Data-Driven ECG Denoising Techniques for Characterising Bipolar Lead Sets along the Left Arm in Wearable Long-Term Heart Rhythm Monitoring
Abstract
:1. Introduction
2. Materials
2.1. Electrode System and Disposition
2.2. Bioamplifiers, Data Acquisition System and Protocol
3. Methods
3.1. Selected Arm-ECG Bipolar Leads
3.2. Raw Data Pre-Processing
3.3. Denoising Control Method: Signal Averaged ECG (SA-ECG)
3.3.1. Reference Ventricular Activity Timing Series
3.3.2. The ECG Signal-Averaging Process on Arm Bipolar Leads
3.3.3. Denoising Performance Metrics
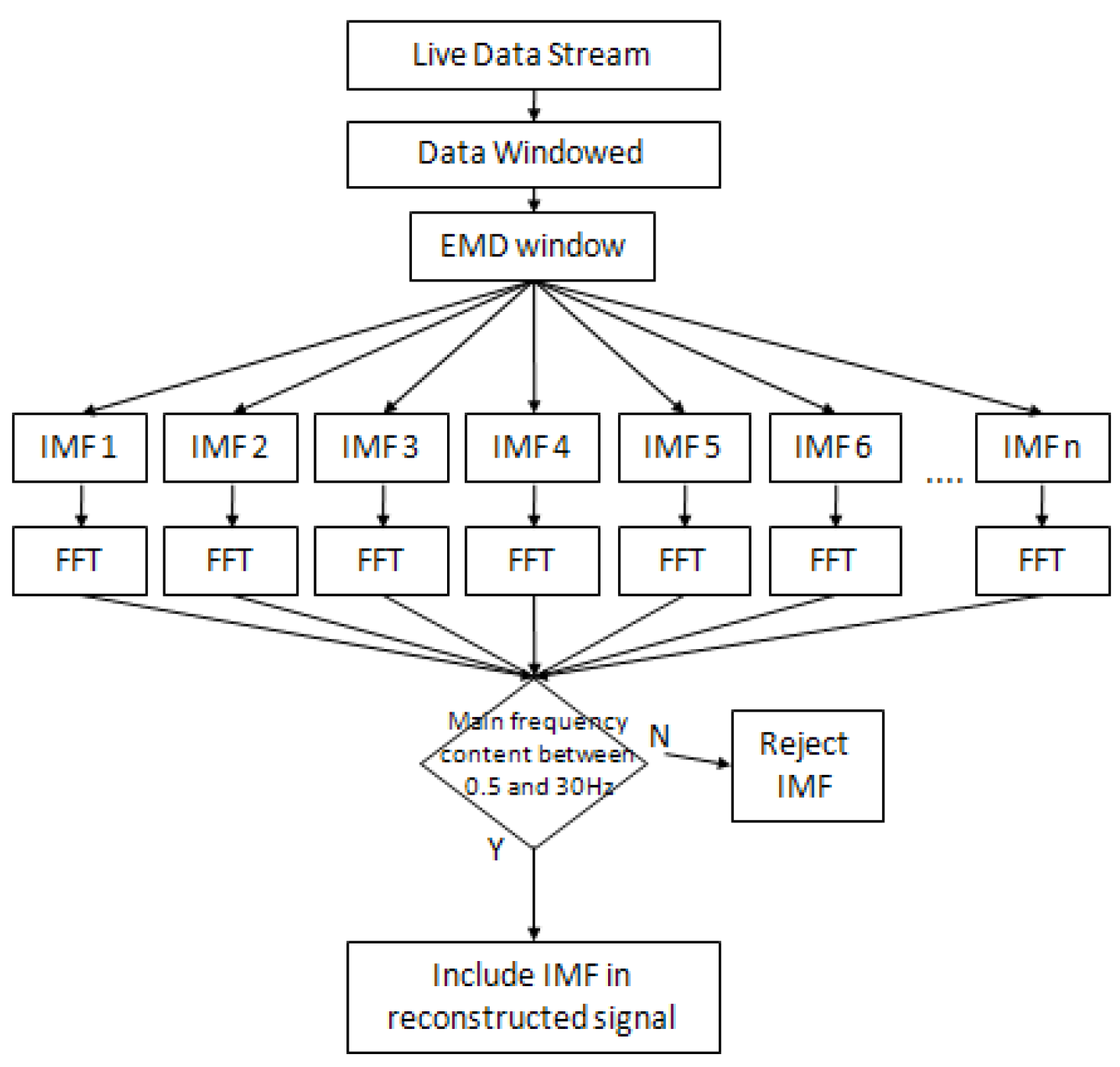
3.4. Empirical Mode Decomposition (EMD)
3.4.1. Implementation Criteria
3.4.2. EMD Implementation Process (Algorithm)
- The signal has at least two extrema; one is the maximum and the other the minimum.
- The time-period scale is defined by the time lapse between the two extrema.
- If the data have no extrema, only the inflection point is recorded, and the extrema can then be estimated by differentiation.
- (1)
- Identify all extrema of x(t).
- (2)
- Using a cubic spline, interpolate between minima and maxima with envelopes (t) and (t).
- (3)
- Compute the mean envelope, , where k is the iteration number.
