Sepsis-Related Lung Injury and the Complication of Extrapulmonary Pneumococcal Pneumonia
Abstract
:1. Introduction
2. Pneumonia and Sepsis (Pathogenesis, Etiology, and Epidemiology)
2.1. Pneumonia
| Class of Pneumonia | Definition/Characteristics | Common Pathogens Associated |
|---|---|---|
| Community-Acquired Pneumonia (CAP) |
|
|
| Hospital-Acquired Pneumonia (HAP) |
| |
| Ventilator-Associated Pneumonia (VAP) |
|
| Name | Characteristics | |
|---|---|---|
| Stage 1 | Congestion | Vascular engorgement, accumulation of alveolar fluid rich in the offending infectious pathogens, heavy and engorged appearance to the lung tissue. |
| Stage 2 | Red-hepatization | Substantive infiltration of blood cells (specifically RBCs and neutrophils) and fibrin into the alveolar fluid, firm and deep red gross appearance of the lung, resembling the liver. |
| Stage 3 | Grey-hepatization | Greyish lobe appearance owing to the presence of fibrino-purulent exudates and broken-down RBCs. |
| Stage 4 | Resolution | Terminal event characterized by the clearing of exudates by resident innate immune mob cells (macrophages) and the potential formation of residual scar tissue (depending on severity). |
2.2. Sepsis
2.3. Epidemiology of Pneumonia and Sepsis
3. Sepsis-Induced Acute Lung Injury
3.1. Acute Lung Injury
3.2. Risk Factors for the Development of ALI
3.3. ALI as a Secondary Complication to Sepsis
3.4. Sepsis-Induced Immunosuppression and the Susceptibility to Secondary Pneumonia
4. Pneumonia and the Risk of Sepsis
4.1. Pneumonia as a Cause of Sepsis
4.2. Pneumococcal Pneumonia
5. The Pneumococcus and Septic Inflammation
5.1. Pneumococcus and the Innate Immune System
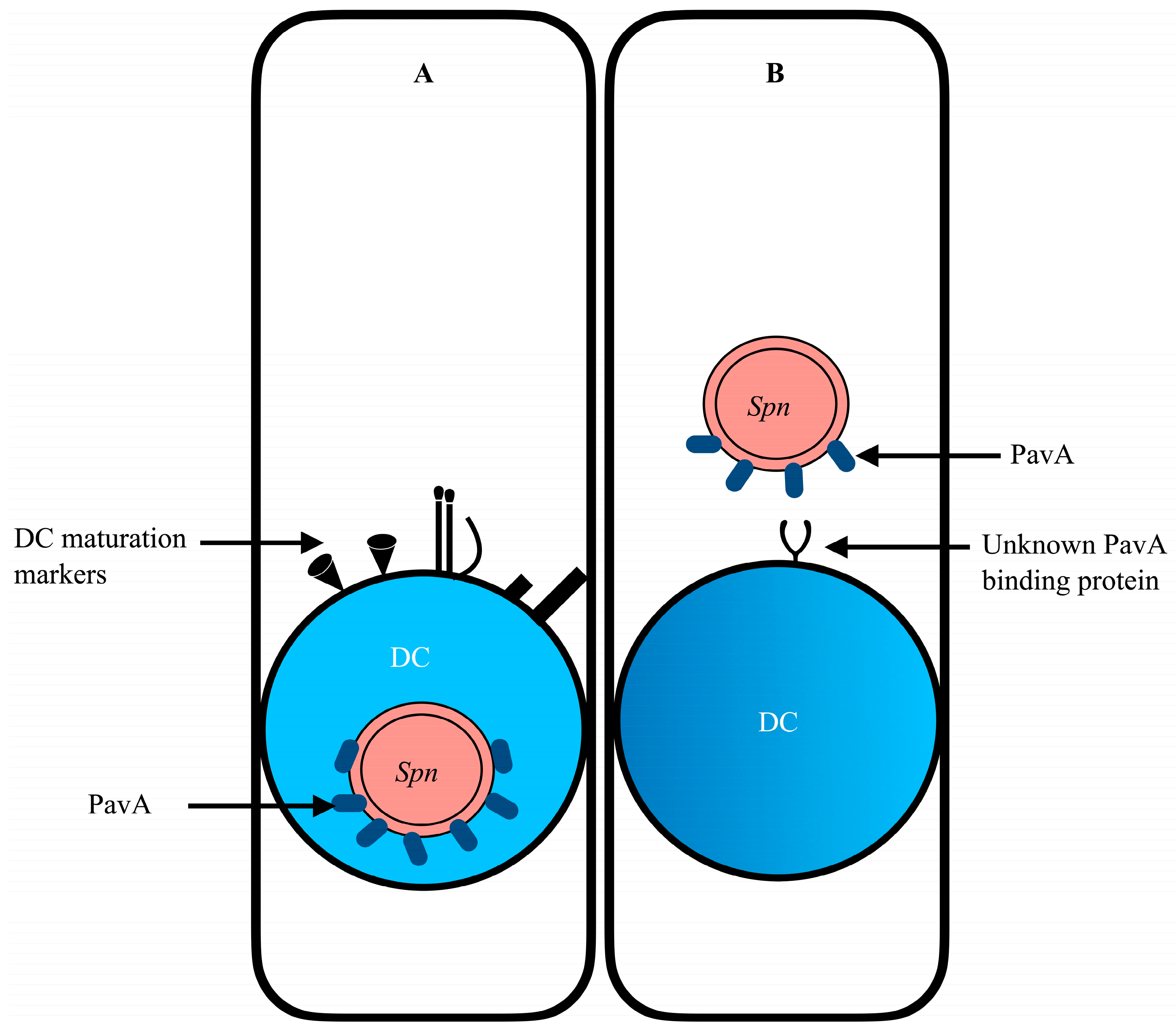
5.2. Cross-Talk between Pneumococcus and Dendritic Cell
5.3. Potential Role of Dendritic Cells in Extrapulmonary Dissemination of Bacteria
5.4. Interleukin 37 (IL-37) and Severe S. pneumoniae Infections
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fleischmann, M.C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Coimbra, J.; Sarda, C.; Rello, J. Burden of Community-Acquired Pneumonia and Unmet Clinical Needs. Adv. Ther. 2020, 37, 1302–1318. [Google Scholar] [CrossRef] [PubMed]
- Bewick, T.; Simmonds, M.; Chikhani, M.; Meyer, J.; Lim, W.S. Pneumonia in the context of severe sepsis: A significant diagnostic problem. Eur. Respir. J. 2008, 32, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Speiser, J.L.; the Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group; Karvellas, C.J.; Shumilak, G.; Sligl, W.I.; Mirzanejad, Y.; Gurka, D.; Kumar, A.; Kumar, A. Predicting in-hospital mortality in pneumonia-associated septic shock patients using a classification and regression tree: A nested cohort study. J. Intensive Care 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Ardanuy, C.; Tubau, F.; Viasus, D.; Dorca, J.; Linares, J.; Gudiol, F.; Carratala, J. Pneumococcal pneumonia presenting with septic shock: Host- and pathogen-related factors and outcomes. Thorax 2010, 65, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R.; Hou, P.C.; Aisiku, I.P.; Frendl, G.; Park, P.K.; Mikkelsen, M.E.; Chang, S.Y.; Gajic, O.; Sevransky, J. Early risk factors and the role of fluid administration in developing acute respiratory distress syndrome in septic patients. Ann. Intensive Care 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E. Host immune response in sepsis due to ventilator-associated pneumonia: How is it different? Crit. Care 2009, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Vashisht, R.; Yilmaz, G.; Bhardwaj, A. Pneumonia Pathology. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mackenzie, G. The definition and classification of pneumonia. Pneumonia 2016, 8, 14. [Google Scholar] [CrossRef]
