monitor hydration status in nursing home residents. Biol. Res. Nurs. 2006, 7, 197–203. [Google Scholar] [CrossRef] [PubMed]
- MedlinePlus: Urine Concentration Test by U.S. National Library of Medicine. Available online: https://medlineplus.gov/ency/article/003608.htm (accessed on 13 August 2017).
- Williamson, M.A.; Snyder, L.M. Wallach’s Interpretation of Diagnostic Tests, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Graff, L. A Handbook of Routine Urinalysis; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1983. [Google Scholar]
- Lentner, C. Units of Measurement, Body Uids, Composition of the Body, Nutrition. In Geigy Scientific Tables; Ciba-Geigy Ltd.: Basle, Switzerland, 1981. [Google Scholar]
- Cheuvront, S.N.; Carter, R., III; Montain, S.J.; Sawka, M.N. Daily body mass variability and stability in active men undergoing exercise-heat stress. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Kenefick, R.W.; Sawka, M.N. Hydration at the work site. J. Am. Coll. Nutr. 2007, 26, 597S–603S. [Google Scholar] [CrossRef] [PubMed]
- Dimant, J. Delivery of nutrition and hydration care in nursing homes: Assessment and interventions to prevent and treat dehydration, malnutrition, and weight loss. J. Am. Med. Dir. Assoc. 2001, 2, 175–182. [Google Scholar] [CrossRef]
- Maughan, R.J.; Shirreffs, S.M.; Leiper, J.B. Errors in the estimation of hydration status from changes in body mass. J. Sport Sci. 2007, 25, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Sollanek, K.J.; Kenefick, R.W.; Cheuvront, S.N.; Axtell, R.S. Potential impact of a 500-mL water bolus and body mass on plasma osmolality dilution. Eur. J. Appl. Physiol. 