- (4)
- Extract the detail .
- (5)
- Repeat (1)–(4) until (t) meets the definition of an IMF, and the IMF converges.
- (6)
- Repeat (1)–(5) to generate a residual (t), (t) = x(t) − (t).
3.5. Ensembled EMD (EEMD)
3.5.1. Implementation Criteria
3.5.2. FFT Thresholding Technique
3.5.3. EEMD Implementation Process (Algorithm)
4. Results
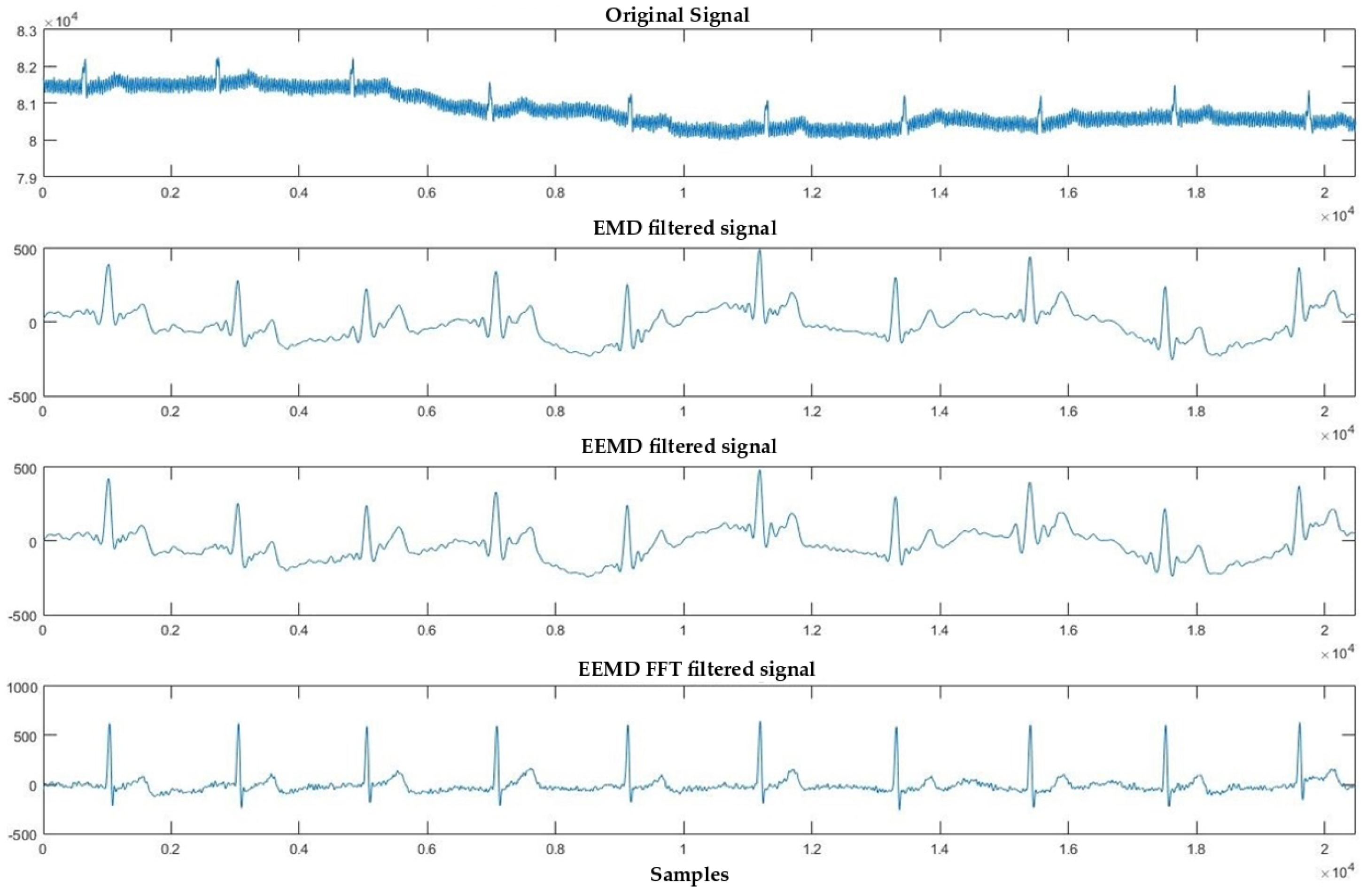
4.1. Denoising Process
4.2. Signal Averaging and EMD, EEMD and EEMD-fft Comparative Study
5. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Katritsis, D.G.; Gersh, B.J.; Camm, A.J. A clinical perspective on sudden cardiac death. Arrhythm. Electrophysiol. Rev. 2016, 5, 177–182. [Google Scholar] [CrossRef]
- Zimetbaum, P.; Josephson, M.E. Evaluation of patients with palpitations. N. Engl. J. Med. 1998, 338, 1369. [Google Scholar] [CrossRef] [PubMed]
- Massot, B.; Noury, N.; Gehin, C.; McAdams, E.T. On designing a ubiquitous sensor network for health monitoring. In Proceedings of the 2013 IEEE 15th International Conference on e-Health Networking, Applications and Services (Healthcom), Lisbon, Portugal, 9–12 October 2013; pp. 310–314. [Google Scholar]
- Shackel, B.; Chidsy, K.D.; Shipley, P. The assessment of chair comfort. Ergonomics 1969, 12, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chien, T.; Liu, S.; Chiang, H. Study of Single-Arm Electrode for ECG Measurement Using Flexible Print Circuit. 2011. Available online: http://eshare.stust.edu.tw/EshareFile/2011_6/2011_6_7f351cdf.pdf (accessed on 23 July 2014).