- Mizgerd, J.P. Respiratory infection and the impact of pulmonary immunity on lung health and disease. Am. J. Respir. Crit. Care Med. 2012, 186, 824–829. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Finelli, L. Community-Acquired Pneumonia Requiring Hospitalization among, U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- Mizgerd, J.P. Pathogenesis of severe pneumonia: Advances and knowledge gaps. Curr. Opin. Pulm. Med. 2017, 23, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Quinton, L.J.; Mizgerd, J.P. Dynamics of lung defense in pneumonia: Resistance, resilience, and remodeling. Annu. Rev. Physiol. 2015, 77, 407–430. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44 (Suppl. S2), S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Brozek, J.L. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. 2016, 63, e61–e111, Erratum in Clin. Infect. 2017, 64, 1298; Erratum in Clin. Infect. 2017, 15, 1435; Erratum in Clin. Infect. 2017, 65, 2161. [Google Scholar] [CrossRef] [PubMed]
- Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N. Engl. J. Med. 2006, 355, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Zinserling, V.A.; Swistunov, V.V.; Botvinkin, A.D.; Stepanenko, L.A.; Makarova, A.E. Lobar (croupous) pneumonia: Old and new data. Infection 2022, 50, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G. Diagnostic tests for agents of community-acquired pneumonia. Clin. Infect. Dis. 2011, 52 (Suppl. S4), S296–S304. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Ewig, S.; Polverino, E.; Marcos, M.A.; Prina, E.; Sellares, J.; Torres, A. Community-acquired pneumonia in outpatients: Etiology and outcomes. Eur. Respir. J. 2012, 40, 931–938. [Google Scholar] [CrossRef]
- Johansson, N.; Kalin, M.; Tiveljung-Lindell, A.; Giske, C.G.; Hedlund, J. Etiology of community-acquired pneumonia: Increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 2010, 50, 202–209. [Google Scholar] [CrossRef]
- Para, R.A.; Fomda, B.A.; Jan, R.A.; Shah, S.; Koul, P.A. Microbial etiology in hospitalized North Indian adults with community-acquired pneumonia. Lung India 2018, 35, 108–115. [Google Scholar] [PubMed]
- Sattar, S.B.A.; Sharma, S. Bacterial Pneumonia. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2021. [Google Scholar]
- Hage, C.A.; Knox, K.S.; Wheat, L.J. Endemic mycoses: Overlooked causes of community-acquired pneumonia. Respir. Med. 2012, 106, 769–776. [Google Scholar] [CrossRef]
- Cilloniz, C.; Martin-Loeches, I.; Garcia-Vidal, C.; San Jose, A.; Torres, A. Microbial Etiology of Pneumonia: Epidemiology, Diagnosis, and Resistance Patterns. Int. J. Mol. Sci. 2016, 17, 2120. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Aznar, R.; Gatell, J.M.; Jiménez, P.; González, J.; Ferrer, A.; Celis, R.; Rodriguez-Roisin, R. