Toward Generating More Diagnostic Features from Photoplethysmogram Waveforms
Abstract
1. Introduction
2. PPG Characteristics Terms
3. VPG Characteristic Terms
4. APG Characteristic Terms
5. Discussion
5.1. Blind Approach
5.2. Insightful Approach
- (1)
- To reduce the effort needed to read and understand research outcomes from different fields.
- (2)
- To enable scientists to focus more on discussing findings and not to worry about discussing the linguistics behind feature extraction.
- (3)
- To enable a universal understanding of outcomes and findings so that they can be easily replicated, evaluated, and validated.
- (4)
- To avoid future differences in feature naming.
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tabara, Y.; Igase, M.; Okada, Y.; Nagai, T.; Miki, T.; Ohyagi, Y.; Matsuda, F.; Kohara, K. Usefulness of the second derivative of the finger photoplethysmogram for assessment of end-organ damage: The J-SHIPP study. Hypertens. Res. 2016, 39, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, K.; Yabe, K.; Kobayashi, T.; Yamashita, Y.; Ibukiyama, C.; Fujita, M.; Sakai, T.; Maeda, K.; Hase, M. Clinical usefulness of the second derivative of a plethysmogram (acceralation plethysmogram). Cardiology 1993, 23, 207–217. [Google Scholar]
- Takazawa, K.; Tanaka, N.; Fujita, M.; Matsuoka, O.; Saiki, T.; Aikawa, M.; Tamura, S.; Ibukiyama, C. Assessment of vasocative agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension 1998, 32, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Kawakami, H.; Yamamoto, H.; Ito, C.; Fujiwara, S.; Sasaki, H.; Kihara, Y. Second derivative of the finger photoplethysmogram and cardiovascular mortality in middle-aged and elderly Japanese women. Hypertens. Res. 2017, 40, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M. Standard Terminologies for Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Shahrbabaki, S.S.; Ahmed, B.; Penzel, T.; Cvetkovic, D. Photoplethysmography derivatives and pulse transit time in overnight blood pressure monitoring. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 16–18 August 2016; pp. 2855–2858. [Google Scholar]
