A Telespirometer for the Developing World
Abstract
:1. Introduction
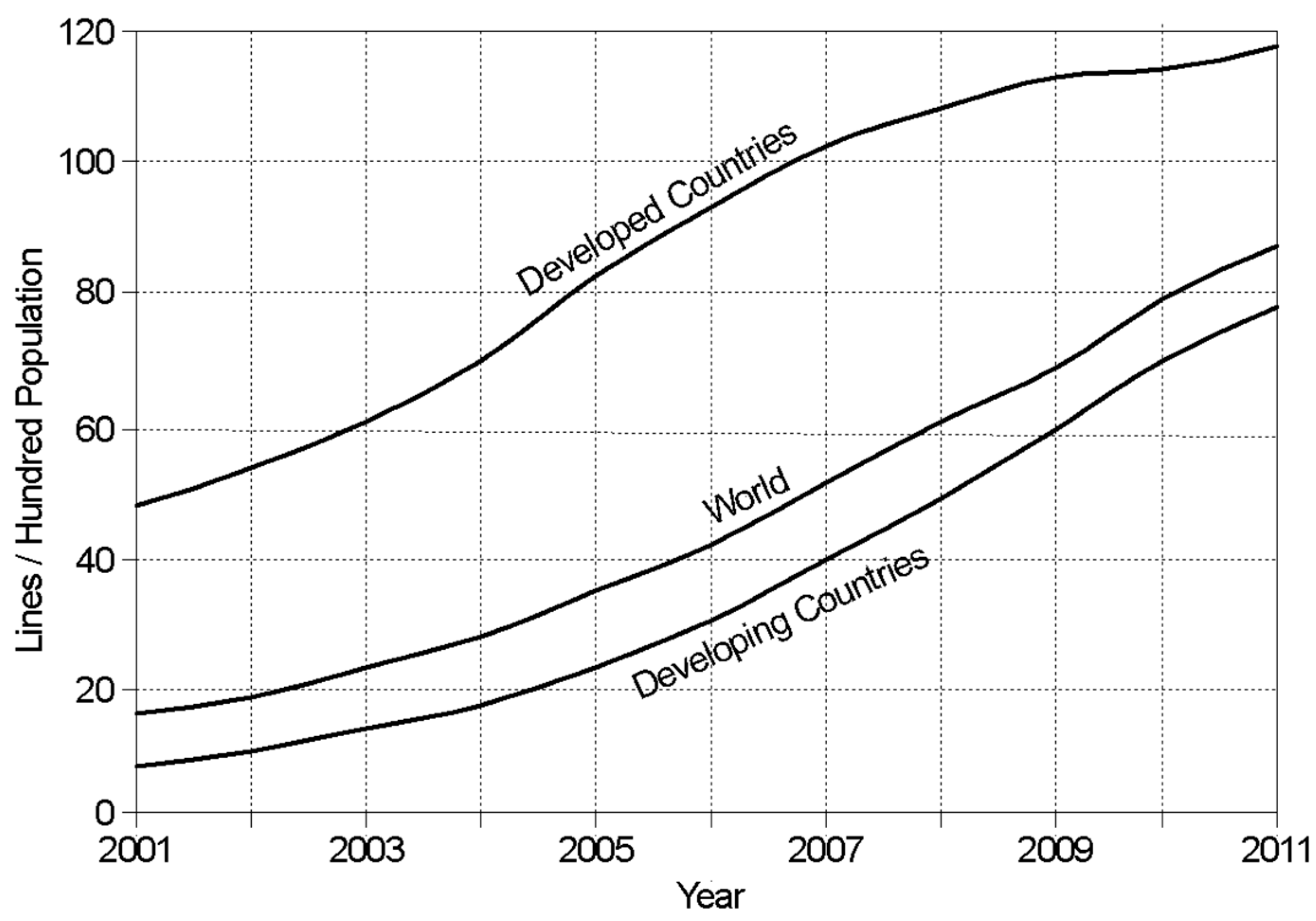
1.1. Using the Cellphone Network
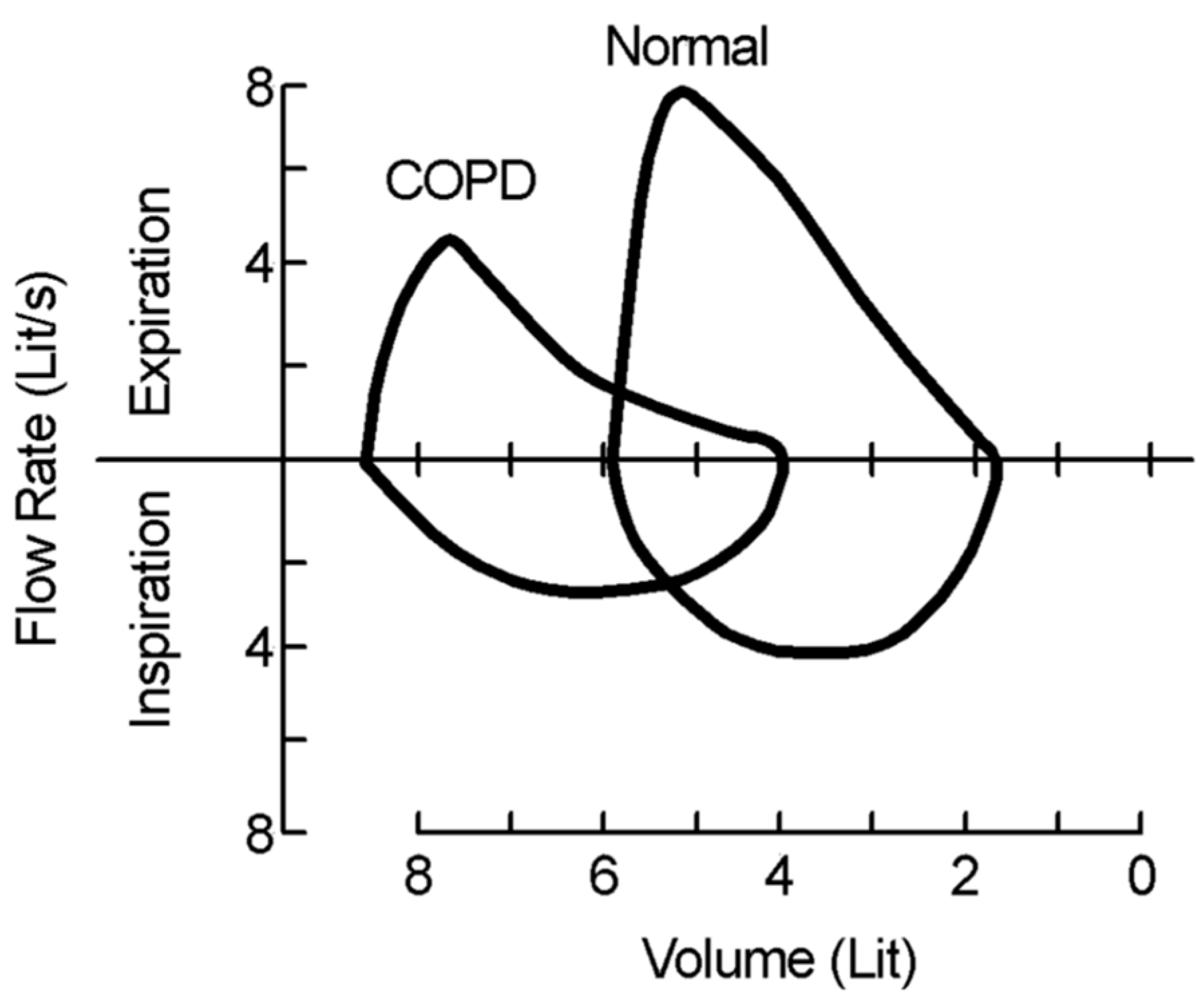
1.2. Recognizing Chronic Obstructive Pulmonary Disease
- Use a COPD questionnaire to identify those at risk.
- Use an electronic device to detect those likely to have a low peak flow.
- Only perform spirometry on those at higher risk of COPD (smoking, biomass fuel exposure).
- Take the time to perform good spirometry.