- Shu, L.; Tung, S.; Kuo, C.; Ma, C. Bared Microtip Arrays for ECG Measurement. Int. J. Autom. Smart Technol. 2016, 6, 177–180. [Google Scholar]
- Chen, K.; Ren, L.; Chen, Z.; Pan, C.; Zhou, W.; Jiang, L. Fabrication of Micro-Needle Electrodes for Bio-Signal Recording by a Magnetization-Induced Self-Assembly Method. Sensors 2016, 16, 1533. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, T.; Kim, D.; Chung, W.K. Curved Microneedle Array-Based sEMG Electrode for Robust Long-Term Measurements and High Selectivity. Sensors 2015, 15, 16265–16280. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Lin, B.; Xu, Z. Electrocardiogram signal denoising based on empirical mode decomposition technique: An overview. J. Instrum. 2017, 12. [Google Scholar] [CrossRef]
- Bosnjak, A.; Kennedy, A.; Linares, P.; Borges, M.; McLaughlin, J.; Escalona, O.J. Performance assessment of dry electrodes for wearable long term cardiac rhythm monitoring: Skin-electrode impedance spectroscopy. In Proceedings of the IEEE Engineering in Medicine and Biology Society, Jeju Island, South Korea, 11–15 July 2017; Volume 39. [Google Scholar]
- Lynn, W.D.; Escalona, O.J.; McEneaney, D.J. A Low Latency Electrocardiographic QRS Activity Recovery Technique for Use on the Upper Left Arm. Electronics 2014, 3, 409–418. [Google Scholar] [CrossRef]
- Lynn, W.D.; Escalona, O.J.; McEneaney, D. Approaching long term cardiac rhythm monitoring using advanced arm worn sensors and ECG recovery techniques. Cardiol. Angiol. 2015, 3, 137–148. [Google Scholar] [CrossRef]
- Lynn, W.D.; Escalona, O.J.; McEneaney, D.J. Arm and wrist surface potential mapping for wearable ECG rhythm recording devices: A pilot clinical study. J. Phys. 2013, 450, 012026. [Google Scholar] [CrossRef]
- Escalona, O.J.; Mendoza, M. Electrocardiographic Waveforms Fitness Check Device Technique for Sudden Cardiac Death Risk Screening. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Orlando, FL, USA, 16–20 August 2016; pp. 3453–3456. [Google Scholar]
- Escalona, O.J.; Mitchell, R.H.; Balderson, D.E. A fast and reliable QRS alignment technique for high-frequency analysis of the signal-averaged ECG. Med. Biol. Eng. Comput. 1993, 31, 137–146. [Google Scholar] [CrossRef]
- Escalona, O.J.; Mendoza, M.; Villegas, G.; Navarro, C. Real-time System for High-resolution ECG Diagnosis Based on 3D Late Potential Fractal Dimension Estimation. Comput. Cardiol. 2011, 38, 789–792. [Google Scholar]
- Escalona, O.J. Analog implementation of the single fiducial point alignment technique for real-time high resolution ECG analysis in the P-R interval. In Proceedings of the Computers in Cardiology 1998, Cleveland, OH, USA, 13–16 September 1998; pp. 229–232. [Google Scholar]
- Flandrin, P.; Rilling, G.; Goncalvés, P. Empirical mode decomposition as a filter bank. IEEE Signal Process. Lett. 2004, 11, 112–114. [Google Scholar] [CrossRef]
- Rilling, G.; Flandrin, P.; Goncalves, P. On Empirical Mode Decomposition and its Algorithm. In Proceedings of the 6th IEEE/EURASIP Workshop on Nonlinear Signal and Image Processing (NSIP’03), Grado, Italy, 8–11 June 2003; pp. 8–11. [Google Scholar]
- Wu, Z.; Huang, N.E.; Chen, X. The multi-dimensional ensemble empirical mode decomposition method. Adv. Adapt. Data Anal. 2009, 1, 339–372. [Google Scholar] [CrossRef]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. A 1998, 454, 903–999. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, N.E. Ensemble empirical mode decomposition: A noise-assisted data analysis method. Adv. Adapt. Data Anal. 2009. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, N.E. A study of the characteristics of white noise using the empirical mode decomposition method. Proc. R. Soc. A 2004, 460, 1597–1611. [Google Scholar] [CrossRef]
- Scilingo, E.P.; Valenza, G. Recent advances on wearable electronics and embedded computing systems for biomedical applications. Electronics 2017, 6, 12. [Google Scholar] [CrossRef]
- Boehm, A.; Yu, X.; Neu, W.; Leonhardt, S.; Teichmann, D. A novel 12-lead ECG T-shirt with active electrodes. Electronics 2016, 5, 75. [Google Scholar] [CrossRef]
- Zhou, Y. An oversampling system for ECG acquisition. J. Biomed. Sci. Eng. 2009, 2, 521–525. [Google Scholar] [CrossRef]
- Olson, D.; Van Ess, D. Method and Apparatus for Increasing the Low Frequency Dynamic Range of a Digital ECG Measuring System—US 6249696 B1. 1999. Available online: http://www.google.ch/patents/US6249696 (accessed on 08 August 2017).