- Elgendi, M. On the Analysis of Fingertip Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Poon, C.; Zhang, Y. A novel parameter from PPG dicrotic notch for estimation of systolic blood pressure using pulse transit time. In Proceedings of the 5th International Summer School and Symposium on Medical Devices and Biosensors (ISSS-MDBS), Hong Kong, China, 1–3 June 2008; pp. 86–88. [Google Scholar]
- Allen, J.; Murray, A. Age-related changes in the characteristics of the photoplethysmographic pulse shape at various body sites. Physiol. Meas. 2003, 24, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yan, B.P.; Zhang, Y.-T.; Liu, J.; Zhao, N.; Tsang, H.K. Pulse Transit Time Based Continuous Cuffless Blood Pressure Estimation: A New Extension and A Comprehensive Evaluation. Sci. Rep. 2017, 7, 11554. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, H.; Ju, K.; Shin, K.; Lee, M.; Shelley, K.; Chon, K.H. Can photoplethysmography variability serve as an alternative approach to obtain heart rate variability information? J. Clin. Monit. Comput. 2008, 22, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Manzo, A.; Delgado, J.; Padilla, K.M.; Trenor, B.; Saiz, J. A computer based photoplethysmographic vascular analyzer through derivatives. In Proceedings of the Computers in Cardiology, Bologna, Italy, 14–17 September 2008; pp. 177–180. [Google Scholar]
- Elgendi, M.; Jonkman, M.; De Boer, F. Measurement of aa intervals at rest in the second derivative plethysmogram. In Proceedings of the 2009 International Symposium on Bioelectronics and Bioinformatics (ISBB), School of Electrical and Computer Engineering, RMIT University, Melbourne, Australia, 9–11 December 2009; p. 76. [Google Scholar]