1.3. Aim and Structure
2. Materials and Methods
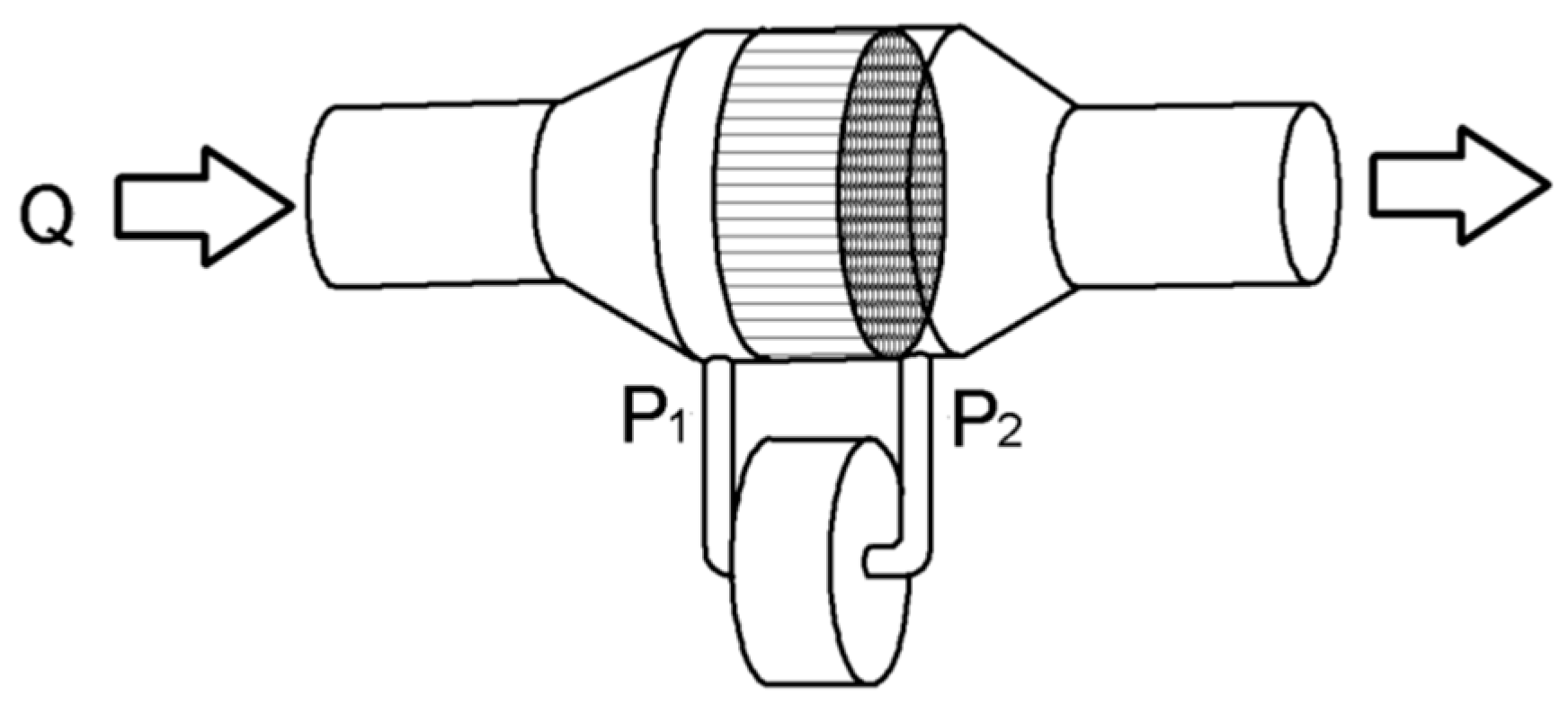
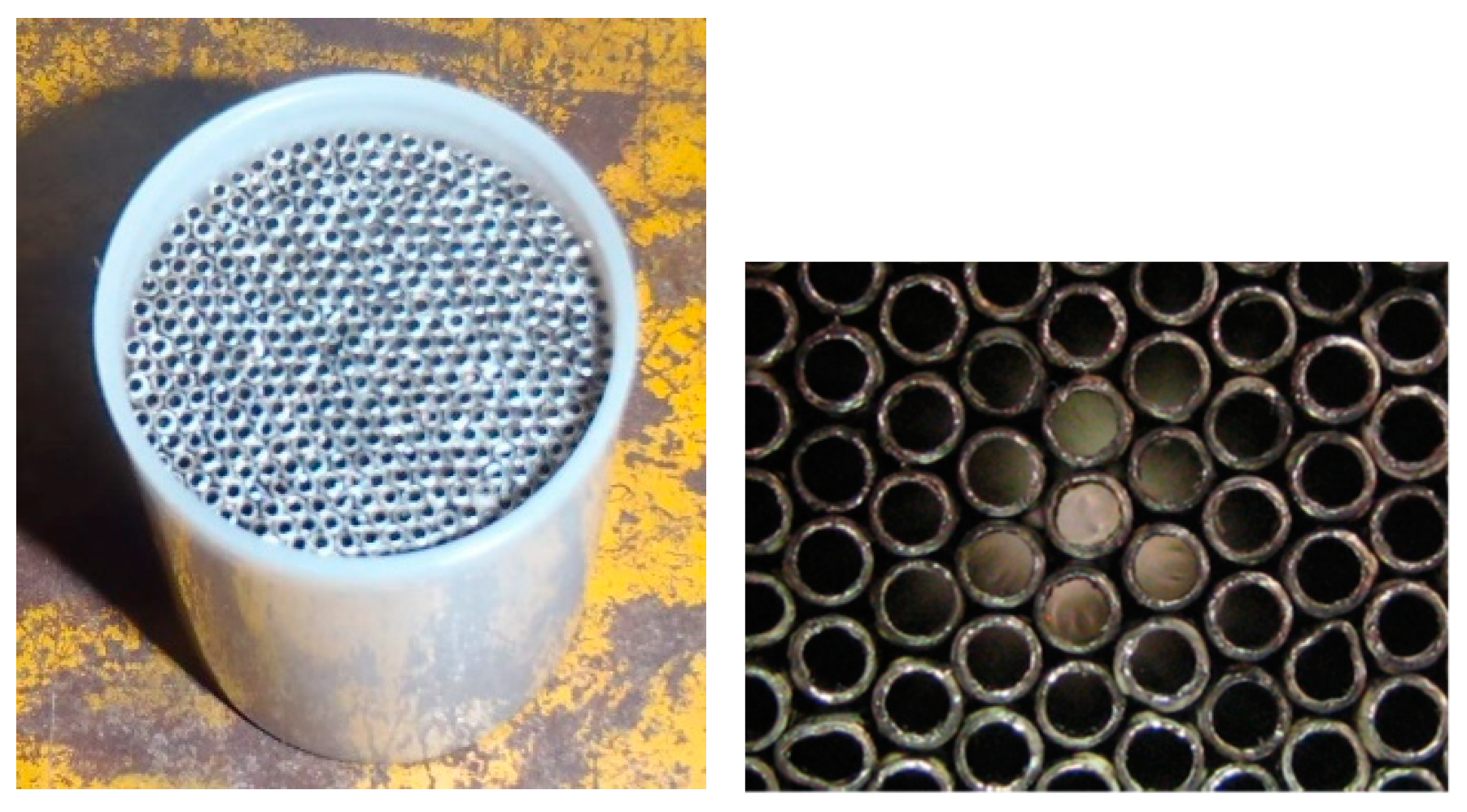
2.1. Fleisch Pneumotachograph
2.2. Theoretical Analysis of the Pneumotachograph
2.3. Performance Assessment
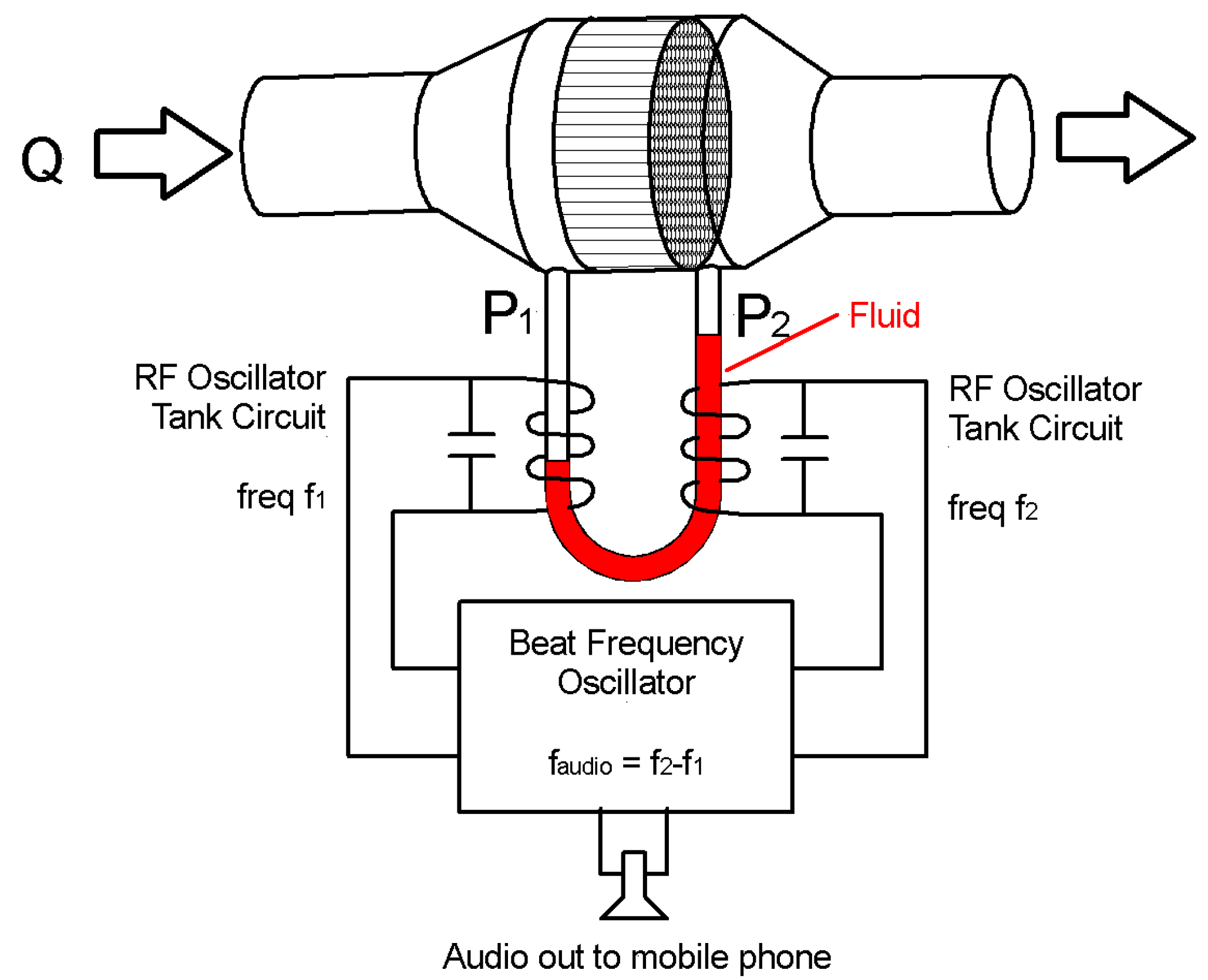
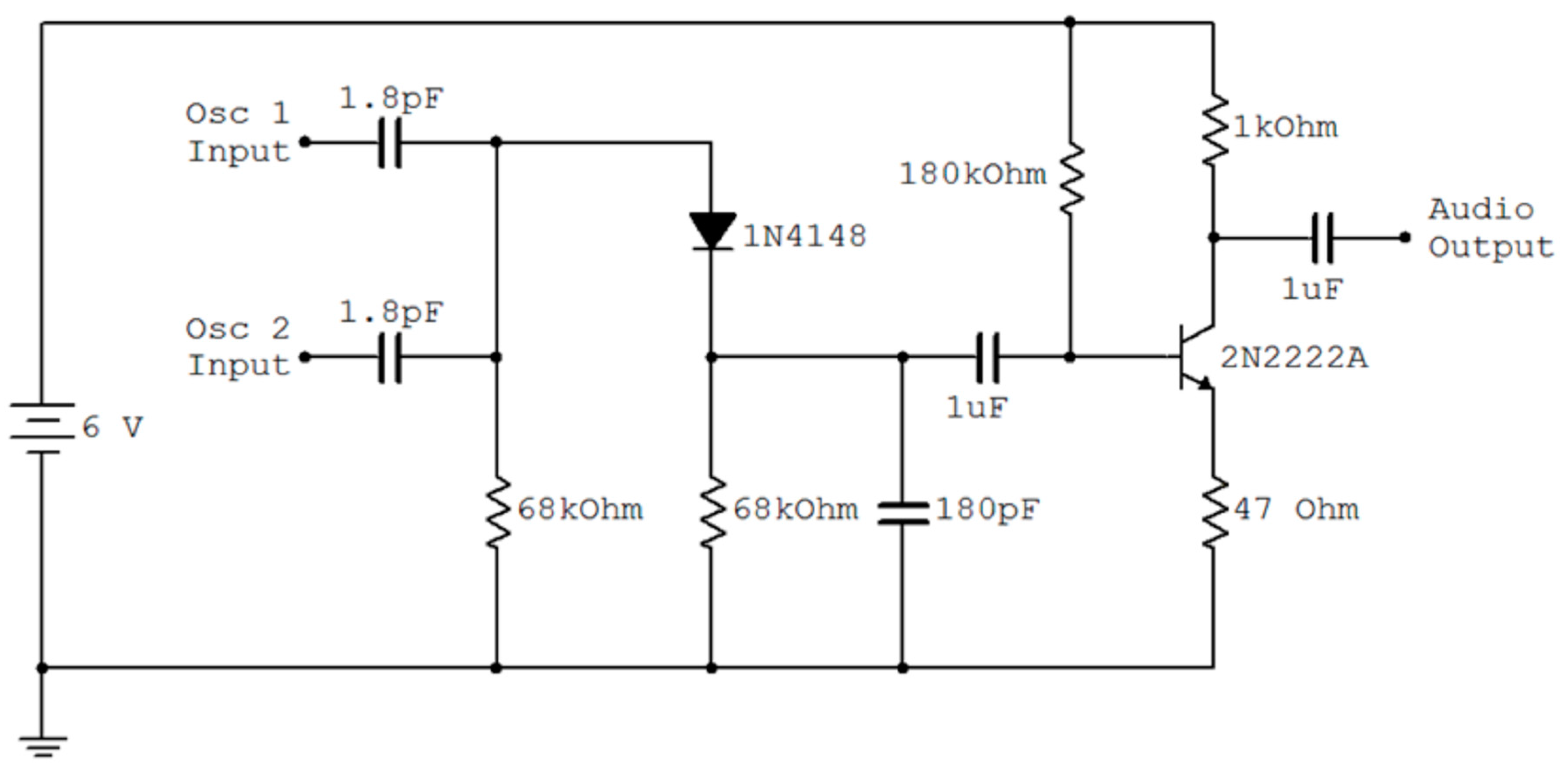
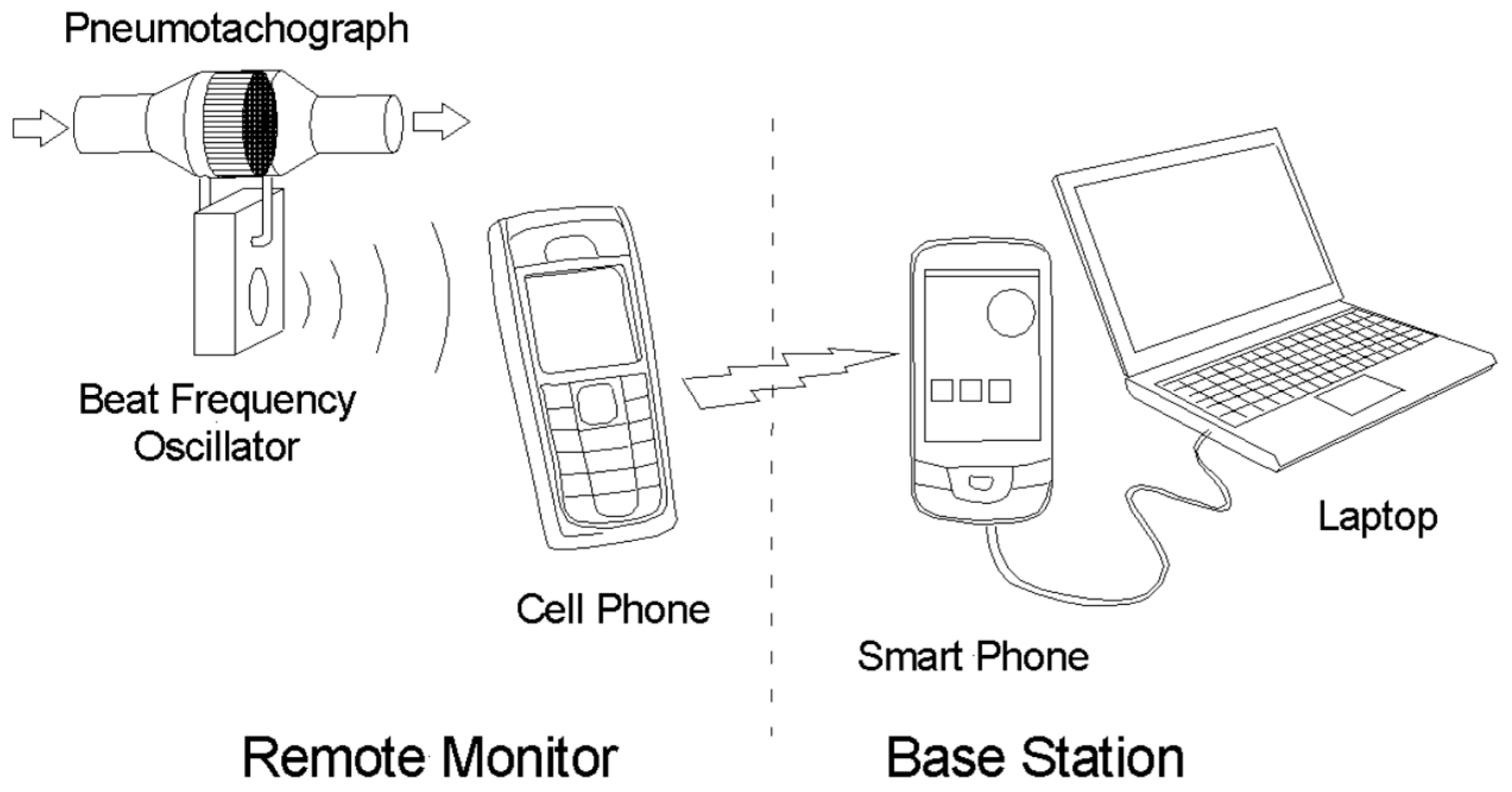
2.4. Interfacing to a Cell Phone
3. Results
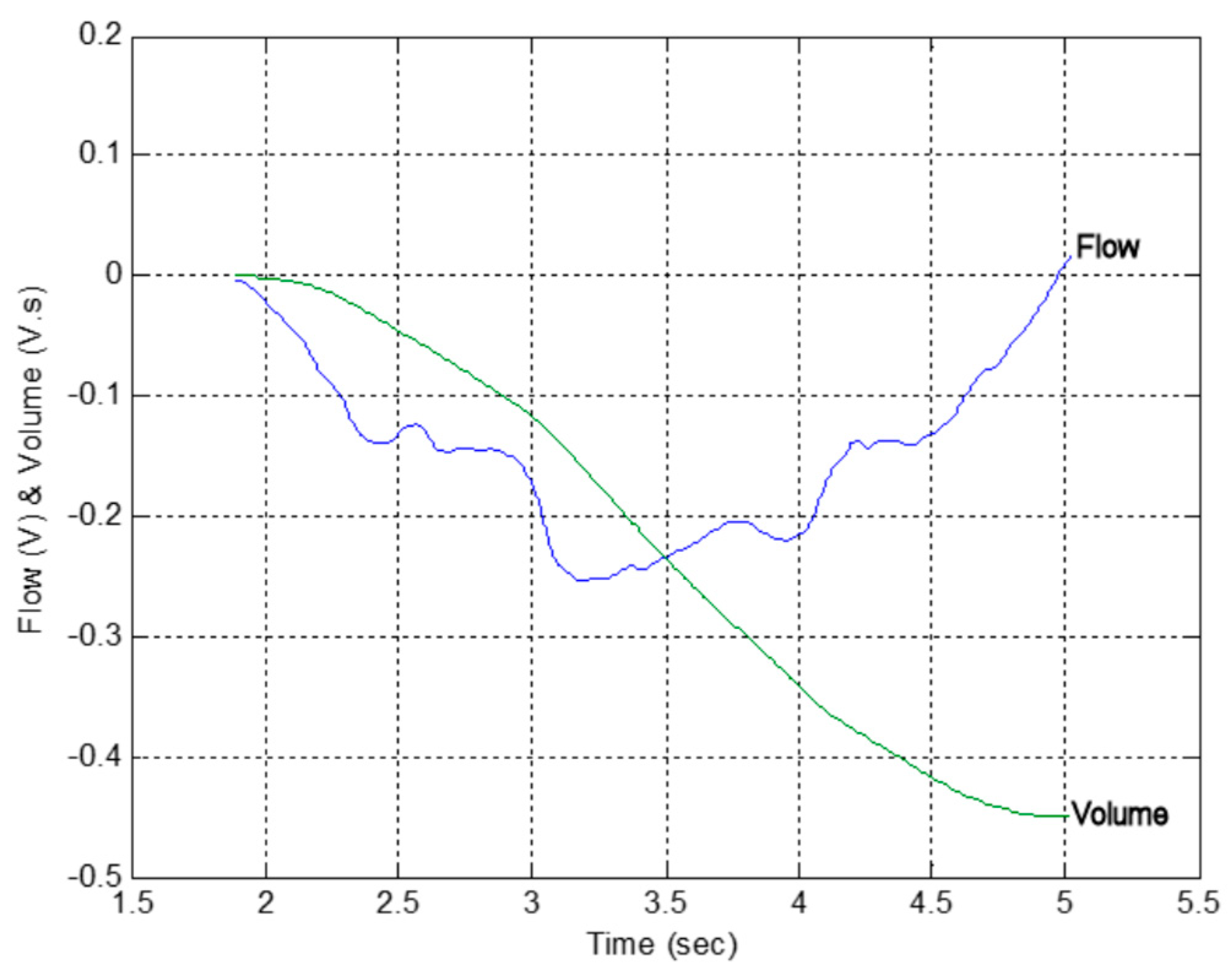
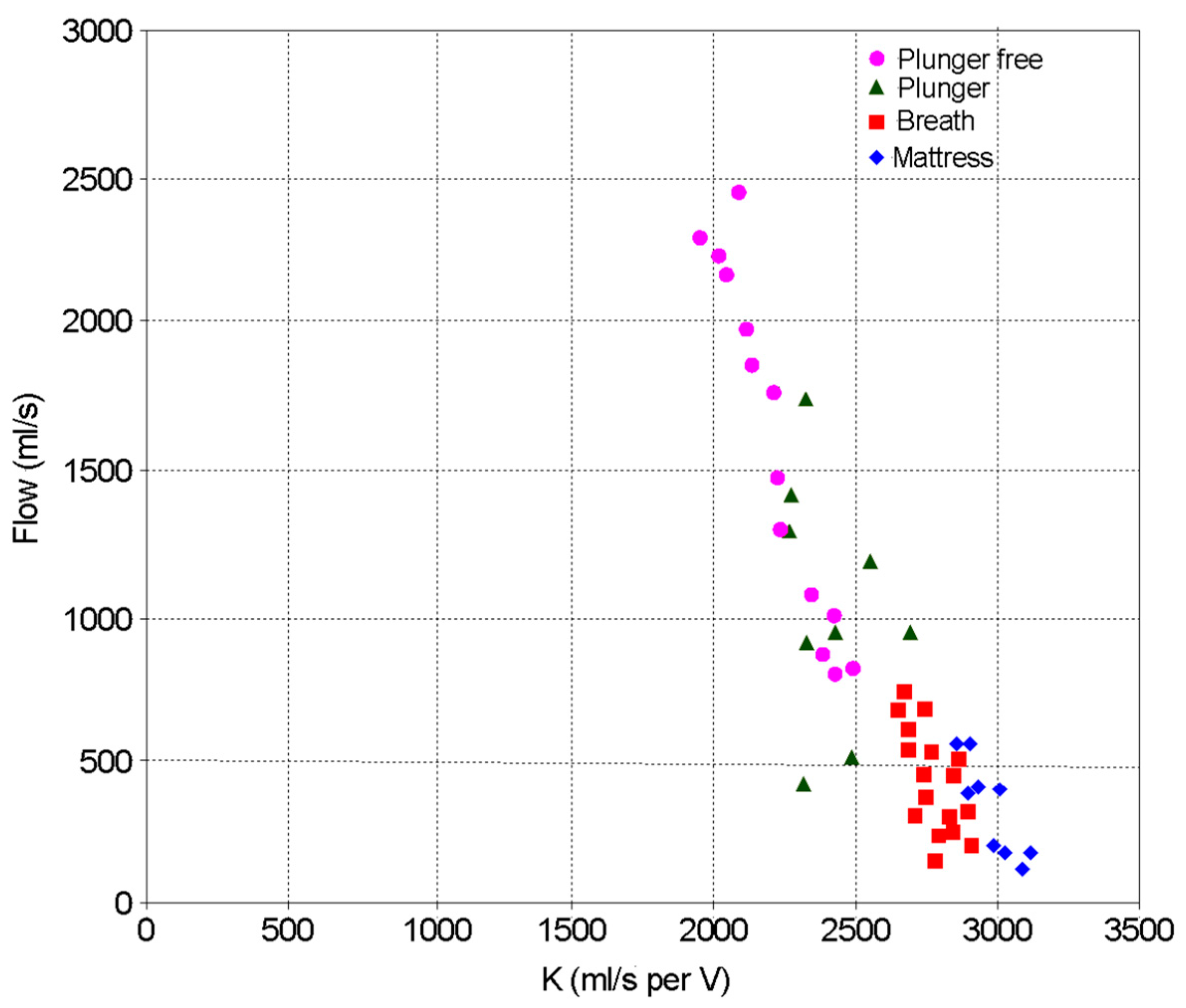
3.1. Calibration of the Fleisch Tube
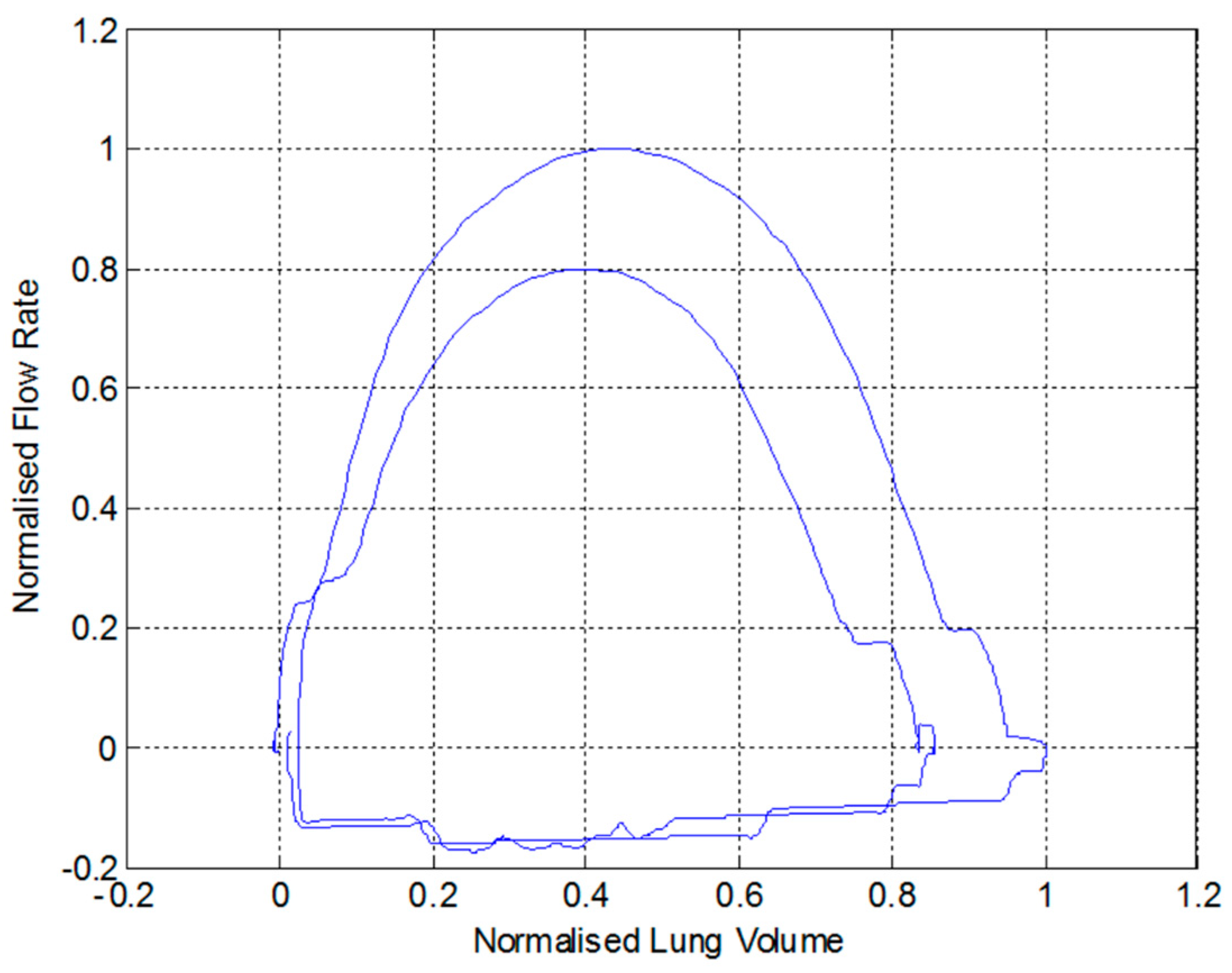
3.2. Comparison with a Lilly Based Spirometer
3.3. Identifying COPD
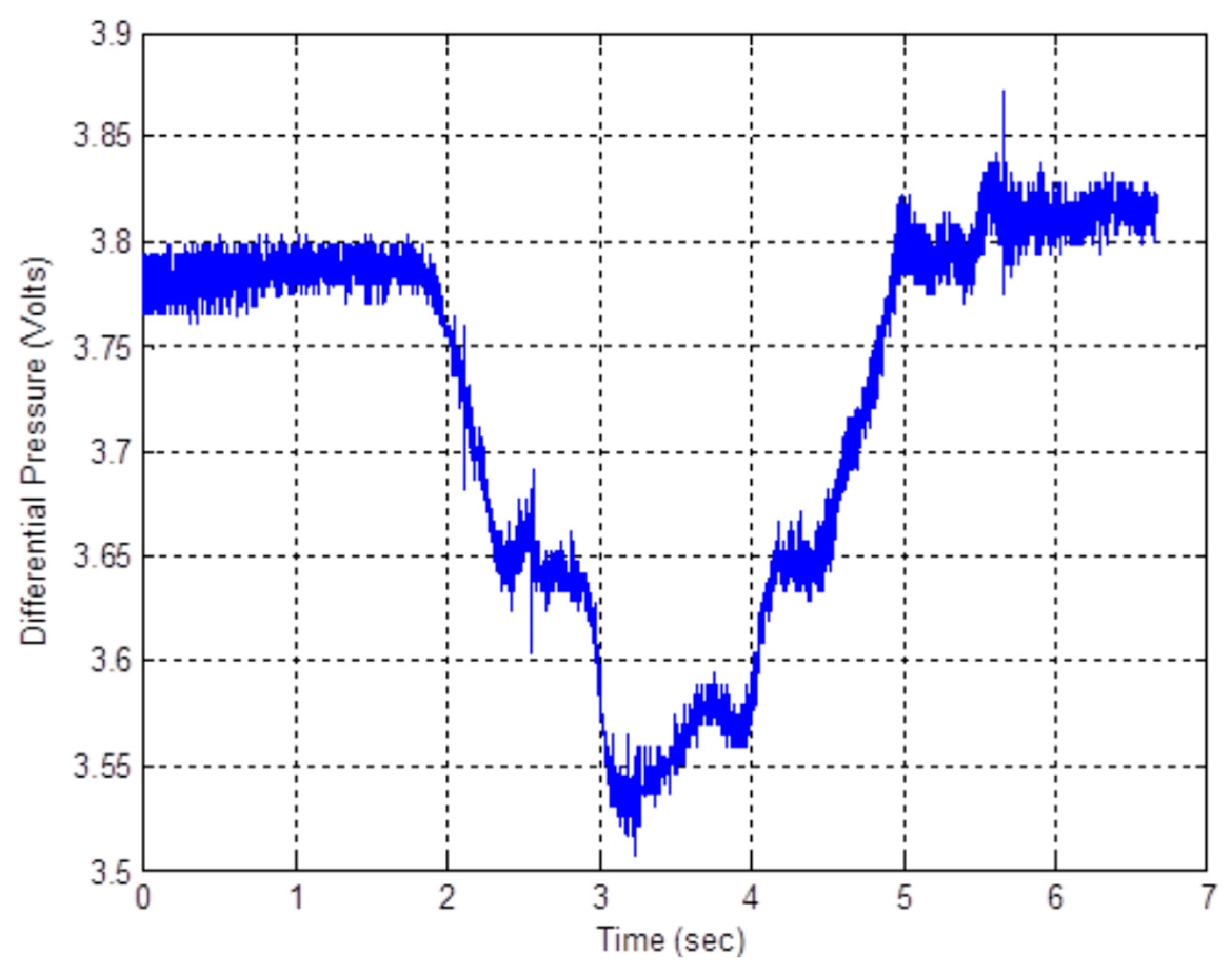
3.4. Measured Performance of the Manometer Based Pressure Transducer
3.5. The Base Station
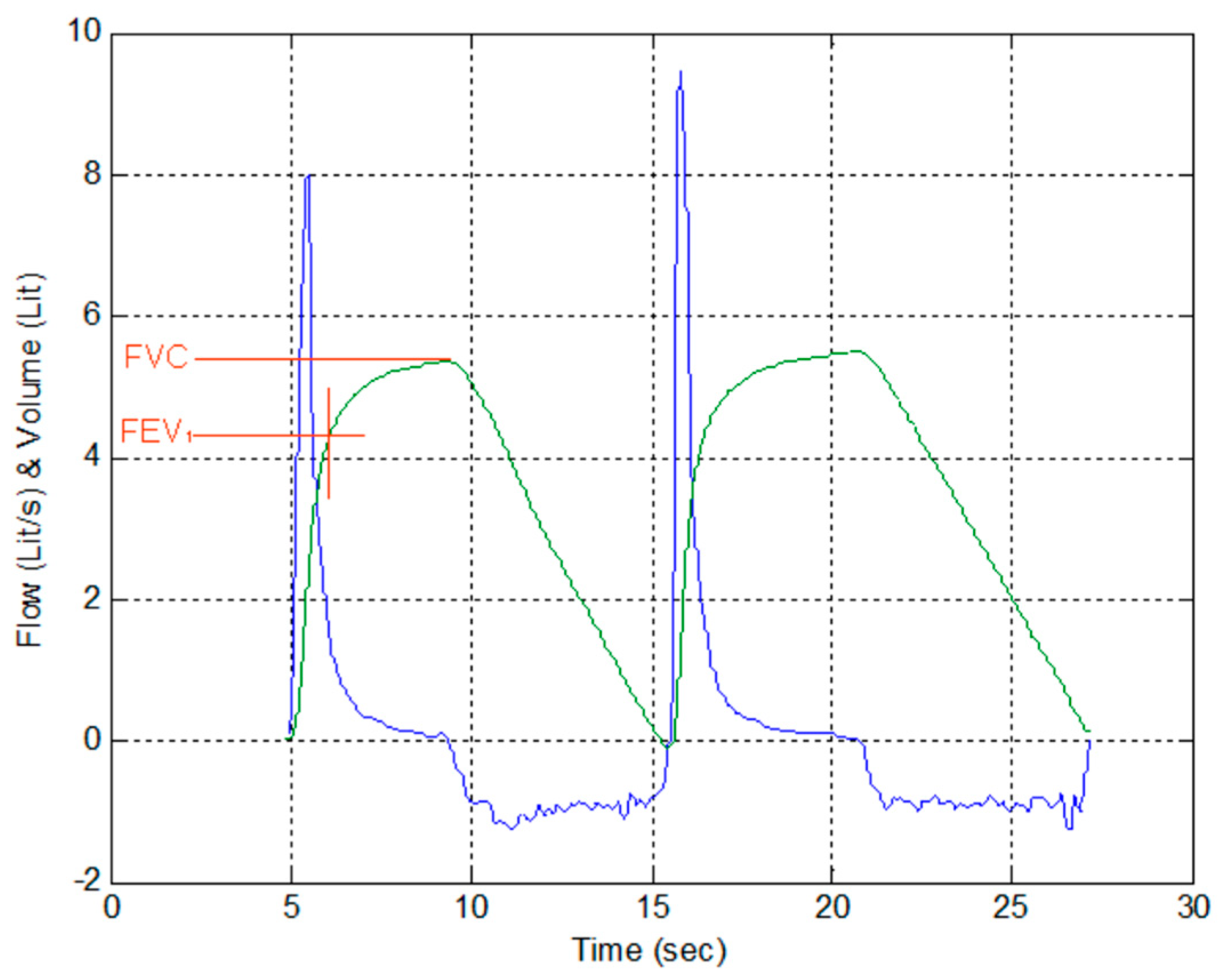
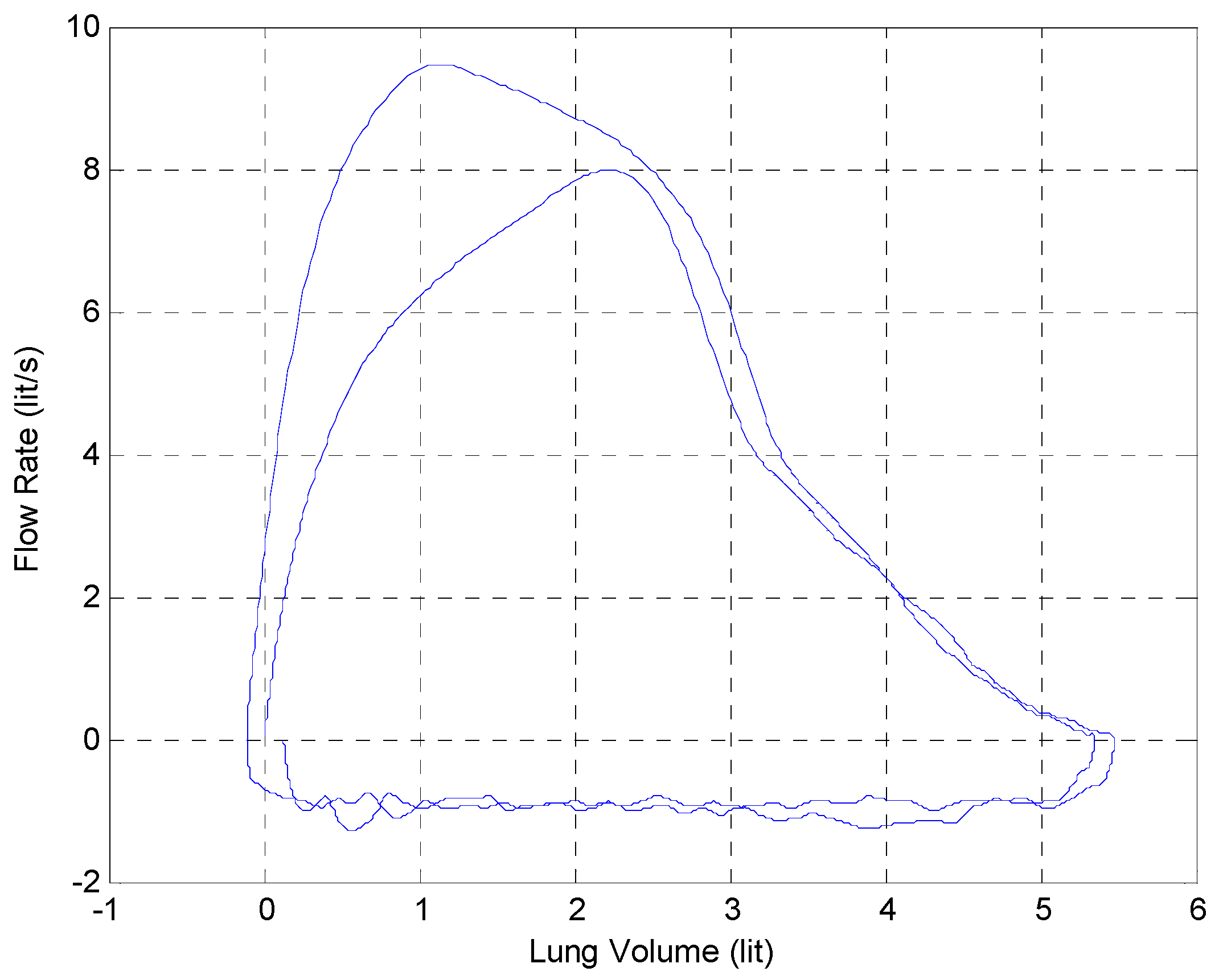