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am. Rev. Respir. Dis. 1990, 142, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Torres, A. Epidemiology of ICU-acquired pneumonia. Curr. Opin. Crit. Care 2018, 24, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Difrancesco, L.F.; Liapikou, A.; Rinaudo, M.; Carbonara, M.; Bassi, G.L.; Gabarrus, A.; Torres, A. Polymicrobial intensive care unit-acquired pneumonia: Prevalence, microbiology, and outcome. Crit. Care 2015, 19, 450. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Figliolini, C.; Trouillet, J.-L.; Kassis, N.; Wolff, M.; Gibert, C.; Chastre, J. Incidence and outcome of polymicrobial ventilator-associated pneumonia. Chest 2002, 121, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef]
- Sartelli, M.; Catena, F.; Di Saverio, S.; Ansaloni, L.; Malangoni, M.; Moore, E.E.; Moore, F.A.; Ivatury, R.; Coimbra, R.; Leppaniemi, A.; et al. Current concept of abdominal sepsis: WSES position paper. World J. Emerg. Surg. 2014, 9, 22. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef]
- Fisher, C.J., Jr.; Zheng, Y. Potential strategies for inflammatory mediator manipulation: Retrospect and prospect. World J. Surg. 1996, 20, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.J., Jr.; Dhainaut, J.-F.A.; Opal, S.M.; Pribble, J.P.; Balk, R.A.; Slotman, G.J.; Iberti, T.J.; Rackow, E.C.; Shapiro, M.J.; Greenman, R.L.; et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 1994, 271, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.L.; Holden, D.C.; Mira, J.C.; Stortz, J.A.; Loftus, T.J.; Mohr, A.M.; Moldawer, L.L.; Moore, F.A.; Larson, S.D.; Efron, P.A. Microbial recognition and danger signals in sepsis and trauma. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863 Pt B, 2564–2573. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Carlet, J.; Cohen, J.; Calandra, T.; Opal, S.M.; Masur, H. Sepsis: Time to reconsider the concept. Crit. Care Med. 2008, 36, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Schefold, J.C.; Hasper, D.; Reinke, P.; Monneret, G.; Volk, H.D. Consider delayed immunosuppression into the concept of sepsis. Crit. Care Med. 2008, 36, 3118. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C. The sepsis syndrome. Definition and general approach to management. Clin. Chest Med. 1996, 17, 175–181. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma. Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Otto, G.P.; Sossdorf, M.; Claus, R.A.; Rödel, J.; Menge, K.; Reinhart, K.; Bauer, M.; Riedemann, N.C. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit. Care 2011, 15, R183. [Google Scholar] [CrossRef]
- Nierhaus, A.; Montag, B.; Timmler, N.; Frings, D.P.; Gutensohn, K.; Jung, R.; Schneider, C.G.; Pothmann, W.; Brassel, A.K.; Esch, J.S.A. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med. 2003, 29, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, G. Precision immunotherapy treatment for sepsis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3142–3149. [Google Scholar] [CrossRef] [PubMed]
- van Ton Peters, A.M.; Kox, M.; Abdo, W.F.; Pickkers, P. Precision Immunotherapy for Sepsis. Front. Immunol. 2018, 9, 1926. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Prout, A.J.; Talisa, V.B.; Carcillo, J.A.; Decker, B.K.; Yende, S. Bacterial and Fungal Etiology of Sepsis in Children in the United States: Reconsidering Empiric Therapy. Crit. Care Med. 2020, 48, e192–e199. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.S.; Carcillo, J.A.; Linde-Zwirble, W.T.; Clermont, G.; Lidicker, J.; Angus, D.C. The epidemiology of severe sepsis in children in the United States. Am. J. Respir. Crit. Care Med. 2003, 167, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Graffunder, E.M.; Venezia, R.A. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J. Antimicrob. Chemother. 2002, 49, 999–1005. [Google Scholar] [CrossRef]