2011, 111, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.S.; Heled, Y.; Margaliot, M.; Shani, Y.; Laor, A.; Margaliot, S.; Shapiro, Y. Hydration status measurement by radio frequency absorptiometry in young athletes—A new method and preliminary results. Physiol. Meas. 2003, 25, 51. [Google Scholar] [CrossRef]
- Francesconi, R.P.; Hubbard, R.W.; Szlyk, P.C.; Schnakenberg, D.; Carlson, D.; Leva, N.; Sils, I.; Hubbard, L.; Pease, V.; Young, J. Urinary and hematologic indexes of hypohydration. J. Appl. Physiol. 1987, 62, 1271–1276. [Google Scholar] [PubMed]
- Guyton, A.C. Textbook of Medical Physiology, 5th ed.; Saunders: Philadelphia, PA, USA, 1976. [Google Scholar]
- Ellis, K.J. Human body composition: In vivo methods. Physiol. Rev. 2000, 80, 649–680. [Google Scholar] [PubMed]
- Schwan, H.P. Electrical properties of tissue and cell suspensions. Adv. Biol. Med. Phys. 1957, 5, 147–209. [Google Scholar] [PubMed]
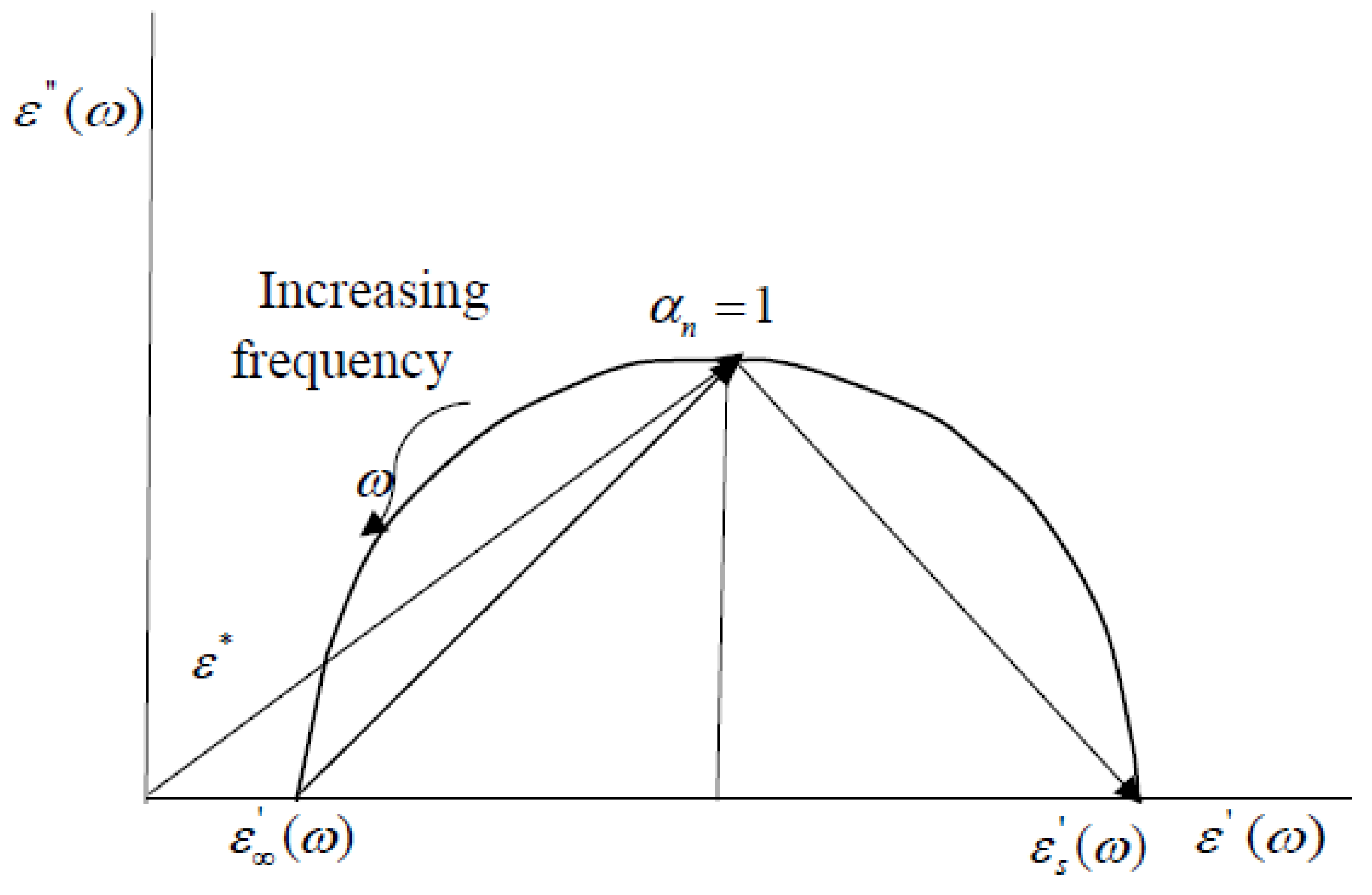
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics—I Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–352. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C. Compilation of the dielectric properties of body tissues at RF and microwave frequencies. In Brooks Air Force Technical Report, Occupational and Environmental Health Directorate, Radiofrequency Radiation Division; N.AL/OE-TR-1996–0037; Brooks Air Force Base: San Antonio, TX, USA, 1996. [Google Scholar]
- Gabriel, C.; Peyman, A. Dielectric measurement: Error analysis and assessment of uncertainty. Phys. Med. Biol. 2006, 51, 6043–6046. [Google Scholar] [CrossRef] [PubMed]
- IEEE Standard for Local and Metropolitan Area Networks Part 15.6: Wireless Body Area Networks; IEEE Std. 802.16.6; IEEE: New York, NY, USA, 2012.