- Kumar, K.S.; Yazdanpanah, B.; Kumar, P.R. Removal of Noise from Electrocardiogram Using Digital FIR and IIR Filters with Various Methods. In Proceedings of the Communications and Signal Processing (ICCSP) Conference, Melmaruvathur, India, 2–4 April 2015; pp. 157–162. [Google Scholar]
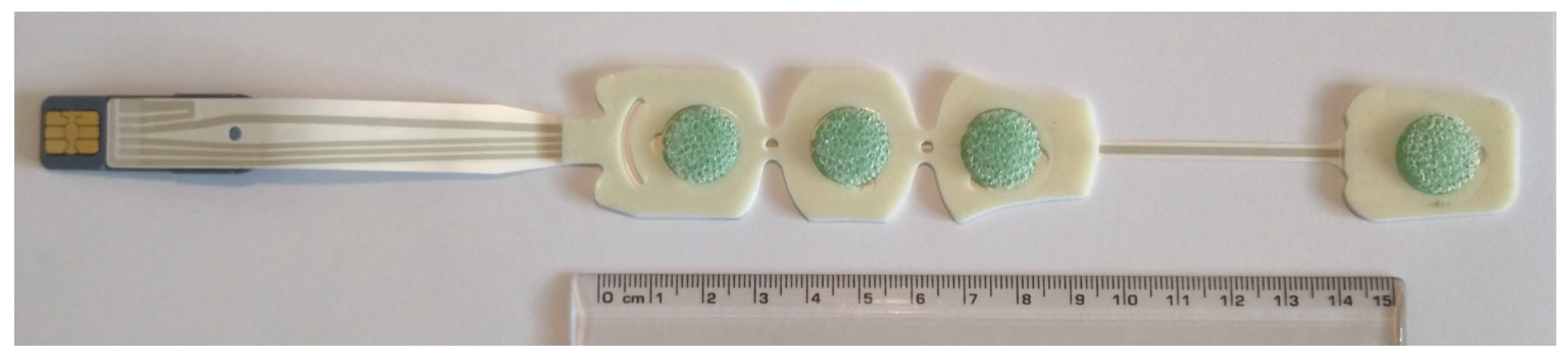




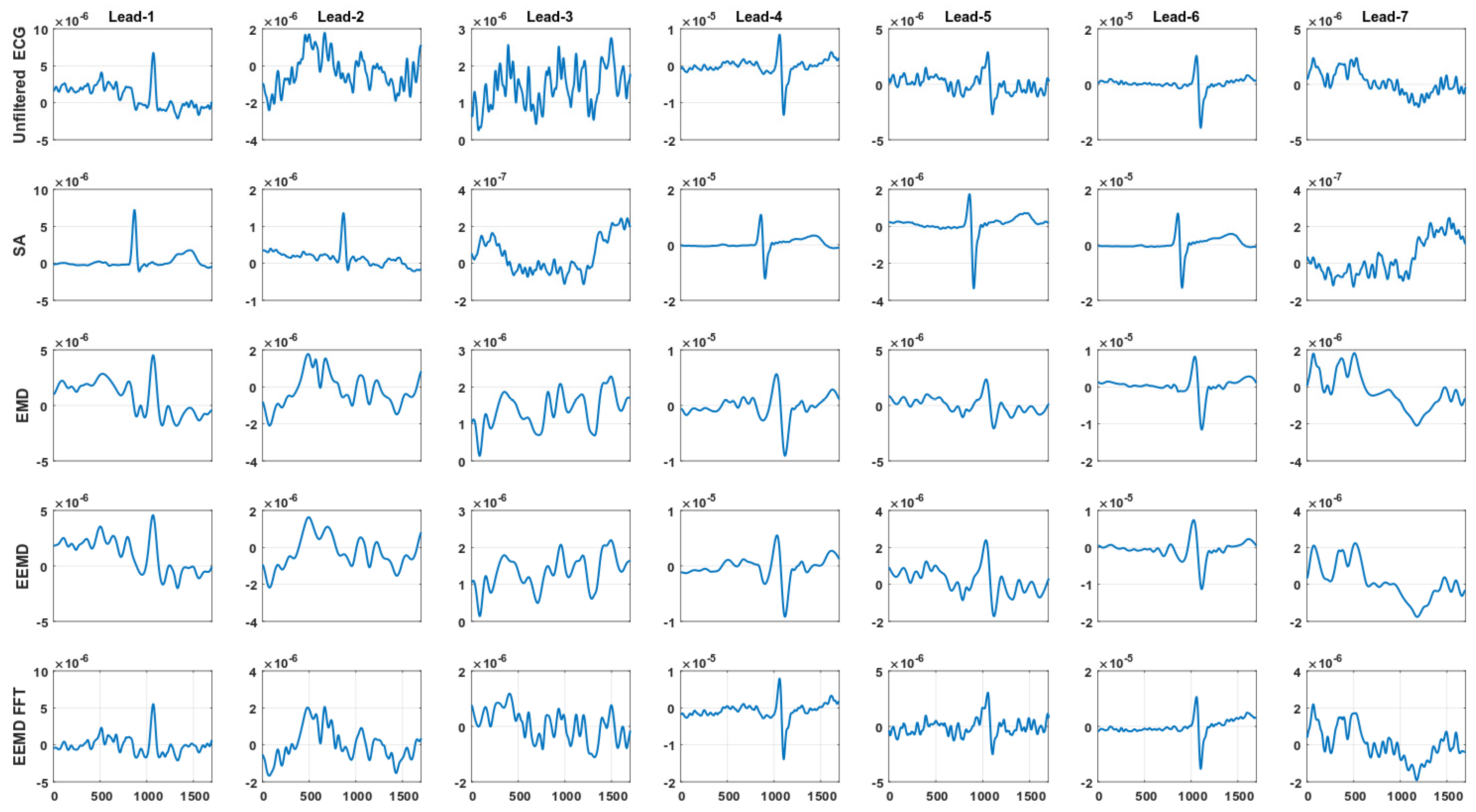
| Transversal left-arm BIS sensor bipolar leads |
| Lead-1 = Ch10–Ch8 → (Upper arm) |
| Lead-2 = Ch7–Ch5 → (Forearm) |
| Lead-3 = Ch4–Ch3 → (Wrist) |
| Axial left-arm BIS sensor bipolar leads |
| Lead-4 = Ch10–Ch6 → (Upper arm-Forearm) |
| Lead-5 = Ch7–Ch2 → (Forearm-Wrist) |
| Lead-6 = Ch10–Ch2 → (Upper arm-Wrist) |
| Lead-7 = Ch1–Ch3 → (Low wrist-Upper wrist) |
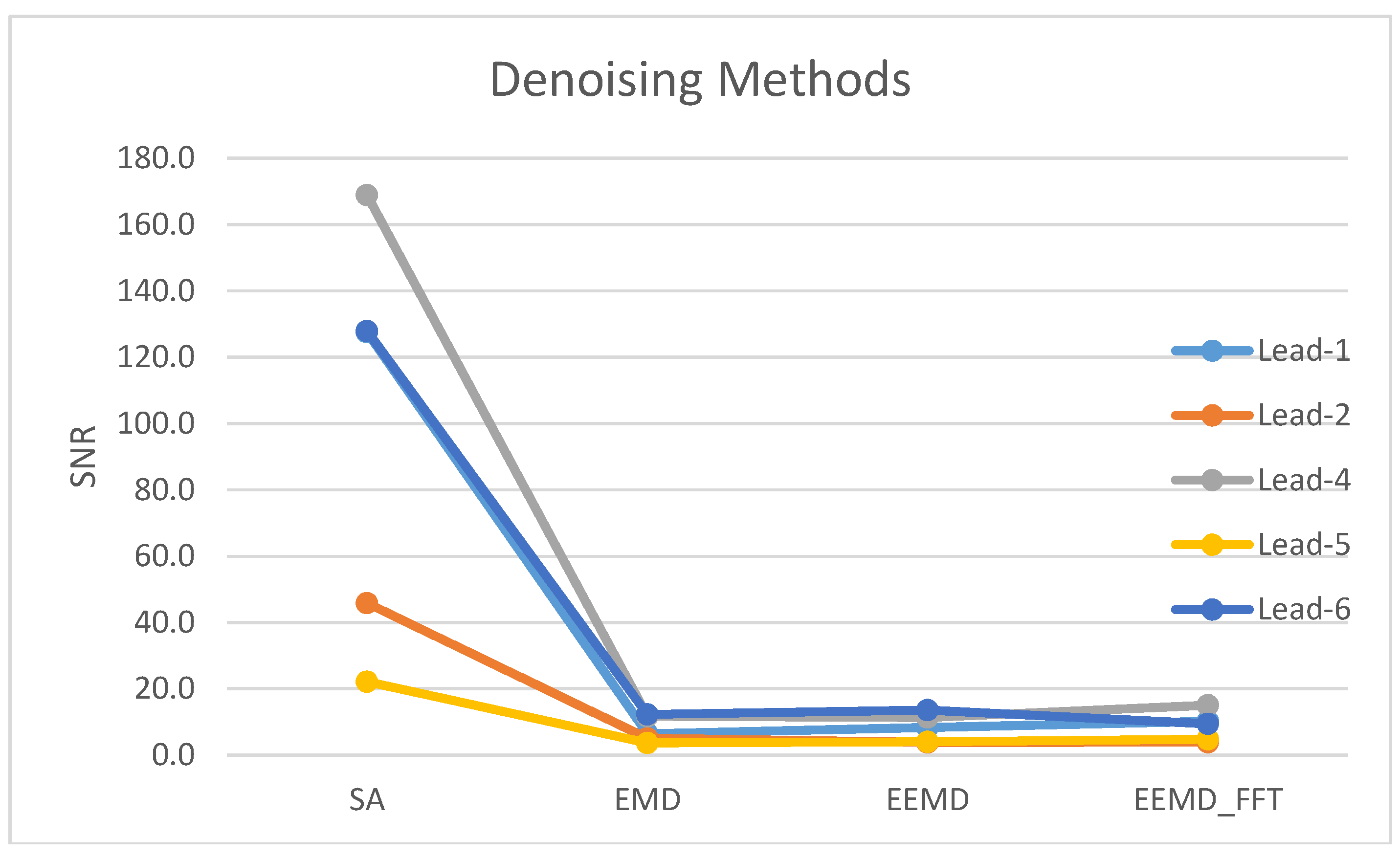
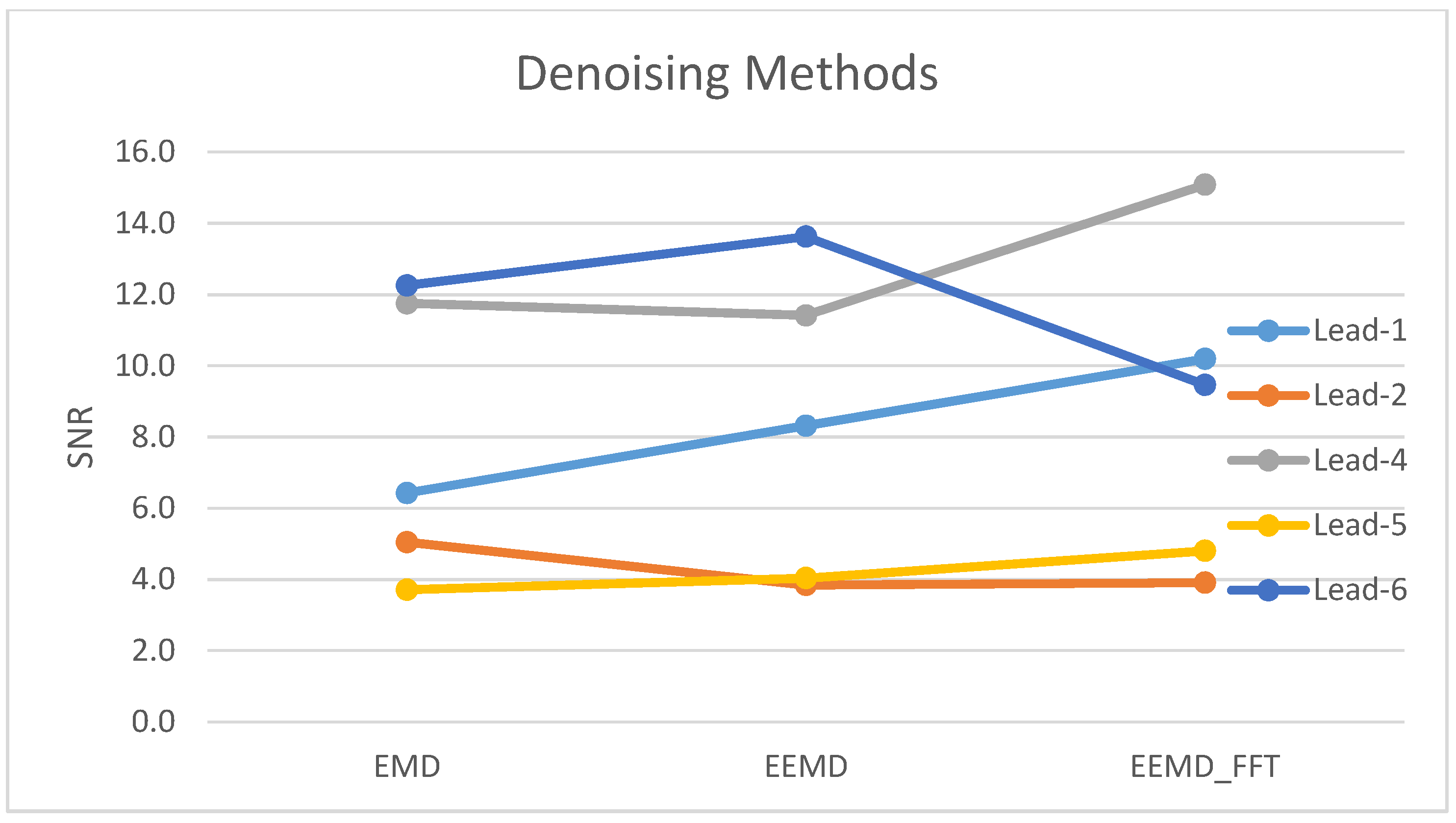
| Method | Lead-1 | Lead-2 | Lead-3 | Lead-4 | Lead-5 | Lead-6 | Lead-7 |
|---|---|---|---|---|---|---|---|
| SA | 102.8 | 33.1 | 2.2 | 114.7 | 21.6 | 95.7 | 2.7 |
| EMD | 8.0 | 3.9 | 3.4 | 9.0 | 3.2 | 9.3 | 2.3 |
| EEMD | 9.1 | 3.5 | 3.1 | 9.0 | 4.0 | 9.8 | 2.1 |
| EEMD-fft | 8.5 | 3.5 | 1.7 | 11.5 | 3.7 | 7.3 | 1.9 |