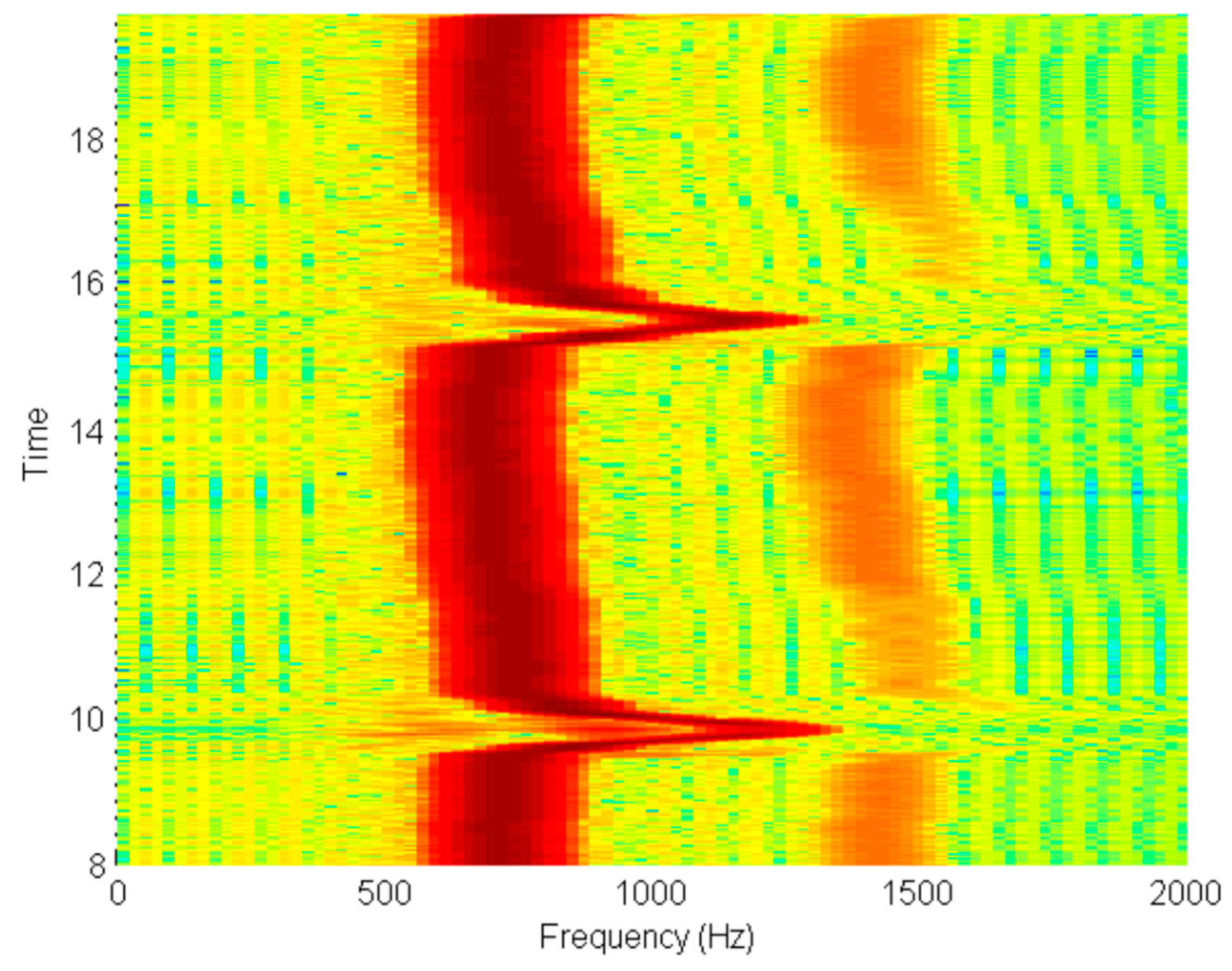
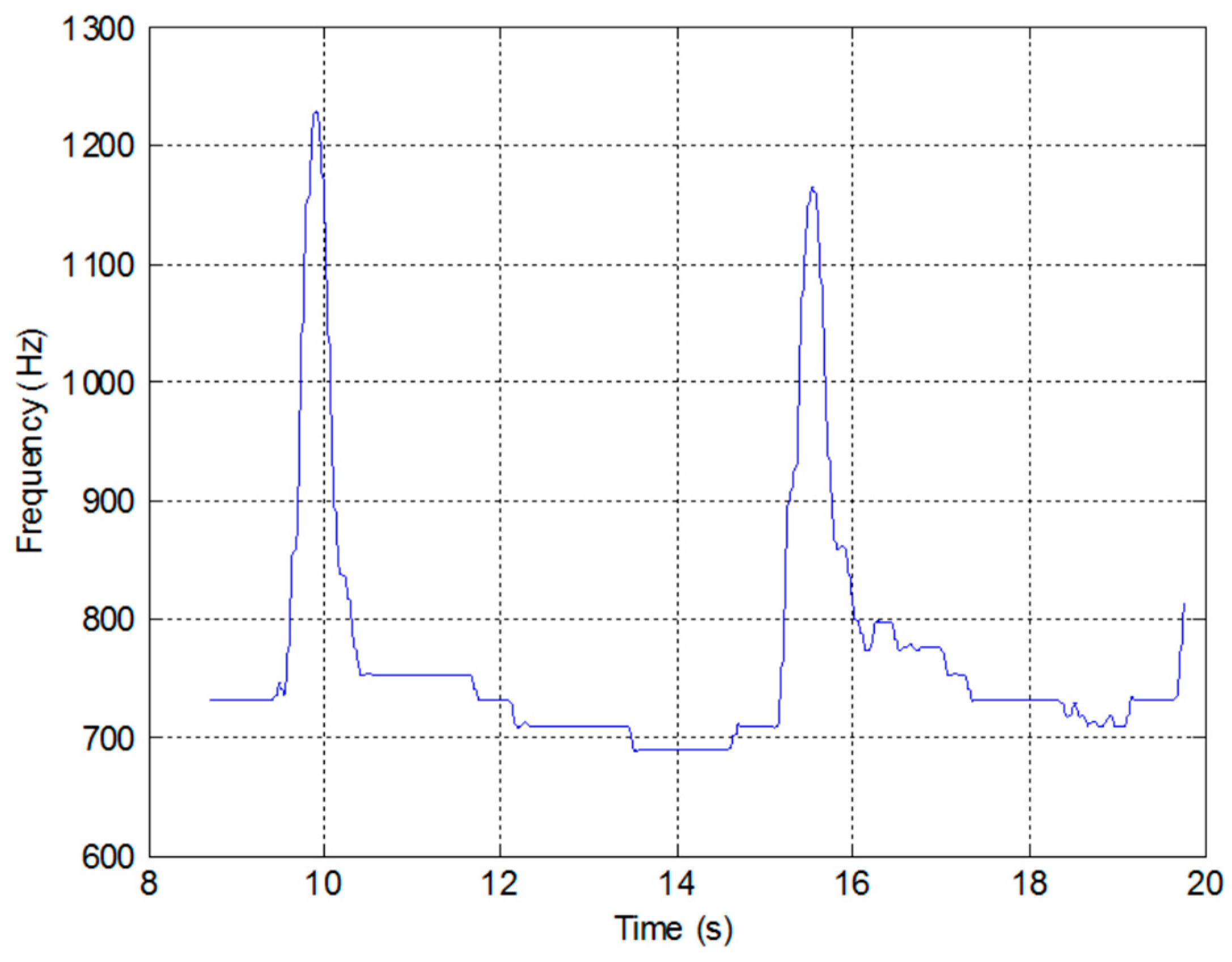
3.6. Measured System Performance
- The liquid in the manometer is topped up (or ideally replaced) so that the levels are half way up the coils.
- A phone conversation is initiated between the remote monitor and the base station, where it is recorded.
- The patient is identified by name and village/district.
- The unit is turned on and calibrated by tipping it forward until the generated tone reaches a maximum, and then backward until it reaches a minimum.
- The unit is then clamped in a level position to ensure that changes in water level are only caused by airflow.
- The patient takes a deep breath and then expels it through the pneumotachograph mouthpiece.
- This is repeated a number of times until the monitor is satisfied that good data have been produced.