- Hardy, K.J.; Hawkey, P.M.; Gao, F.; Oppenheim, B.A. Methicillin resistant Staphylococcus aureus in the critically ill. Br. J. Anaesth. 2004, 92, 121–130. [Google Scholar] [CrossRef]
- Palavutitotai, N.; Jitmuang, A.; Tongsai, S.; Kiratisin, P.; Angkasekwinai, N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PLoS ONE 2018, 13, e0193431. [Google Scholar] [CrossRef]
- Chavez-Bueno, S.; McCulloh, R.J. Current trends in epidemiology and antimicrobial resistance in neonatal sepsis. In Annual Update in Intensive Care and Emergency Medicine; Springer: Cham, Switzerland, 2018; pp. 39–51. [Google Scholar]
- Dolin, H.H.; Papadimos, T.J.; Chen, X.; Pan, Z.K. Characterization of Pathogenic Sepsis Etiologies and Patient Profiles: A Novel Approach to Triage and Treatment. Microbiol. Insights 2019, 12, 1178636118825081. [Google Scholar] [CrossRef]
- Ljungström, L.R.; Jacobsson, G.; Claesson, B.E.B.; Andersson, R.; Enroth, H. Respiratory viral infections are underdiagnosed in patients with suspected sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Palmeri, A.; Amato, T.; Immordino, R.; Distefano, S.; Giammanco, A. Detection of bacterial and yeast species with the Bactec 9120 automated system with routine use of aerobic, anaerobic, and fungal media. J. Clin. Microbiol. 2008, 46, 4029–4033. [Google Scholar] [CrossRef] [PubMed]
- Opota, O.; Croxatto, A.; Prod’hom, G.; Greub, G. Blood culture-based diagnosis of bacteraemia: State of the art. Clin. Microbiol. Infect. 2015, 21, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Partouche, H.; Lepoutre, A.; Vaure, C.B.D.; Poisson, T.; Toubiana, L.; Gilberg, S. Incidence of all-cause adult community-acquired pneumonia in primary care settings in France. Med. Mal. Infect. 2018, 48, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sterrantino, C.; Trifirò, G.; Lapi, F.; Pasqua, A.; Mazzaglia, G.; Piccinni, C.; Cricelli, C.; Rossi, A.; Blasi, F. Burden of community-acquired pneumonia in Italian general practice. Eur. Respir. J. 2013, 42, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Lopardo, G.D.; Fridman, D.; Raimondo, E.; Albornoz, H.; Lopardo, A.; Bagnulo, H.; Goleniuk, D.; Sanabria, M.; Stamboulian, D. Incidence rate of community-acquired pneumonia in adults: A population-based prospective active surveillance study in three cities in South America. BMJ Open 2018, 8, e019439. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544, Erratum in Lancet 2017, 389, e1. [Google Scholar]
- World Health Organization. Global Health Estimates 2016: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000–2016; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- UNICEF. UNICEF, Levels & Trends in Child Mortality; Report 2017; The United Nations Children’s Fund: New York, NY, USA, 2017. [Google Scholar]
- UNICEF Data, Pneumonia in Children Statistics. Available online: https://data.unicef.org/topic/child-health/pneumonia/#:~:text=Pneumonia%20kills%20more%20children%20than,or%20around%202%2C000%20every%20day (accessed on 27 October 2023).