- Elgendi, M.; Norton, I.; Brearley, M.; Abbott, D.; Schuurmans, D. Detection of a and b waves in the acceleration photoplethysmogram. Biomed. Eng. Online 2014, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M.; Norton, I.; Brearley, M.; Abbott, D.; Schuurmans, D. Systolic peak detection in acceleration photoplethysmograms measured from emergency responders in tropical conditions. PLoS ONE 2013, 8, e76585. [Google Scholar]
- Elgendi, M. Detection of c, d, and e waves in the acceleration photoplethysmogram. Comput. Methods Programs Biomed. 2014, 117, 125–136. [Google Scholar]
- Elgendi, M.; Jonkman, M.; De Boer, F. Applying the APG to measure Heart Rate Variability. In Proceedings of the 2nd International Conference on Computer and Automation Engineering (ICCAE), Singapore, 26–28 February 2010; pp. 514–517. [Google Scholar]
- Homma, S.; Ito, S.; Koto, T.; Ikegami, H. Relationship between accelerated plethysmogram, blood pressure and arterior elasticity. Jpn. Soc. Phys. Fit. Sport Med. 1992, 41, 98–107. [Google Scholar]
- Nousou, N.; Urase, S.; Maniwa, Y.; Fujimura, K.; Fukui, Y. Classification of Acceleration Plethysmogram Using Self-Organizing Map. In Proceedings of the ISPACS 2006 International Symposium on Intelligent Signal Processing and Communications, Tottori, Japan, 12–15 December 2006; pp. 681–684. [Google Scholar]
- Tokutaka, H.; Maniwa, Y.; Gonda, E.; Yamamoto, M.; Kakihara, T.; Kurata, M.; Fujimura, K.; Shigang, L.; Ohkita, M. Construction of A General Physical Condition Judgment System Using Acceleration Plethysmogram Pulse-Wave Analysis; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5629, pp. 307–315. [Google Scholar]