- Zimmerman, T.G. Personal area networks: Near-field intrabody communication. IBM Syst. 1996, 3–4, 609–617. [Google Scholar] [CrossRef]
- Song, Y.; Hao, Q.; Zhang, K.; Wang, M.; Chu, Y.; Kang, B. The simulation method of the galvanic coupling intrabody communication with different signal transmission paths. IEEE Trans. Instrum. Meas. 2011, 60, 1257–1266. [Google Scholar] [CrossRef]
- Asogwa, C.O.; Teshome, A.; Collins, S.; Lai, D. A Circuit Model of Real Time Human Body Hydration. IEEE Trans. Biomed. Eng. 2016, 63, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhu, H.; Yuan, J. Electric-field intra-body communication channel modeling with finite-element method. IEEE Trans. Biomed. Eng. 2011, 58, 705–712. [Google Scholar] [PubMed]
- Wegmueller, M.S.; Kuhn, A.; Froehlich, J.; Oberle, M.; Felber, N.; Kuster, N.; Fichtner, W. An attempt to model the human body as a communication channel. IEEE Trans. Biomed. Eng. 2007, 54, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Dielectric and Electronic Properties of Biological Materials; Wiley Press: New York, NY, USA, 1979; pp. 47–53. [Google Scholar]
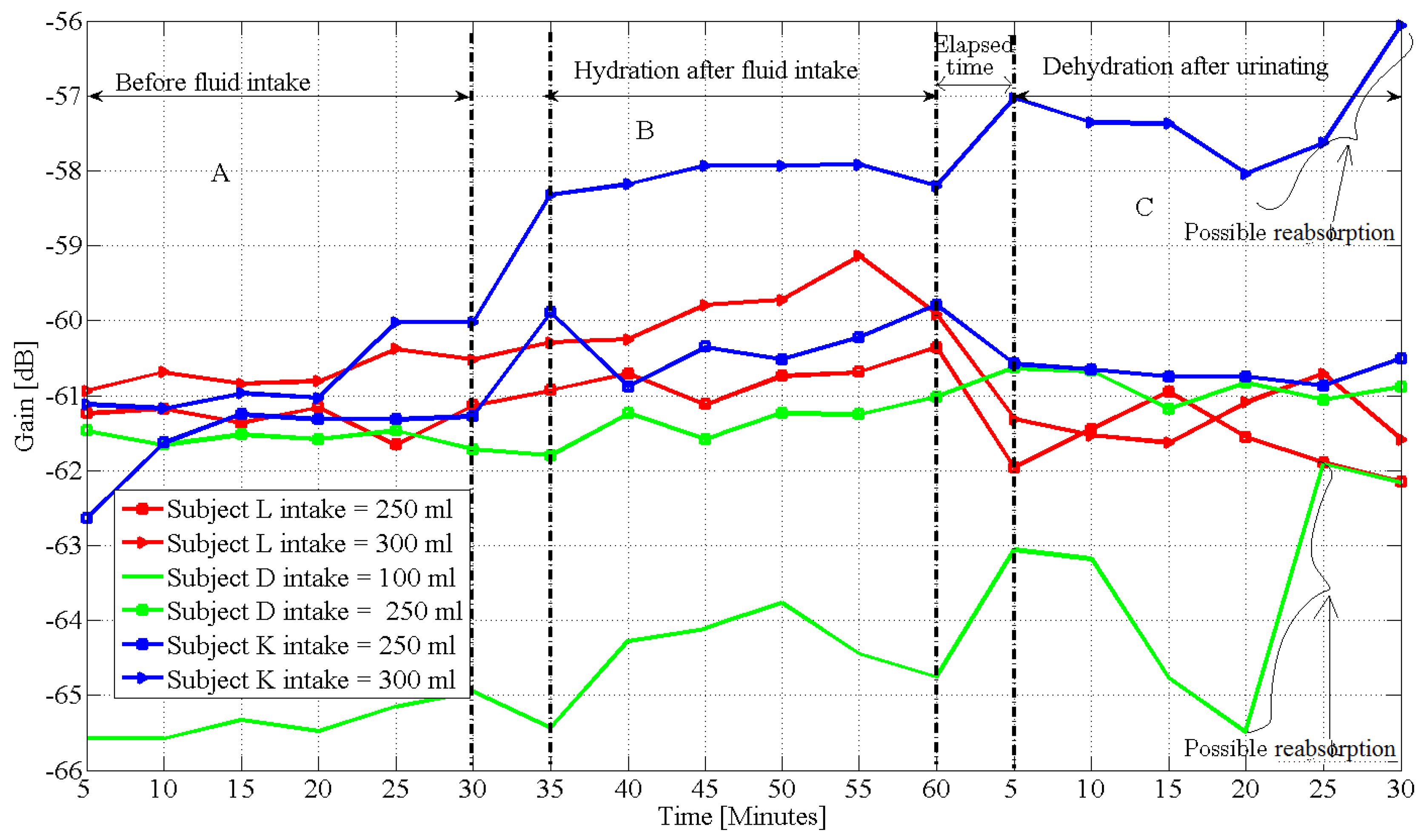
- Wang, J.; Zilic, Z.; Shu, Y. Evaluation of an RF wearable device for non-invasive real-time hydration monitoring. In Proceedings of the 2017 IEEE 14th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Eindhoven, The Netherlands, 9–12 May 2017; pp. 91–94. [Google Scholar]
- Shanholtzer, B.A.; Patterson, S.M. Use of bioelectrical impedance in hydration status assessment: Reliability of a new tool in psychophysiology research. Int. J. Psychophysiol. 2003, 49, 217–226. [Google Scholar] [CrossRef]
- Asselin, M.C.; Kriemler, S.; Chettle, D.R.; Webber, C.E.; Bar-Or, O.; McNeill, F.E. Hydration status assessed by multi-frequency bioimpedance analysis. Appl. Radiat. Isotopes 1998, 49, 495–497. [Google Scholar] [CrossRef]
- Nyboer, J. Workable volume and flow concepts of bio-segments by electrical impedance plethysmography. TIT. J. Life Sci. 1972, 2, 1–13. [Google Scholar] [PubMed]
- Kreider, J.; Nyboer, M.M.; Hannapel, L. Electrical impedance plethysmography: A physical and physiologic approach to peripheral vascular study. Circulation 2 1950, 6, 811–821. [Google Scholar]
- Van Loan, M.D.; Withers, P.; Matthie, J.; Mayclin, P.L. Use of bioimpedance spectroscopy to determine extracellular fluid, intracellular fluid, total body water, and fat-free mass. Basic Life Sci. 1993, 60, 67–70. [Google Scholar] [PubMed]
- Wabel, P.; Chamney, P.; Moissl, U.; Jirka, T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009, 27, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Zouridakis, A.; Simos, Y.V.; Verginadis, I.I.; Charalabopoulos, K.; Ragos, V.; Dounousi, E.; Boudouris, G.; Karkabounas, S.; Evangelou, A.; Peschos, D. Correlation of bioelectrical impedance analysis phase angle with changes in oxidative stress on end-stage renal disease patients, before, during, and after dialysis. Ren. Fail 2016, 38, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Asogwa, C.O.; Collins, S.F.; Mclaughlin, P.; Lai, D.T. A Galvanic Coupling Method for Assessing Hydration Rates. Electronics 2016, 5, 39. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Deurenberg, P.; Andreoli, A.; Sasso, G.F.; Palestini, M.; Docimo, R. Multifrequency impedance in the assessment of body water losses during dialysis. Kidney Blood Press Res. 1994, 17, 326–332. [Google Scholar] [CrossRef]
- Ellis, K.J.; Bell, S.J.; Chertow, G.M.; Chumlea, W.C.; Knox, T.A.; Kotler, D.P.; Lukaski, H.C.; Schoeller, D.A. Bioelectrical impedance methods in clinical research: A follow-up to the NIH Technology Assessment Conference. Nutrition 1999, 15, 874–880. [Google Scholar] [CrossRef]
- Jaffrin, M.Y.; Morel, H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med. Eng. Phys. 2008, 30, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R.F.; Gudivaka, R.; Schoeller, D.A. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am. J. Clin. Nutr. 1996, 64, 423S–427S. [Google Scholar] [PubMed]
- Ionescu, M.A.; Guerin, H. System for the Remote Monitoring of the Hydration Status of a Living Being. U.S. Patent 14/840,852, 3 March 2016. Ecole polytechnique fédérale de Lausanne (EPFL). [Google Scholar]
- Ramos, J.; Ausín, J.L.; Torelli, G.; Duque-Carrillo, J.F. A wireless sensor network for fat and hydration monitoring by bioimpedance analysis. In Proceedings of the 6th IEEE International Workshop on Wearable Micro and Nano Technologies for Personalized Health (pHealth), Oslo, Norway, 24–26 June 2009; pp. 49–52. [Google Scholar]
- Huang, X.; Liu, Y.; Cheng, H.; Shin, W.J.; Fan, J.A.; Liu, Z.; Lu, C.J.; Kong, G.W.; Chen, K.; Patnaik, D.; et al. Materials and designs for wireless epidermal sensors of hydration and strain. Adv. Funct. Mater. 2014, 24, 3846–3854. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, H.; Chen, K.; Zhang, Y.; Zhang, Y.; Liu, Y.; Zhu, C.; Ouyang, S.C.; Kong, G.W.; Yu, C.; et al. Epidermal impedance sensing sheets for precision hydration assessment and spatial mapping. IEEE Trans. Biomed. Eng. 2013, 60, 2848–2857. [Google Scholar] [CrossRef] [PubMed]
- Solovei, D.; Žák, J.; Majzlíková, P.; Sedláček, J.; Hubálek, J. Chemical sensor platform for non-invasive monitoring of activity and dehydration. Sensors 2015, 15, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Myers, A.; Malhotra, A.; Lin, F.; Bozkurt, A.; Muth, J.F.; Zhu, Y. A Wearable Hydration Sensor with Conformal Nanowire Electrodes. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
| Method | Strength | Weakness | Comment |
|---|---|---|---|
| Urine Colour Analysis (UCA) | Very simple No speciality skill is required | Subjective Limited accuracy | 1. No standard colour code 2. Affected by dietary intakes, drugs and supplements [26] |
| Urine Specific Gravity | More reliable than UCA 3. Inexpensive | Result can be altered by medication | 1. Limited accuracy |