- Ramirez, J.A.; Wiemken, T.L.; Peyrani, P.; Arnold, F.W.; Kelley, R.; Mattingly, W.A.; Nakamatsu, R.; Pena, S.; Guinn, B.E.; Furmanek, S.P.; et al. Adults Hospitalized with Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin. Infect. Dis. 2017, 65, 1806–1812. [Google Scholar] [CrossRef]
- Gibson, G.J.; Loddenkemper, R.; Lundbäck, B.; Sibille, Y. Respiratory health and disease in Europe: The new European Lung White Book. Eur. Respir. J. 2013, 42, 559–563. [Google Scholar] [CrossRef]
- Aston, S.J.; Rylance, J. Community-Acquired Pneumonia in Sub-Saharan Africa. Semin. Respir. Crit. Care Med. 2016, 37, 855–867. [Google Scholar]
- Rello, J.; Manuel, O.; Eggimann, P.; Richards, G.; Wejse, C.; Petersen, J.E.; Zacharowski, K.; Leblebicioglu, H. Management of infections in critically ill returning travelers in the intensive care unit-II: Clinical syndromes and special considerations in immunocompromised patients. Int. J. Infect. Dis. 2016, 48, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-C.; Shen, C.-F.; Chen, S.-J.; Chen, H.-M.; Wang, Y.-C.; Chang, W.-S.; Chang, Y.-T.; Chen, W.-Y.; Huang, C.-Y.; Kuo, C.-C.; et al. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 172–199. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, T.P.; Ewan, V.; Wilson, P.; Simpson, A.J. The Intensive Care Society recommended bundle of interventions for the prevention of ventilator-associated pneumonia. J. Intensive Care Soc. 2016, 17, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Hespanhol, V.; Bárbara, C. Pneumonia mortality, comorbidities matter? Pulmonology 2020, 26, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Naghavi, M. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.E.; Saito, H. Sepsis in old age: Review of human and animal studies. Aging Dis. 2014, 5, 126–136. [Google Scholar] [CrossRef]
- Brakenridge, S.C.; Efron, P.A.; Stortz, J.A.; Ozrazgat-Baslanti, T.; Ghita, G.; Wang, Z.; Bihorac, A.; Mohr, A.M.; Brumback, B.A.; Moldawer, L.L.; et al. The impact of age on the innate immune response and outcomes after severe sepsis/septic shock in trauma and surgical intensive care unit patients. J. Trauma. Acute Care Surg. 2018, 85, 247–255. [Google Scholar] [CrossRef]
- Kotfis, K.; Wittebole, X.; Jaschinski, U.; Solé-Violán, J.; Kashyap, R.; Leone, M.; Nanchal, R.; Fontes, L.E.; Sakr, Y.; Vincent, J.-L. A worldwide perspective of sepsis epidemiology and survival according to age: Observational data from the ICON audit. J. Crit. Care 2019, 51, 122–132. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Kramer, A.A.; Knaus, W.A. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit. Care 2013, 17, R81. [Google Scholar] [CrossRef] [PubMed]
- Thaver, D.; Zaidi, A.K. Burden of neonatal infections in developing countries: A review of evidence from community-based studies. Pediatr. Infect. Dis. J. 2009, 28 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.C.; Blencowe, H.; Zaidi, A.; Ganatra, H.; Syed, S.; Engmann, C.; Newton, C.R.; Vergnano, S.; Stoll, B.J.; Cousens, S.N.; et al. Neonatal severe bacterial infection impairment estimates in South Asia, sub-Saharan Africa, and Latin America for 2010. Pediatr. Res. 2013, 74 (Suppl. S1), 73–85. [Google Scholar] [CrossRef]
- Ranjeva, S.L.; Warf, B.C.; Schiff, S.J. Economic burden of neonatal sepsis in sub-Saharan Africa. BMJ Glob. Health 2018, 3, e000347. [Google Scholar] [CrossRef]
- Arefian, H.; Heublein, S.; Scherag, A.; Brunkhorst, F.M.; Younis, M.Z.; Moerer, O.; Fischer, D.; Hartmann, M. Hospital-related cost of sepsis: A systematic review. J. Infect. 2017, 74, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Langa, K.M.; Iwashyna, T.J. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 2015, 313, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress in adults. Lancet 1967, 2, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.; Al-Haddad, M. Acute lung injury and acute respiratory distress syndrome, Continuing Education in Anaesthesia. Crit. Care Pain 2009, 9, 152–156. [Google Scholar]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Spragg, R. The American-European Consensus Conference on, A.R.D.S. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149 Pt 1, 818–824. [Google Scholar] [CrossRef]
