ERBB1- and ERBB2-Positive Medullary Thyroid Carcinoma: A Case Report
Abstract
:1. Introduction
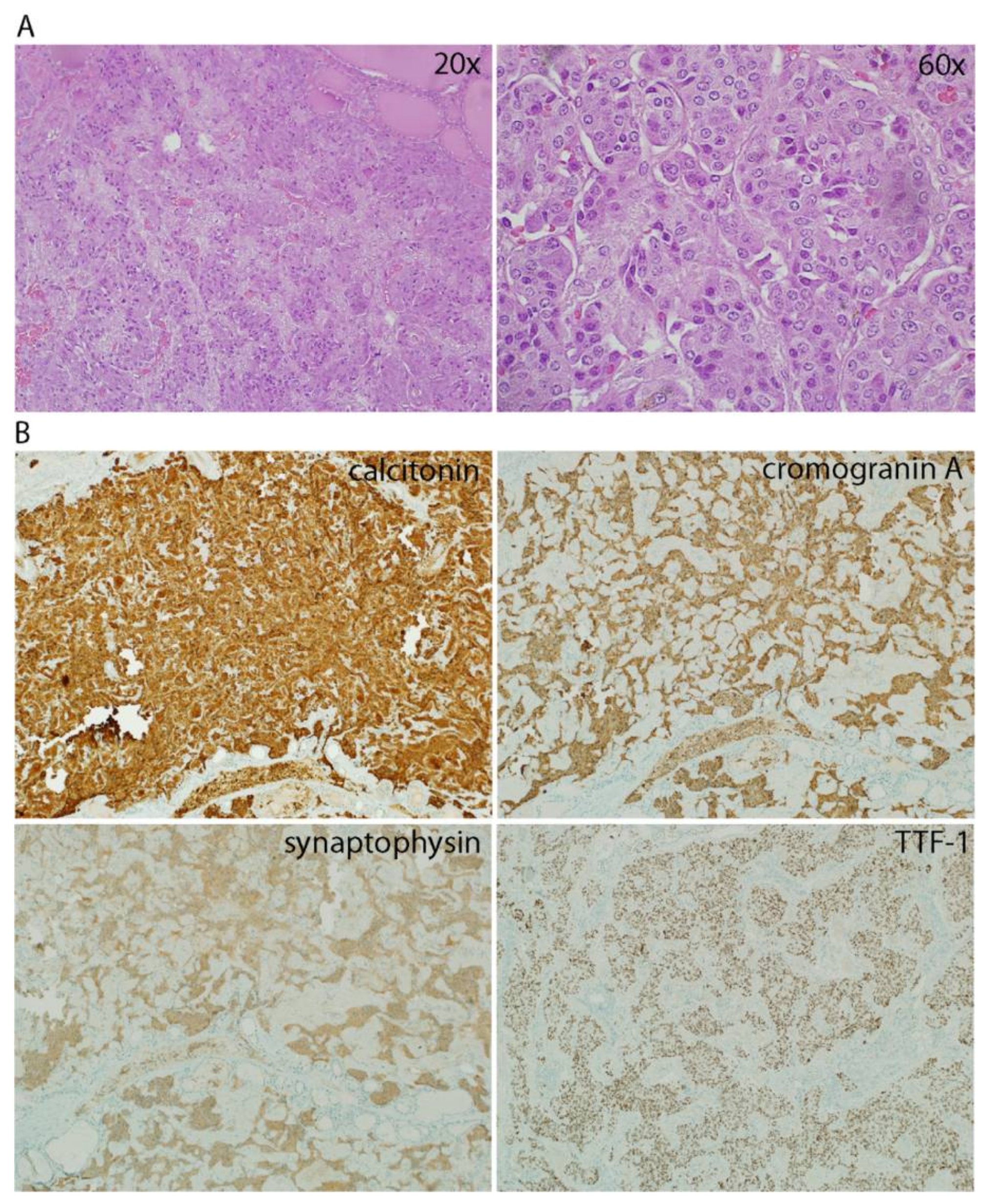
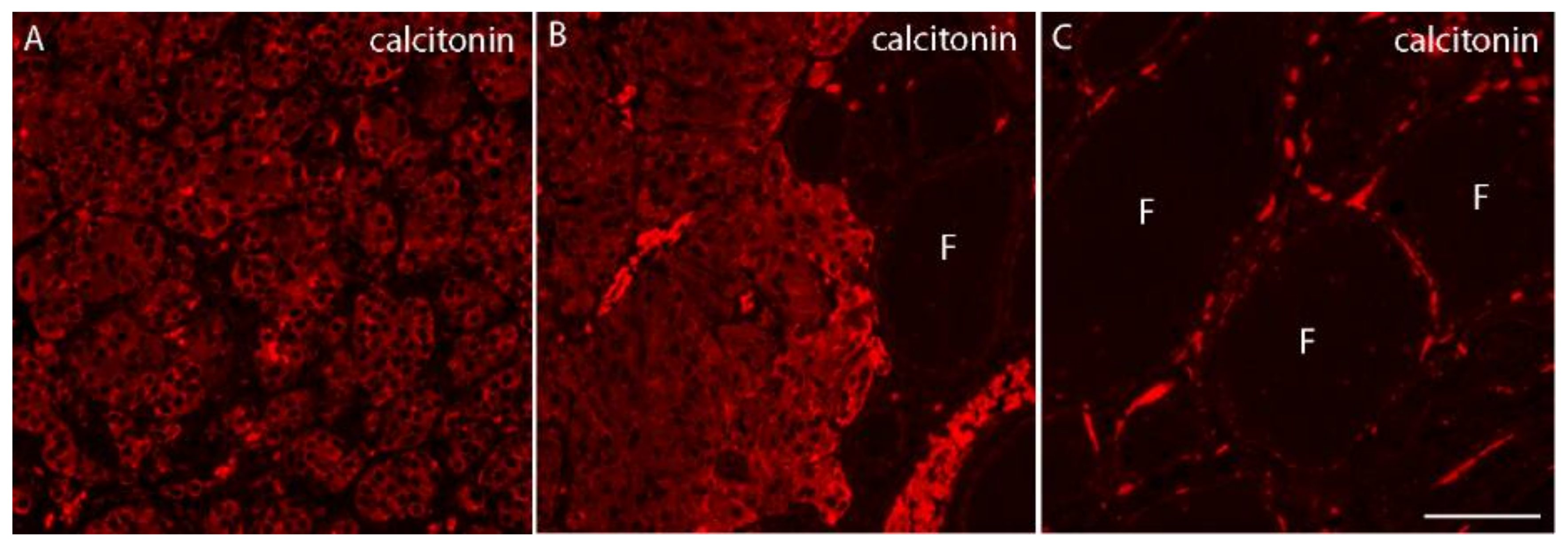
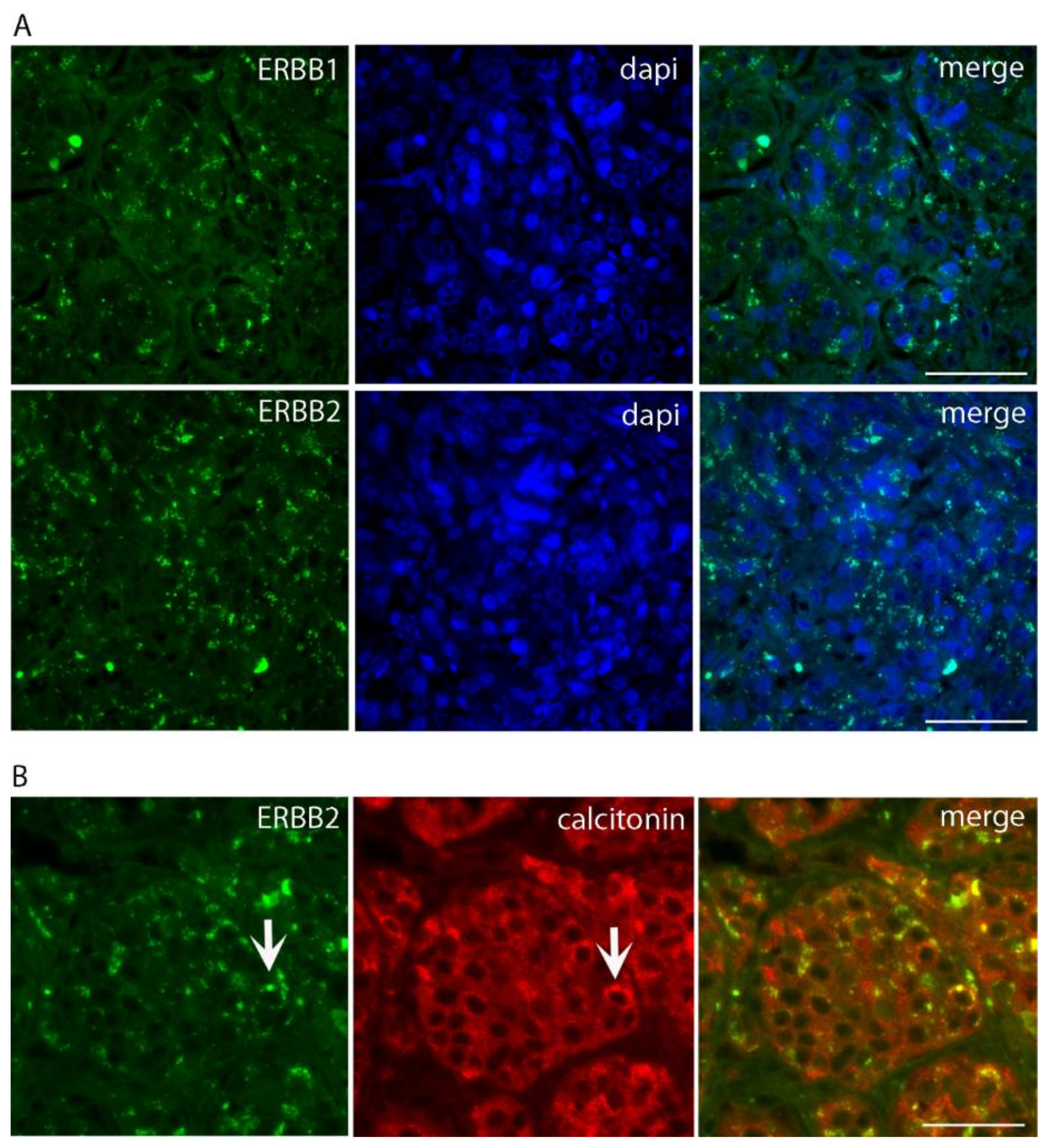
2. Case Presentation
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johansson, E.; Andersson, L.; Ornros, J.; Carlsson, T.; Ingeson-Carlsson, C.; Liang, S.; Dahlberg, J.; Jansson, S.; Parrillo, L.; Zoppoli, P.; et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development 2015, 142, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Bottici, V.; Luchetti, F.; Di Coscio, G.; Romei, C.; Grasso, L.; Miccoli, P.; Iacconi, P.; Basolo, F.; Pinchera, A.; et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: Experience in 10,864 patients with nodular thyroid disorders. J. Clin. Endocrinol. Metab. 2004, 89, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, P.; Minuto, M.N.; Ugolini, C.; Molinaro, E.; Basolo, F.; Berti, P.; Pinchera, A.; Elisei, R. Clinically unpredictable prognostic factors in the outcome of medullary thyroid cancer. Endocr.-Relat. Cancer 2007, 14, 1099–1105. [Google Scholar]
- Khurana, R.; Agarwal, A.; Bajpai, V.K.; Verma, N.; Sharma, A.K.; Gupta, R.P.; Madhusudan, K.P. Unraveling the amyloid associated with human medullary thyroid carcinoma. Endocrinology 2004, 145, 5465–5470. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.R.; Dravid, C.S.; Digumarti, R.; Dandekar, M. Targeted Therapy for Medullary Thyroid Cancer: A Review. Front. Oncol. 2017, 7, 238. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J. Expression and Localization of Neuregulin-1 (Nrg1) and ErbB2/ErbB4 Receptors in Main Endocrine Organs of the Rhesus Monkey. Int. J. Endocrinol. Metab. 2013, 11, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Hynes, N.E.; MacDonald, G. ErbB receptors and signaling pathways in cancer. Curr. Opin. Cell. Biol. 2009, 21, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Utrilla, J.C.; Martin-Lacave, I.; San Martin, M.V.; Fernandez-Santos, J.M.; Galera-Davidson, H. Expression of c-erbB-2 oncoprotein in human thyroid tumours. Histopathology 1999, 34, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Antona, C.; Pallares, J.; Montero-Conde, C.; Inglada-Perez, L.; Castelblanco, E.; Landa, I.; Leskela, S.; Leandro-Garcia, L.J.; Lopez-Jimenez, E.; Leton, R.; et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr.-Relat. Cancer 2010, 17, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell. Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kameya, T.; Shimosato, Y.; Adachi, I.; Abe, K.; Kasai, N.; Kimura, K.; Baba, K. Immunohistochemical and ultrastructural analysis of medullary carcinoma of the thyroid in relation to hormone production. Am. J. Pathol. 1977, 89, 555–574. [Google Scholar] [PubMed]
- Huang, S.N.; Goltzman, D. Electron and immunoelectron microscopic study of thyroidal medullary carcinoma. Cancer 1978, 41, 2226–2235. [Google Scholar] [CrossRef]
- Engedal, N.; Mills, I.G. Endosomal signaling and oncogenesis. Meth. Enzymol. 2014, 535, 179–200. [Google Scholar] [PubMed]