- The phone conversation is ended.
- The mouthpiece is sterilized to be ready for the next patient.
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wootton, R.; Bonnardot, L. In what circumstances is telemedicine appropriate in the developing world? J. R. Soc. Med. (JSRM) 2010, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Bristol, N. Are Cell Phones Leading the mHealth Revolution? Global Health. 2009. Available online: http://globalmd.org/c/journal_articles/view_article_content?articleId=225&version=1.0 (accessed on 5 February 2020).
- Wootton, R. (Ed.) Telehealth in the Developing World; Royal Society of Medicine Press Ltd. & International Development Research Centre: London, UK, 2009. [Google Scholar]
- Hersh, W.; Margolis, A.; Quiros, F.; Otero, P. Building a Health Informatics Workforce in Developing Countrires. Health Aff. 2010, 29, 274–277. [Google Scholar] [CrossRef] [PubMed]
- ITU. 2011. Available online: https://www.itu.int/en/ITU-D/Statistics/Pages/stat/default.aspx (accessed on 5 February 2020).
- Hernandez, C.; Mallow, J.; Narsavage, G. Delivering telemedicineinterventions in chronicrespiratory disease. Breathe 2014, 10, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Lane, N.D.; Miluzzo, E.; Hong, L.; Peebles, D.; Choudhury, T.; Campbell, A.T. A survey of mobile phone sensing. IEEE Commun. Mag. 2010, 48, 140–150. [Google Scholar] [CrossRef]
- Crilly, P.; Muthukkumarasamy, V. Using smart phones and body sensors to deliver pervasive mobile personal healthcare. In Proceedings of the 2010 Sixth International Conference on Intelligent Sensors, Sensor Networks and Information Processing, Brisbane, Australia, 7–10 December 2010; pp. 291–296. [Google Scholar]