- Ranieri, V.I.T.O.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Rubenfeld, G.D.; Herridge, M.S. Epidemiology and outcomes of acute lung injury. Chest 2007, 131, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Bakowitz, M.; Bruns, B.; McCunn, M. Acute lung injury and the acute respiratory distress syndrome in the injured patient. Scand. J. Trauma. Resusc. Emerg. Med. 2012, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Chaiwat, O.; Lang, J.D.; Vavilala, M.S.; Wang, J.; MacKenzie, E.J.; Jurkovich, G.J.; Rivara, F.P. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology 2009, 110, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.M.; Matthay, M.A.; Kangelaris, K.N.M.; Siew, E.D.; Janz, D.R.; Bernard, G.R.; May, A.K.; Jacob, P.; Havel, C.; Benowitz, N.L.; et al. Cigarette Smoke Exposure and the Acute Respiratory Distress Syndrome. Crit. Care Med. 2015, 43, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.P.; Wang, F.; Jones, T.K.; Palakshappa, J.A.; Anderson, B.J.; Shashaty, M.G.S.; Dunn, T.G.; Johansson, E.D.; Riley, T.R.; Lim, B.; et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: Evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018, 44, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Lemos-Filho, L.B.; Mikkelsen, M.E.; Martin, G.S.; Dabbagh, O.; Adesanya, A.; Gentile, N.; Gong, M.N. Sex, race, and the development of acute lung injury. Chest 2013, 143, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Mannino, D.M. Race and gender differences in acute respiratory distress syndrome deaths in the United States: An analysis of multiple-cause mortality data (1979–1996). Crit. Care Med. 2002, 30, 1679–1685. [Google Scholar] [CrossRef]
- Erickson, S.E.; Martin, G.S.; Davis, J.L.; Matthay, M.A.; Eisner, M.D.; NIH NHLBI ARDS Network. Recent trends in acute lung injury mortality: 1996–2005. Crit. Care Med. 2009, 37, 1574–1579. [Google Scholar] [CrossRef]
- Herrero, R.; Martin-Loeches, I.; Artigas, A. The complex interaction between sepsis and lung injury. In Annual Update in Intensive Care and Emergency Medicine; Springer: Berlin/Heidelberg, Germany, 2012; pp. 149–159. [Google Scholar]
- Hu, Q.; Hao, C.; Tang, S. From sepsis to acute respiratory distress syndrome (ARDS): Emerging preventive strategies based on molecular and genetic researches. Biosci. Rep. 2020, 40, BSR20200830. [Google Scholar] [CrossRef]
- Chavez, A.; Smith, M.; Mehta, D. New insights into the regulation of vascular permeability. Int. Rev. Cell Mol. Biol. 2011, 290, 205–248. [Google Scholar] [PubMed]
- Bice, T.; Cox, C.E.; Carson, S.S. Cost and health care utilization in ARDS--different from other critical illness? Semin. Respir. Crit. Care Med. 2013, 34, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.-C.; Gong, M.N.; Zhai, R.; Chen, F.; Bajwa, E.K.; Clardy, P.F.; Gallagher, D.C.; Thompson, B.T.; Christiani, D.C. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest 2010, 138, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Skrupky, L.P.; Kerby, P.W.; Hotchkiss, R.S. Advances in the management of sepsis and the understanding of key immunologic defects. Anesthesiology 2011, 115, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.; Dezfulian, C.; Collard, H.R.; Saint, S. Clinical and economic consequences of ventilator-associated pneumonia: A systematic review. Crit. Care Med. 2005, 33, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.W.; Knatz, N.L.; Vetterly, C.; Tomarello, S.; Wewers, M.D.; Volk, H.D.; Carcillo, J.A. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011, 37, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Sundar, K.M.; Sires, M. Sepsis induced immunosuppression: Implications for secondary infections and complications. Indian. J. Crit. Care Med. 2013, 17, 162–169. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Coopersmith, C.M.; McDunn, J.E.; Ferguson, T.A. The sepsis seesaw: Tilting toward immunosuppression. Nat. Med. 2009, 15, 496–497. [Google Scholar] [CrossRef]
- Blanco, J.; Muriel-Bombín, A.; Sagredo, V.; Taboada, F.; Gandía, F.; Tamayo, L.; Collado, J.; García-Labattut, A.; Carriedo, D.; Valledor, M.; et al. Incidence, organ dysfunction and mortality in severe sepsis: A Spanish multicentre study. Crit. Care 2008, 12, R158. [Google Scholar] [CrossRef]
- Montull, B.; Menéndez, R.; Torres, A.; Reyes, S.; Méndez, R.; Zalacaín, R.; Capelastegui, A.; Rajas, O.; Borderías, L.; Martin-Villasclaras, J.; et al. Predictors of Severe Sepsis among Patients Hospitalized for Community-Acquired Pneumonia. PLoS ONE 2016, 11, e0145929. [Google Scholar] [CrossRef]