- Gosnell, J.E.; Duh, Q.Y. Medullary Thyroid Carcinoma-We Should Do Better. JAMA Surg. 2018, 153, 59. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, S.; Reagh, J.; Bullock, M.; Aniss, A.; Clifton-Bligh, R.; Learoyd, D.; Robinson, B.; Delbridge, L.; Sidhu, S.; Gill, A.J.; et al. Medullary Thyroid Carcinoma: Survival Analysis and Evaluation of Mutation-Specific Immunohistochemistry in Detection of Sporadic Disease. World J. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rendl, G.; Manzl, M.; Hitzl, W.; Sungler, P.; Pirich, C. Long-term prognosis of medullary thyroid carcinoma. Clin. Endocrinol. 2008, 69, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Tashijan, A.H., Jr.; Howland, B.G.; Melvin, K.E., Jr.; Hill, C.S. Immunoassay of human calcitonin. N. Engl. J. Med. 1970, 283, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Sand, M.; Gelos, M.; Sand, D.; Bechara, F.G.; Bonhag, G.; Welsing, E.; Mann, B. Serum calcitonin negative medullary thyroid carcinoma. World J. Surg. Oncol. 2006, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Massicotte, M.H.; Nascimento, C.L.; Chougnet, C.; Baudin, E.; Leboulleux, S. Kinase inhibitors for advanced medullary thyroid carcinoma. Clinics (Sao Paulo) 2012, 67 (Suppl. 1), 125–129. [Google Scholar] [CrossRef]
- Nelkin, B. Recent advances in the biology and therapy of medullary thyroid carcinoma. F1000Research 2017, 6, 2184. [Google Scholar] [CrossRef] [PubMed]
- Tampaki, E.C.; Tampakis, A.; Droeser, R.; Patsouris, E.; Kouraklis, G. Cabozantinib and Vandetanib in medullary thyroid carcinoma: Mitochondrial function and its potential as a therapeutic target towards novel strategies to design anti-CSCs drugs. Cancer Biol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Ruffilli, I.; Santini, F.; Minuto, M.; Galleri, D.; Miccoli, P. New targeted therapies for thyroid cancer. Curr. Genom. 