| Lead-1 | Lead-2 | Lead-3 | Lead-4 | Lead-5 | Lead-6 | Lead-7 | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | EMD | EEMD | EEMD fft | SA | EEMD fft | EEMD | EEMD | SA | EMD | EEMD fft | EEMD | SA | EMD | EEMD | EEMD fft | SA | EMD | EEMD | EEMD fft | SA | EMD | EEMD | EEMD fft | SA | EMD | EEMD | EEMD fft | |
| Case 1. | 203.1 | 15.5 | 23.1 | 16.8 | 16.5 | 4.7 | 5.4 | 1.6 | 0.9 | 1.1 | 1.1 | 1.6 | 164.2 | 29.0 | 26.4 | 14.4 | 5.1 | 1.2 | 1.6 | 4.7 | 68.4 | 6.5 | 5.8 | 7.2 | 0.8 | 1.0 | 1.1 | 1.6 |
| Case 10. | 206.6 | 9.3 | 5.7 | 3.4 | 24.3 | 2.4 | 3.9 | 2.2 | 1.2 | 1.3 | 1.4 | 2.7 | 142.2 | 34.8 | 22.8 | 25.2 | 54.2 | 26.6 | 15.1 | 14.7 | 617.0 | 48.0 | 56.2 | 48.2 | 1.7 | 1.2 | 1.0 | 2.2 |
| Case 13. | 20.7 | 8.9 | 9.0 | 11.0 | 9.7 | 4.6 | 9.0 | 4.7 | 2.3 | 3.7 | 3.8 | 1.7 | 12.6 | 9.4 | 9.5 | 14.2 | 2.3 | 3.7 | 3.7 | 1.5 | 2.4 | 3.8 | 3.7 | 1.6 | 1.9 | 2.1 | 2.5 | 0.7 |
| Case 15. | 182.9 | 1.5 | 1.3 | 3.7 | 177.6 | 32.2 | 8.5 | 9.1 | 0.9 | 2.5 | 2.7 | 0.5 | 419.3 | 2.2 | 1.9 | 6.8 | 37.4 | 6.8 | 16.3 | 20.9 | 145.4 | 3.0 | 3.5 | 5.5 | 1.5 | 1.8 | 2.3 | 1.4 |
| Case 24. | 31.5 | 1.7 | 1.7 | 1.0 | 9.4 | 1.9 | 2.7 | 1.4 | 0.6 | 1.7 | 1.9 | 2.1 | 106.1 | 7.1 | 12.2 | 7.3 | 9.7 | 0.9 | 1.0 | 1.0 | 58.4 | 8.1 | 8.7 | 5.6 | 1.3 | 0.4 | 0.9 | 1.8 |
| Case 26. | 212.9 | 7.9 | 8.8 | 5.2 | 26.7 | 1.7 | 1.7 | 0.7 | 0.6 | 1.1 | 1.9 | 1.2 | 135.9 | 13.1 | 12.2 | 7.2 | 8.8 | 1.3 | 1.2 | 0.9 | 45.4 | 11.2 | 11.1 | 5.4 | 0.7 | 1.5 | 1.6 | 1.3 |