| Densitometry | Easy to perform | Measures TBW only | over simplified result |
| Body mass measurement | Self-administered Simple and inexpensive | Limited accuracy Measures TBW only | 1. Accuracy depends on sensitivity of scale |
| SF-BIA | Good for TBW | 1. Cannot differentiate ECF from ICF 2. It is based on a 2-C model of human body | 1. Over simplified 2. Subject to uncertainties 3. Does not respond quickly to acute changes in body fluid level [59] 4. Measures only extra-cellular fluid [60] |
| MF-BIA | Measures ECW and ICW, Good for whole body healthy normal-weight people fluid measurement in | 1. Does not respond to acute changes in fluid level from hydration or dehydration | 1. Different devices used for different body parts 2. Not suitable as a wearable device |
| BIS | 1. Measures ECW, ICW, FFM, FM, TBW 2. Broad frequency range( 1–1000 kHz) 3. Segmental BIS calculates body composition segment by segment 4. Bulky, not suitable as a wearable device | 1. Needs further refinement particularly for populations with abnormal body geometry (e.g., very high BMI) 2. Assumes constant specific resistivity of the fluid compartment measured 3. Did not include the effects of temperature and metabolism [62] on the assumptions | 1. Theoretically differentiates ECW from ICF 2. Quantifies body cell mass 3. Impedance data in the frequency spectrum is fit into Cole’s model [61] 4. Uses statistical modelling equations based on Hanai mixture theory to calculate resistances at different frequencies |
| Analysis of Blood Sample | Higher accuracy than previous methods | 1. Result is affected by postural changes and exercise 2. Complicated and risky 3. Does not evaluate the amount of water by tissue compartment [33] 4. Does not respond quickly to mild reduction of water [34] | Measures the concentration of electrolyte substance in the blood and comparison with a reference |
| Isotope Dilution | High accuracy, 1. Tracks the distribution of water in the body Suitable for laboratory research | 1. Complicated and expensive 2. Requires different isotopes for different tissue compartments 3. Large time consuming 4. Can be affected by metabolism 5. Insensitive to 1 kg loss in TBW [36] | Assumes uniform circulation of tracers and risks overdose |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asogwa, C.O.; Lai, D.T.H. A Review on Opportunities To Assess Hydration in Wireless Body Area Networks. Electronics 2017, 6, 82. https://doi.org/10.3390/electronics6040082
Asogwa CO, Lai DTH. A Review on Opportunities To Assess Hydration in Wireless Body Area Networks. Electronics. 2017; 6(4):82. https://doi.org/10.3390/electronics6040082
Chicago/Turabian StyleAsogwa, Clement Ogugua, and Daniel T. H. Lai. 2017. "A Review on Opportunities To Assess Hydration in Wireless Body Area Networks" Electronics 6, no. 4: 82. https://doi.org/10.3390/electronics6040082