- Pantelopoulos, A.; Bourbakis, N.G. A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2010, 40, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Hollin, I.; Kachnowski, S. A review of the use of mobile phone text messaging in clinical and healthy behaviour interventions. J. Telemed. Telecare 2010, 17, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Burgos, F.; Disdier, C.; deSantamaria, E.; Galdiz, B.; Roger, N.; Rivera, M.; Hervàs, R.; Durán-Tauleria, E.; Garcia-Aymerich, J.; Roca, J.; et al. Telemedicine enhances quality of forced spirometry in primary care. Eur. Respir. J. 2012, 39, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Brennan, T. Integrating Low-Cost Sensors with Mobile Phones for Remote Monitoring of Long-Term Condition in Resource Constrained Environments. In Proceedings of the International Biomedical and Bioelectronics Workshop, Sydney, Australia, 22–24 August 2011. [Google Scholar]
- Benlamri, R.; Docksteader, L. MORF: A Mobile Health-Monitoring Platform. IT Prof. 2010, 12, 18–25. [Google Scholar] [CrossRef]
- Tzu-Hao, Y.; Hsing-Fang, T.; Sih-Yin, L.; Ching-Shuen, C.; Shih-Tsang, T.; Jiun-Hung, L. Portable oximeter for acute mountain sickness. In Proceedings of the 2010 International Conference on Electronics and Information Engineering (ICEIE), Kyoto, Japan, 1–3 August 2010. [Google Scholar]
- Reeder, B.; David, A. Health at hand: A systematic review of smart watch uses for health and wellness. J. Biomed. Inform. 2016, 63, 269–276. [Google Scholar] [CrossRef]
- Ong, J. Medical Detection and Diagnosis with Smartwatches. Smartwatch News. 2019. Available online: https://www.smartwatches4u.com/medical-detection-and-diagnosis/ (accessed on 20 December 2019).