| Case 39. | 1.7 | 2.7 | 2.7 | 8.0 | 36.0 | 4.1 | 6.5 | 15.8 | 2.8 | 3.2 | 2.1 | 2.5 | 115.4 | 17.1 | 25.2 | 58.4 | 14.4 | 3.7 | 7.1 | 4.8 | 124.8 | 30.9 | 42.0 | 29.1 | 1.4 | 0.4 | 0.7 | 1.2 |
| Case 44. | 60.4 | 0.2 | 0.2 | 11.2 | 29.0 | 2.1 | 2.0 | 2.9 | 2.2 | 13.7 | 15.0 | 1.1 | 327.4 | 0.2 | 0.2 | 19.0 | 49.7 | 0.2 | 0.2 | 8.0 | 226.1 | 0.1 | 0.1 | 11.1 | 2.5 | 1.2 | 0.9 | 1.4 |
| Case 47. | 117.8 | 14.3 | 11.1 | 10.8 | 36.3 | 2.9 | 3.3 | 4.9 | 0.9 | 3.8 | 3.0 | 2.2 | 272.0 | 9.1 | 9.1 | 10.1 | 46.3 | 6.5 | 4.2 | 3.6 | 277.2 | 16.0 | 21.4 | 10.3 | 1.8 | 1.7 | 1.8 | 2.1 |
| Case 5. | 130.4 | 11.3 | 44.1 | 31.8 | 24.9 | 1.7 | 1.7 | 0.8 | 0.8 | 0.3 | 0.5 | 0.5 | 160.3 | 20.2 | 15.2 | 8.6 | 8.9 | 1.6 | 0.4 | 1.0 | 63.8 | 12.8 | 15.6 | 7.3 | 1.4 | 1.0 | 0.8 | 0.4 |
| Case 54. | 2.6 | 0.5 | 0.5 | 22.3 | 151.9 | 0.8 | 0.9 | 4.2 | 1.7 | 1.9 | 1.5 | 0.7 | 322.4 | 0.3 | 0.3 | 20.8 | 38.7 | 0.9 | 1.2 | 1.3 | 152.0 | 1.2 | 1.3 | 4.1 | 4.4 | 4.5 | 4.6 | 1.3 |
| Case 6. | 2.8 | 6.9 | 8.0 | 11.4 | 8.1 | 1.8 | 2.1 | 3.1 | 0.6 | 9.6 | 5.5 | 1.5 | 92.8 | 16.8 | 17.0 | 6.6 | 5.9 | 2.2 | 6.6 | 0.9 | 94.1 | 17.2 | 19.7 | 5.3 | 1.4 | 1.2 | 1.6 | 0.6 |