- Cillóniz, C.; Dominedò, C.; Ielpo, A.; Ferrer, M.; Gabarrús, A.; Battaglini, D.; Torres, A. Risk and Prognostic Factors in Very Old Patients with Sepsis Secondary to Community-Acquired Pneumonia. J. Clin. Med. 2019, 8, 961. [Google Scholar] [CrossRef] [PubMed]
- Bozóky, G.; Ruby, E. Community-acquired pneumonia as a cause of sepsis. Trends Med. 2019, 19, 1–4. [Google Scholar] [CrossRef]
- Giuliano, K.K.; Baker, D. Sepsis in the Context of Nonventilator Hospital-Acquired Pneumonia. Am. J. Crit. Care 2020, 29, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.; Gursel, G. Predictive factors for septic shock in patients with ventilator-associated pneumonia. South. Med. J. 2008, 101, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Dominedò, C.; Magdaleno, D.; Ferrer, M.; Gabarrús, A.; Torres, A. Pure Viral Sepsis Secondary to Community-Acquired Pneumonia in Adults: Risk and Prognostic Factors. J. Infect. Dis. 2019, 220, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Jin, C.; Wu, J.; Zhu, S.; Liu, Y.J.; Chen, J. Guards at the gate: Physiological and pathological roles of tissue-resident innate lymphoid cells in the lung. Protein Cell 2017, 8, 932. [Google Scholar] [CrossRef]
- Dockrell, D.H.; Whyte, M.K.B.; Mitchell, T.J. Pneumococcal pneumonia: Mechanisms of infection and resolution. Chest 2012, 142, 482–491. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Fernandez-Sabe, N.; Carratala, J.; Diaz, V.; Verdaguer, R.; Dorca, J.; Manresa, F.; Gudiol, F. Early mortality in patients with community-acquired pneumonia: Causes and risk factors. Eur. Respir. J. 2008, 32, 733–739. [Google Scholar] [CrossRef]
- Cillóniz, C.; Ewig, S.; Polverino, E.; Muñoz-Almagro, C.; Marco, F.; Gabarrús, A.; Menéndez, R.; Mensa, J.; Torres, A. Pulmonary complications of pneumococcal community-acquired pneumonia: Incidence, predictors, and outcomes. Clin. Microbiol. Infect. 2012, 18, 1134–1142. [Google Scholar] [CrossRef]
- Feldman, C.; Anderson, R. Bacteremic pneumococcal pneumonia: Current therapeutic options. Drugs 2011, 71, 131–153. [Google Scholar] [CrossRef]
- Weinberger, D.M.; Harboe, Z.B.; Sanders, E.A.M.; Ndiritu, M.; Klugman, K.P.; Rückinger, S.; Dagan, R.; Adegbola, R.; Cutts, F.; Johnson, H.L.; et al. Association of serotype with risk of death due to pneumococcal pneumonia: A meta-analysis. Clin. Infect. Dis. 2010, 51, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Lujan, M.; Gallego, M.; Fontanals, D.; Mariscal, D.; Rello, J. Prospective observational study of bacteremic pneumococcal pneumonia: Effect of discordant therapy on mortality. Crit. Care Med. 2004, 32, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Aspa, J.; Rajas, O.; de Castro, F.R.; Huertas, M.C.; Borderías, L.; Cabello, F.J.; Tábara, J.; Hernández-Flix, S.; Martinez-Sanchis, A.; Torres, A. Impact of initial antibiotic choice on mortality from pneumococcal pneumonia. Eur. Respir. J. 2006, 27, 1010–1019. [Google Scholar] [CrossRef]
- Naucler, P.; Darenberg, J.; Morfeldt, E.; Ortqvist, A.; Henriques Normark, B. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax 2013, 68, 571–579. [Google Scholar] [CrossRef]
- Dietl, B.; Henares, D.; Boix-Palop, L.; Muñoz-Almagro, C.; Garau, J.; Calbo, E. Related Factors to Streptococcus pneumoniae Invasive Infection and Clinical Manifestations: The Potential Role of Nasopharyngeal Microbiome. Front. Med. 2021, 8, 650271. [Google Scholar] [CrossRef]
- Kloek, A.T.; Brouwer, M.C.; van de Beek, D. Host genetic variability and pneumococcal disease: A systematic review and meta-analysis. BMC Med. Genomics. 2019, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef]
- Hippenstiel, S.; Opitz, B.; Schmeck, B.; Suttorp, N. Lung epithelium as a sentinel and effector system in pneumonia--molecular mechanisms of pathogen recognition and signal transduction. Respir. Res. 2006, 7, 97. [Google Scholar] [CrossRef]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and localization of Toll-like receptor signaling complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef]
- Gardiner, E.E.; Andrews, R.K. Neutrophil extracellular traps (NETs) and infection-related vascular dysfunction. Blood Rev. 2012, 26, 255–259. [Google Scholar] [CrossRef]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology, 12th ed.; Garland Science: New York, NY, USA, 2017. [Google Scholar]