- Nosta, J. A Smart Device, Ultrasound, and Telemedicine Combine to Drive a New Level of Collaboration and Care. Forbes. 26 March 2018. Available online: https://www.forbes.com/sites/johnnosta/2018/03/26/a-smart-device-ultrasound-and-telemedicine-combine-to-drive-a-new-level-of-collaboration-and-care/#7d2772f83a2c (accessed on 20 December 2019).
- Larson, E.; Goel, M.; Redfield, M.; Boriello, G.; Rosenfeld, M.; Patel, S. Tracking Lung Function on any Phone. In Proceedings of the DEV’13, Bangalore, India, 11–12 January 2013. [Google Scholar]
- Zhou, P.; Yang, L.; Huang, Y.-X. A Smart Phone Based Handheld Wireless Spirometer with Functions and Precision Comparable to Laboratory Spirometers. Sensors 2019, 19, 2487. [Google Scholar] [CrossRef] [Green Version]
- Wootton, R. Telemedicine support for the developing world. J. Telemed. Telecare 2008, 2008, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Shak, H.; Nordyke, B. 2004 COPD: Consequences of an Underrecognised Disease. Managed Healthcare Executive. Available online: http://managedhealthcareexecutive.modernmedicine.com (accessed on 20 December 2019).
- vanGemert, F.; vanderMolen, T.; Jones, R.; Chavannes, N. The impact of asthma and COPD in sub-Saharan Africa. Prim. Care Respir. J. 2011, 20, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Bruderman, I.; Abboud, S. Telespirometry: Novel System for Home Monitoring of Asthmatic Patients. Telemed. J. 1997, 3, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Jannett, T.C.; Prashanth, S.; Mishra, S.; Ved, V.; Mangalvedhekar, A.; Deshpande, J. An intelligent telemedicine system for remote spirometric monitoring. In Proceedings of the Thirty-Fourth Southeastern Symposium on System Theory (Cat. No.02EX540), Huntsville, AL, USA, 19 March 2002; pp. 53–56. [Google Scholar]
- Polisena, J.; Tran, K.; Cimon, K.; Hutton, B.; McGill, S.; Palmer, K.; Scott, R.E. Home telehealth for chronic obstructive pulmonary disease: A systematic review and meta-analysis. J. Telemed. Telecare 2010, 16, 120–127. [Google Scholar] [CrossRef]
- Arne, M.; Lisspers, K.; Stallberg, B.; Boman, G.; Hedenstrom, H.; Janson, C.; Emtner, M. How often is diagnosis of COPD confirmed with spirometry? Respir. Med. 2010, 104, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Minas, M.; Hatzoglou, C.; Karetsi, E.; Papaooannou, A.; Tanou, K.; Tsaroucha, R.; Gogou, E.; Gourgoulianis, K.I.; Kostikas, K. COPD prevalence and the differences between newly and previously diagnosed COPD patients in a spirometry program. Prim. Care Respir. J. 2010, 19, 363–370. [Google Scholar] [CrossRef]
- Barnes, T.; Fromer, L. Spirometry use: Detection of chronic obstructive pulmonary disease in the primary care setting. Clin. Interv. Aging 2011, 6, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, R.; Moore, V.; Purkait, S.; Goon, P.; Warburton, C.; Chakrabarti, B.; Calverley, P.M.A. Feasibility of performing valid spirometry in rural India: Preliminary results from a population study assessing the prevalence of COPD. Thorax 2010, 65. [Google Scholar] [CrossRef] [Green Version]
- Keller, J. Viscous flow through a grating or lattice of cylinders. J. Fluid Mech. 1963, 18, 94–96. [Google Scholar] [CrossRef]
- Malmberg, L.; Hedman, J.; Sovijarvi, A. Accuracy and repeatability of a pocket turbine spirometer: Comparison with a rolling seal flow-volume spirometer. Clin. Physiol. 1993, 13, 89–98. [Google Scholar] [CrossRef]
- Hughes, T. Hot Thermistor Spirometry for the Artificial Ventilation of Students. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1981. [Google Scholar]
- Araujo, G.A.L.; Freire, R.C.S.; Silva, J.F.; Oliveira, A.; Jaguaribe, E.F. Breathing flow measurement with constant temperature hot-wire anemometer for forced oscillations technique. In Proceedings of the 21st IEEE Instrumentation and Measurement Technology Conference (IEEE Cat. No.04CH37510), Como, Italy, 18–20 May 2004; pp. 730–733. [Google Scholar]
- Sutera, S.; Skalak, R. The History of Poiseuille’s Law. Annu. Rev. Fluid Mech. 1993, 25, 1–19. [Google Scholar] [CrossRef]
- Reynolds, O. An experimental investigation of the circumstances which determine whether the motion of water shall be direct or sinuous, and the law of resistance in parallel channels. Philos. Trans. R. Soc. 1883, 174, 935–982. [Google Scholar]
- Pfitzner, J. Poiseuille and his law. Anaesthesia 1976, 31, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Omega. Differential Pressure Transducers. 2011. Available online: https://www.omega.nl/pressure/pdf/PX160.pdf (accessed on 20 December 2019).
- Tang, Y.; Turner, M.; Yem, J.; Baker, B. Calibration of pneumotachographs using a calibrated syringe. J. Appl. Physiol. 2003, 95, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Johns, D.; Pierce, R. McGraw-Hill’s Pocket Guide to Spirometry; McGraw-Hill: Tokyo, Japan, 2003. [Google Scholar]
- Miller, M. Chronic obstructive pulmonary disease and ‘150 years of blowing’. Hosp. Med. 1998, 59, 719–722. [Google Scholar]
- Miller, M.; Sigsgaard, T. Prevention of thermal and condensation errors in pheumatachographic recordings of the maximal forced expiratory manoeuvre. Eur. Respir. J. 1994, 7, 198–201. [Google Scholar] [CrossRef]
- Rohde&Schwarz. Application Note: Acoustic Measurements on GSM Mobile Phones with Audio Analyzer UPL and Digital Radiocommunication Tester CMD. 2010. Available online: http://www2.rohde-schwarz.com/file_1158/1ga39_0e.pdf (accessed on 20 December 2019).
- Schultz, S. Calibration of Permittivity Sensors to Measure Contaminants in Water and Biodiesel Fuel. Ph.D. Thesis, Department of Biological and Agricultural Engineering, Kansas State University, Manhattan, KS, USA, 2009. [Google Scholar]
- Oliver, B.; Cage, J. Electronic Measurements and Instrumentation; McGraw-Hill Kogakusha, Ltd.: Tokyo, Japan, 1971; pp. 340–342. [Google Scholar]
- Woodward, G. (Ed.) The Radio Amateur’s Handbook; American Radio Relay League: Newington, CT, USA, 1982. [Google Scholar]
- Knight, D. The Self-Resonance and Self-Capacitance of Solenoid Coils. 2010. Available online: http://www.g3ynh.info/zdocs/magnetics/appendix/self-res.html (accessed on 20 December 2019).
- Millman, J.; Halkias, C. Integrated Electronics; McGraw-Hill Kogakusha, Ltd.: Tokyo, Japan, 1972; pp. 490–492. [Google Scholar]
- Hartley, R. Oscillation Generator. U.S. Patent 3,147,615, 1 June 1915. [Google Scholar]
- Nathell, L.; Nathell, M.; Malmberg, P.; Larsson, K. COPD diagnosis related to different guidelines and spirometry techniques. Respir. Res. 2007, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hamid, Q.; Shannon, J.; Martin, J. Physiologic Basis of Respiratory Disease; BC Becker Inc.: Hamilton, ON, Canada, 2005. [Google Scholar]
- Bellamy, D.; Booker, R. Chronic Obstructive Pulmonary Disease in Primary Care, 3rd ed.; Class Publishing: London, UK, 2004. [Google Scholar]
- RadonSoft. Spectral Pro Analyzer. Available online: https://play.google.com/store/apps/details?id=radonsoft.net.spectralviewpro&hl=en (accessed on 20 December 2019).






© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brooker, G. A Telespirometer for the Developing World. Electronics 2020, 9, 275. https://doi.org/10.3390/electronics9020275
Brooker G. A Telespirometer for the Developing World. Electronics. 2020; 9(2):275. https://doi.org/10.3390/electronics9020275
Chicago/Turabian StyleBrooker, Graham. 2020. "A Telespirometer for the Developing World" Electronics 9, no. 2: 275. https://doi.org/10.3390/electronics9020275