2011, 12, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Albanell, J.; Codony, J.; Rovira, A.; Mellado, B.; Gascon, P. Mechanism of action of anti-HER2 monoclonal antibodies: Scientific update on trastuzumab and 2C4. Adv. Exp. Med. Biol. 2003, 532, 253–268. [Google Scholar] [PubMed]
- Martin-Lacave, I.; Utrilla, J.C. Expression of a neu/c-erbB-2-like product in neuroendocrine cells of mammals. Histol. Histopathol. 2000, 15, 1027–1033. [Google Scholar] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minuto, M.; Varaldo, E.; Marcocci, G.; De Santanna, A.; Ciccone, E.; Cortese, K. ERBB1- and ERBB2-Positive Medullary Thyroid Carcinoma: A Case Report. Diseases 2018, 6, 25. https://doi.org/10.3390/diseases6020025
Minuto M, Varaldo E, Marcocci G, De Santanna A, Ciccone E, Cortese K. ERBB1- and ERBB2-Positive Medullary Thyroid Carcinoma: A Case Report. Diseases. 2018; 6(2):25. https://doi.org/10.3390/diseases6020025
Chicago/Turabian StyleMinuto, Michele, Emanuela Varaldo, Gianluca Marcocci, Amleto De Santanna, Ermanno Ciccone, and Katia Cortese. 2018. "ERBB1- and ERBB2-Positive Medullary Thyroid Carcinoma: A Case Report" Diseases 6, no. 2: 25. https://doi.org/10.3390/diseases6020025
APA StyleMinuto, M., Varaldo, E., Marcocci, G., De Santanna, A., Ciccone, E., & Cortese, K. (2018). ERBB1- and ERBB2-Positive Medullary Thyroid Carcinoma: A Case Report. Diseases, 6(2), 25. https://doi.org/10.3390/diseases6020025