- Imanaga, I.; Hara, H.; Koyanagi, S.; Tanaka, K. Correlation between wave components of the second derivative of plethysmogram and arterial distensibility. Jpn. Heart J. 1998, 39, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.J.; Kim, J.S.; Kim, Y.S.; Lee, H.B.; Park, K.S. Second Derivative of Photoplethysmography for Estimating Vascular Aging. In Proceedings of the 6th International Special Topic Conference on Information Technology Applications in Biomedicine, Tokyo, Japan, 8–11 November 2007; pp. 70–72. [Google Scholar]
- Watanabe, N.; Bando, Y.K.; Kawachi, T.; Yamakita, H.; Futatsuyama, K.; Honda, Y.; Yasui, H.; Nishimura, K.; Kamihara, T.; Okumura, T.; et al. Development and Validation of a Novel Cuff-Less Blood Pressure Monitoring Device. JACC Basic Trans. Sci. 2017, 2, 631–642. [Google Scholar] [CrossRef]
- Palkovits, S.; Told, R.; Schmidl, D.; Boltz, A.; Napora, K.J.; Lasta, M.; Kaya, S.; Werkmeister, R.M.; Popa-Cherecheanu, A.; Garhöfer, G.; et al. Regulation of retinal oxygen metabolism in humans during graded hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1412–H1418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aiba, Y.; Ohshiba, S.; Horiguchi, S.; Morioka, I.; Miyashita, K.; Kiyota, I.; Endo, G.; Takada, H.; Iwata, H. Peripheral Hemodynamics Evaluated by Acceleration Plethysmography in Workers Exposed to Lead. Ind. Health 1999, 37, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-C.; Huang, P.-W.; Hung, C.-S.; Shan, S.-M.; Lin, Y.-H.; Shieh, J.-S.; Lai, D.-M.; Wu, A.-Y.; Jeng, J.-S. Identification of Atrial Fibrillation by Quantitative Analyses of Fingertip Photoplethysmogram. Sci. Rep. 2017, 7, 45644. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Sun, M. Optical blood pressure estimation with photoplethysmography and FFT-based neural networks. Biomed. Opt. Express 2016, 7, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Von Wowern, E.; Östling, G.; Nilsson, P.M.; Olofsson, P. Digital photoplethysmography for assessment of arterial stiffness: Repeatability and comparison with applanation tonometry. PLoS ONE 2015, 10, e0135659. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, Z.; Liu, G.; Elgendi, M. A new, short-recorded photoplethysmogram dataset for blood pressure monitoring in China. Sci. Data 2018, 5, 180020. [Google Scholar] [CrossRef] [PubMed]
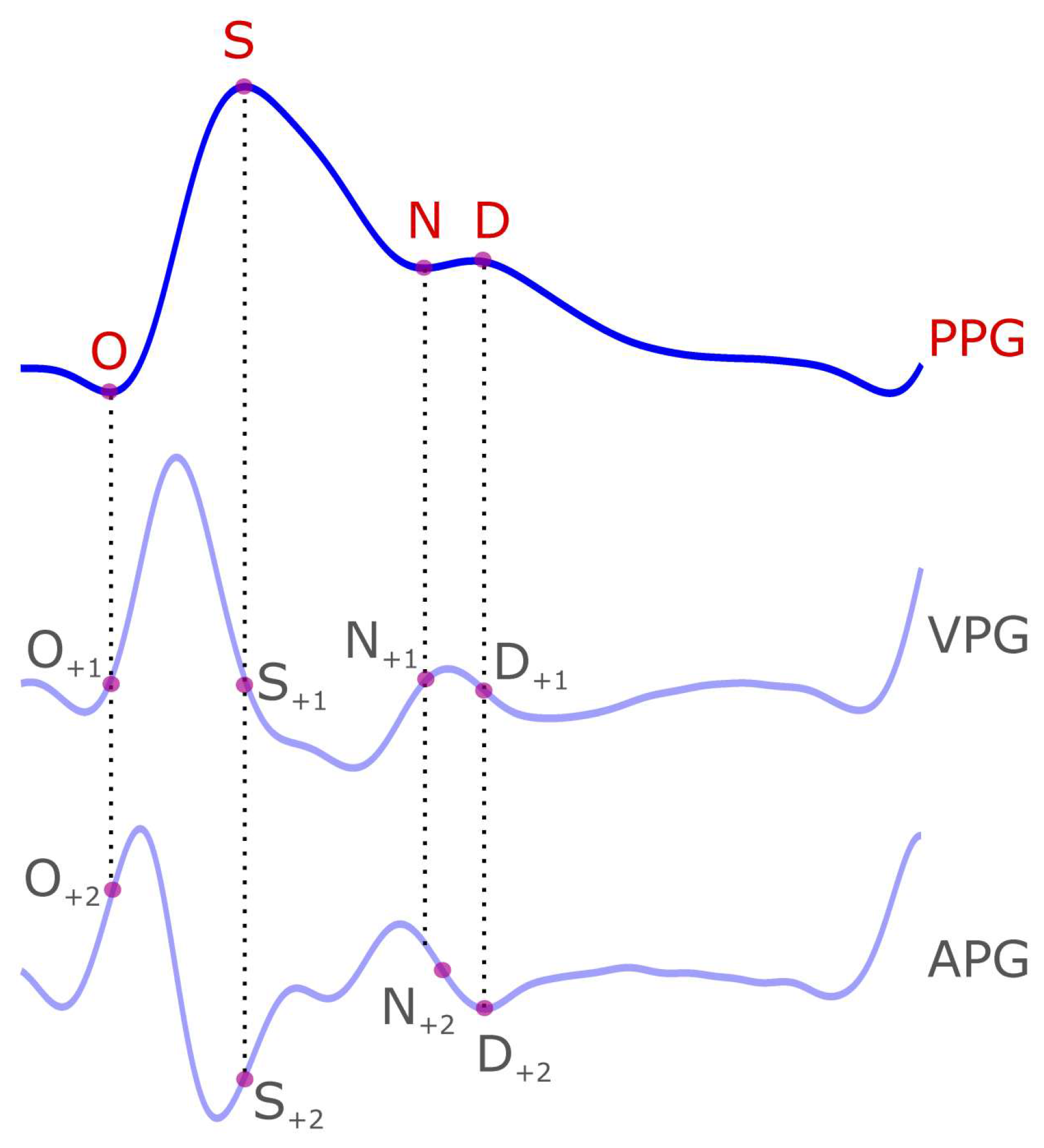
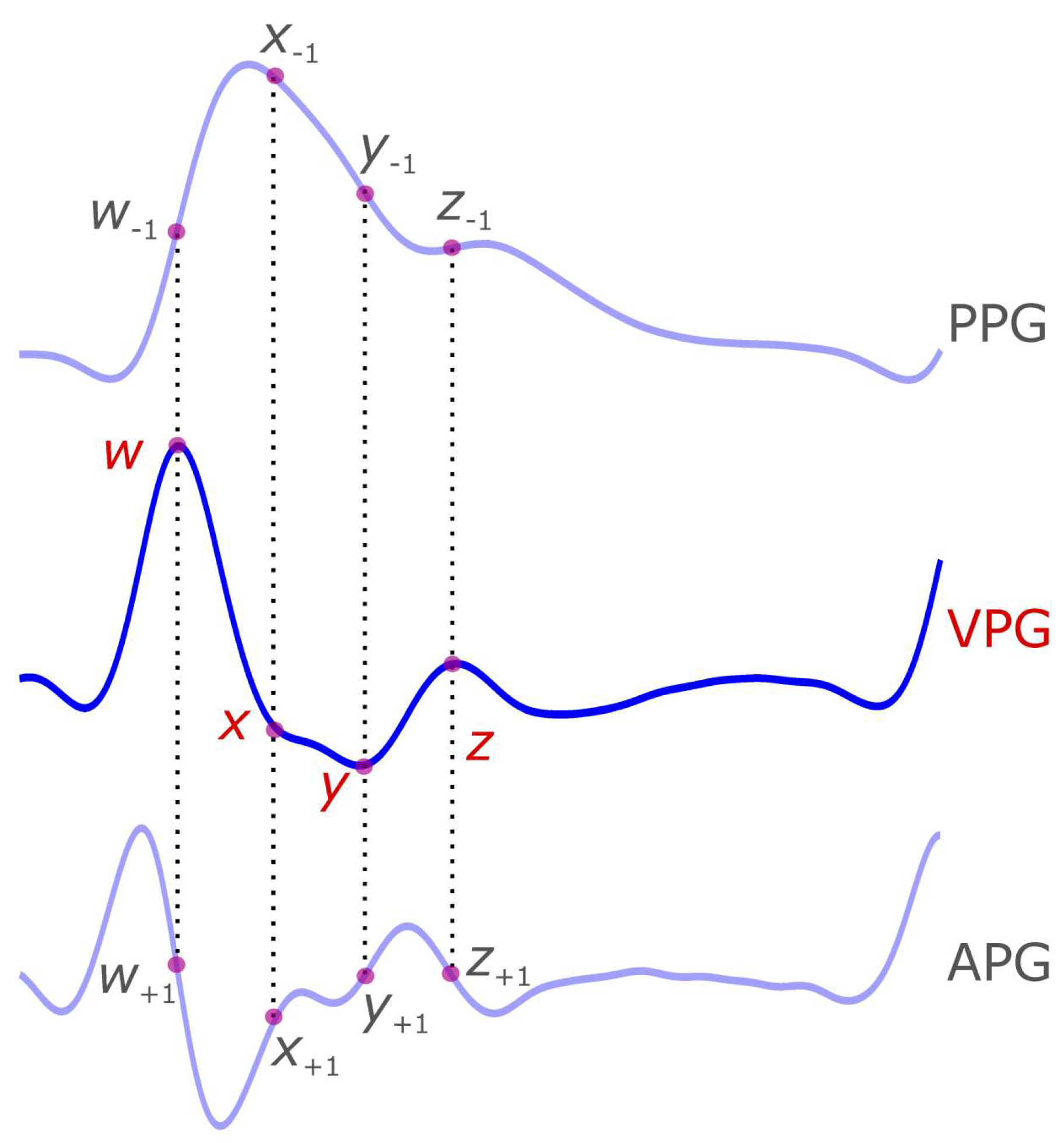
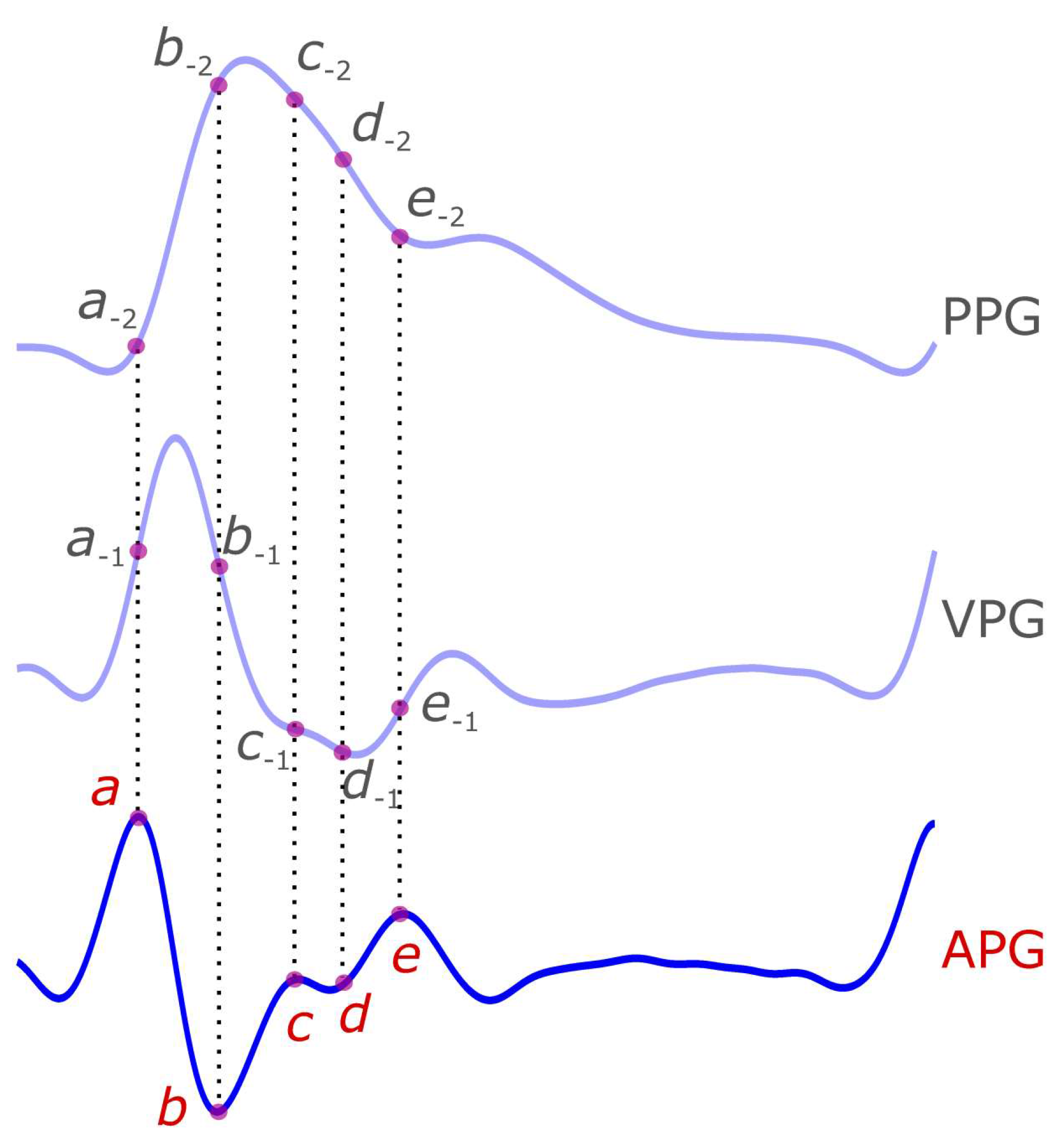
| Signal | Wave Name | Suggested Name |
|---|---|---|
| Photoplethysmogram (PPG) | systolic notch | O |
| systolic main peak | S | |
| diastolic notch or dicrotic notch | N | |
| dicrotic peak or diastolic peak | D | |
| First Derivative Photoplethysmogram (VPG) | Max slope point in systole | u |
| Min slope point in diastolic | v | |
| Max slope in diastole or MDS | w | |
| Second Derivative Photoplethysmogram (APG) | a wave | a |
| b wave | b | |
| c wave | c | |
| d wave | d | |
| e wave | e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgendi, M.; Liang, Y.; Ward, R. Toward Generating More Diagnostic Features from Photoplethysmogram Waveforms. Diseases 2018, 6, 20. https://doi.org/10.3390/diseases6010020
Elgendi M, Liang Y, Ward R. Toward Generating More Diagnostic Features from Photoplethysmogram Waveforms. Diseases. 2018; 6(1):20. https://doi.org/10.3390/diseases6010020
Chicago/Turabian StyleElgendi, Mohamed, Yongbo Liang, and Rabab Ward. 2018. "Toward Generating More Diagnostic Features from Photoplethysmogram Waveforms" Diseases 6, no. 1: 20. https://doi.org/10.3390/diseases6010020
APA StyleElgendi, M., Liang, Y., & Ward, R. (2018). Toward Generating More Diagnostic Features from Photoplethysmogram Waveforms. Diseases, 6(1), 20. https://doi.org/10.3390/diseases6010020