- Dockrell, D.H.; Brown, J.S. Streptococcus Pneumoniae Interactions with Macrophages and Mechanisms of Immune Evasion. In Streptococcus Pneumoniae; Chapter 21; Brown, J., Hammerschmidt, S., Orihuela, C., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 401–422. [Google Scholar]
- Kang, Y.S.; Kim, J.Y.; Bruening, S.A.; Pack, M.; Charalambous, A.; Pritsker, A.; Park, C.G. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA 2004, 101, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Darkwah, S.; Nago, N.; Appiah, M.G.; Myint, P.K.; Kawamoto, E.; Shimaoka, M.; Park, E.J. Differential Roles of Dendritic Cells in Expanding CD4 T Cells in Sepsis. Biomedicines 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hemmi, H. Dendritic cells: Translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006, 311, 17–58. [Google Scholar] [PubMed]
- Heesters, B.A.; Carroll, M.C. The Role of Dendritic Cells in S.pneumoniae Transport to Follicular Dendritic Cells. Cell Rep. 2016, 16, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-S.; Do, Y.; Lee, H.-K.; Park, S.H.; Cheong, C.; Lynch, R.M.; Loeffler, J.M.; Steinman, R.M.; Park, C.G. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell 2006, 125, 47–58. [Google Scholar] [CrossRef]
- Reis e Sousa, C. Dendritic cells as sensors of infection. Immunity 2001, 14, 495–498. [Google Scholar] [CrossRef]
- Pron, B.; Boumaila, C.; Jaubert, F.; Berche, P.; Milon, G.; Geissmann, F.; Gaillard, J. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell. Microbiol. 2001, 3, 331–340. [Google Scholar] [CrossRef]
- Wykes, M.N.; Horne-Debets, J. Dendritic cells: The Trojan horse of malaria? Int. J. Parasitol. 2012, 42, 583–587. [Google Scholar] [CrossRef]
- Noske, N.; Kämmerer, U.; Rohde, M.; Hammerschmidt, S. Pneumococcal interaction with human dendritic cells: Phagocytosis, survival, and induced adaptive immune response are manipulated by PavA. J. Immunol. 2009, 183, 1952–1963. [Google Scholar] [CrossRef]
- Holmes, A.R.; McNab, R.; Millsap, K.W.; Rohde, M.; Hammerschmidt, S.; Mawdsley, J.L.; Jenkinson, H.F. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol. Microbiol. 2001, 41, 1395–1408. [Google Scholar] [CrossRef]
- Wu, D.D.; Li, T.; Ji, X.Y. Dendritic Cells in Sepsis: Pathological Alterations and Therapeutic Implications. J. Immunol. Res. 2017, 2017, 3591248. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, Z.; Jin, H.; Yan, J.; Liang, H.P. Alterations of dendritic cells in sepsis: Featured role in immunoparalysis. BioMed Res. Int. 2015, 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Flohé, S.B.; Agrawal, H.; Schmitz, D.; Gertz, M.; Schade, F.U. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J. Leukoc. Biol. 2006, 79, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Faivre, V.; Lukaszewicz, A.C.; Alves, A.; Charron, D.; Payen, D.; Haziot, A. Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PLoS ONE 2012, 7, e47209. [Google Scholar] [CrossRef]
- Rosendahl, A.; Bergmann, S.; Hammerschmidt, S.; Goldmann, O.; Medina, E. Lung dendritic cells facilitate extrapulmonary bacterial dissemination during pneumococcal pneumonia. Front. Cell Infect. Microbiol. 2013, 3, 21. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Torres, A.; Blasi, F.; Dartois, N.; Akova, M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax 2015, 70, 984–989. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef]
- Schauer, A.E.; Klassert, T.E.; von Lachner, C.; Riebold, D.; Schneeweiß, A.; Stock, M.; Müller, M.M.; Hammerschmidt, S.; Bufler, P.; Seifert, U.; et al. IL-37 Causes Excessive Inflammation and Tissue Damage in Murine Pneumococcal Pneumonia. J. Innate Immun. 2017, 9, 403–418. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darkwah, S.; Kotey, F.C.N.; Ahenkorah, J.; Adutwum-Ofosu, K.K.; Donkor, E.S. Sepsis-Related Lung Injury and the Complication of Extrapulmonary Pneumococcal Pneumonia. Diseases 2024, 12, 72. https://doi.org/10.3390/diseases12040072
Darkwah S, Kotey FCN, Ahenkorah J, Adutwum-Ofosu KK, Donkor ES. Sepsis-Related Lung Injury and the Complication of Extrapulmonary Pneumococcal Pneumonia. Diseases. 2024; 12(4):72. https://doi.org/10.3390/diseases12040072
Chicago/Turabian StyleDarkwah, Samuel, Fleischer C. N. Kotey, John Ahenkorah, Kevin Kofi Adutwum-Ofosu, and Eric S. Donkor. 2024. "Sepsis-Related Lung Injury and the Complication of Extrapulmonary Pneumococcal Pneumonia" Diseases 12, no. 4: 72. https://doi.org/10.3390/diseases12040072