| Case 62. | 84.7 | 3.4 | 7.5 | 8.5 | 32.0 | 2.9 | 2.0 | 2.9 | 6.0 | 1.6 | 1.4 | 1.8 | 95.7 | 8.8 | 11.4 | 5.4 | 11.3 | 0.4 | 0.4 | 0.3 | 50.5 | 5.3 | 6.4 | 2.4 | 27.2 | 1.3 | 1.4 | 4.7 |
| Case 63. | 385.4 | 7.1 | 5.9 | 5.7 | 27.5 | 1.4 | 1.2 | 0.6 | 0.4 | 1.1 | 1.3 | 4.6 | 90.3 | 18.2 | 15.0 | 6.0 | 0.1 | 0.6 | 0.6 | 3.8 | 1.0 | 3.7 | 4.2 | 4.1 | 3.0 | 3.3 | 3.6 | 2.8 |
| Case 64. | 51.2 | 7.6 | 7.2 | 11.5 | 21.2 | 1.9 | 2.5 | 3.4 | 1.7 | 5.5 | 4.4 | 2.2 | 118.1 | 4.0 | 4.3 | 9.4 | 9.5 | 2.6 | 2.7 | 1.8 | 21.5 | 4.6 | 4.0 | 2.7 | 0.6 | 6.4 | 9.0 | 10.8 |
| Case 65. | 53.5 | 3.1 | 3.5 | 8.8 | 60.2 | 2.8 | 4.0 | 4.1 | 0.5 | 2.5 | 2.8 | 0.8 | 113.4 | 7.2 | 8.2 | 18.1 | 19.4 | 1.2 | 1.5 | 0.7 | 132.3 | 11.4 | 6.1 | 4.2 | 0.7 | 9.3 | 4.6 | 1.7 |
| Case 7. | 214.2 | 8.1 | 3.8 | 4.5 | 81.1 | 2.0 | 2.0 | 1.1 | 6.4 | 0.4 | 0.3 | 0.5 | 214.2 | 6.8 | 6.5 | 12.8 | 9.2 | 0.8 | 1.4 | 0.6 | 87.4 | 7.9 | 4.8 | 3.6 | 0.2 | 0.9 | 0.7 | 0.5 |
| Case 9. | 333.3 | 5.7 | 5.7 | 7.7 | 52.4 | 19.0 | 9.7 | 7.0 | 0.7 | 1.2 | 2.3 | 0.9 | 138.3 | 7.3 | 8.0 | 21.3 | 67.3 | 5.6 | 7.4 | 15.8 | 134.8 | 29.0 | 30.8 | 12.5 | 1.7 | 1.6 | 1.2 | 0.7 |
| Avg. (N = 18) | 127.5 | 6.4 | 8.3 | 10.2 | 45.8 | 5.0 | 3.8 | 3.9 | 1.7 | 3.1 | 2.9 | 1.6 | 168.9 | 11.7 | 11.4 | 15.1 | 22.1 | 3.7 | 4.0 | 4.8 | 127.9 | 12.3 | 13.6 | 9.5 | 3.0 | 2.3 | 2.2 | 2.1 |
| Method | Lead-1 | Lead-2 | Lead-3 | Lead-4 | Lead-5 | Lead-6 | Lead-7 |
|---|---|---|---|---|---|---|---|
| SA | 29 | 27 | 1 | 34 | 20 | 31 | 0 |
| EMD | 22 | 11 | 0 | 29 | 7 | 25 | 0 |
| EEMD | 23 | 11 | 0 | 29 | 7 | 23 | 0 |
| EEMD-fft | 27 | 13 | 0 | 33 | 9 | 30 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalona, O.J.; Lynn, W.D.; Perpiñan, G.; McFrederick, L.; McEneaney, D.J. Data-Driven ECG Denoising Techniques for Characterising Bipolar Lead Sets along the Left Arm in Wearable Long-Term Heart Rhythm Monitoring. Electronics 2017, 6, 84. https://doi.org/10.3390/electronics6040084
Escalona OJ, Lynn WD, Perpiñan G, McFrederick L, McEneaney DJ. Data-Driven ECG Denoising Techniques for Characterising Bipolar Lead Sets along the Left Arm in Wearable Long-Term Heart Rhythm Monitoring. Electronics. 2017; 6(4):84. https://doi.org/10.3390/electronics6040084
Chicago/Turabian StyleEscalona, Omar J., William D. Lynn, Gilberto Perpiñan, Louise McFrederick, and David J. McEneaney. 2017. "Data-Driven ECG Denoising Techniques for Characterising Bipolar Lead Sets along the Left Arm in Wearable Long-Term Heart Rhythm Monitoring" Electronics 6, no. 4: 84. https://doi.org/10.3390/electronics6040084





