Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate and Its Analogs Modified in the Benzene Moiety of the Molecule as New Analgesics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. General Procedure for the Synthesis of Methyl Anthranilates (2a–p)
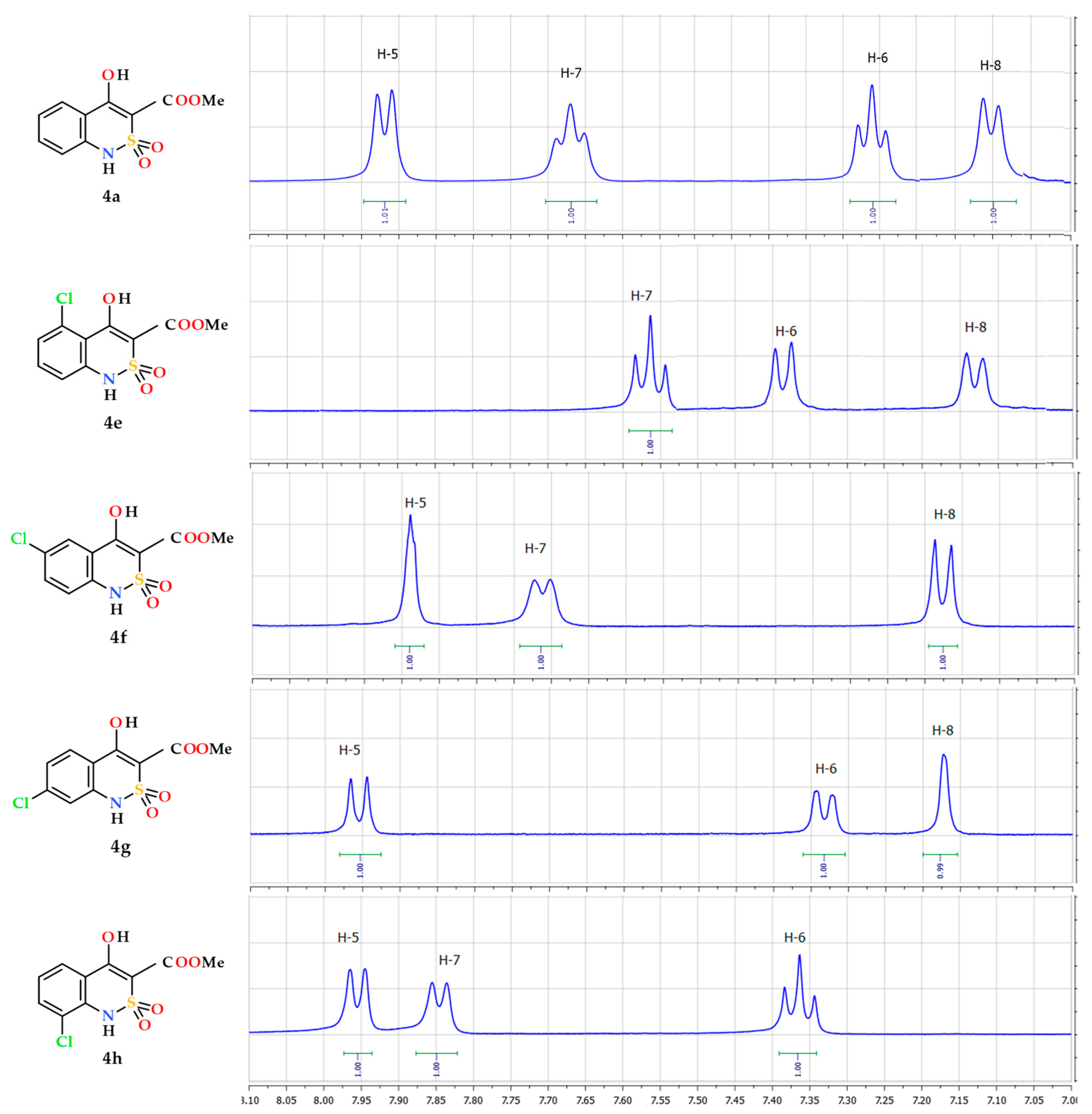
2.3. General Procedure for the Synthesis of Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates (4a–p)
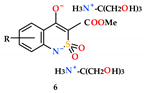
2.4. General Procedure for the Synthesis of Mono-and Disubstituted Salts of Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylates and Tris(hydroxymethyl)aminomethane (5–6)
2.5. X-ray Structural Analysis of Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate (4a)
2.6. X-ray Structural Analysis of Mono-Substituted Salt of Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate and Tris(hydroxymethyl)aminomethane Monohydrate (5a)
2.7. Pharmacology
Analgesic Test
3. Results and Discussion
3.1. Chemistry
3.2. The Molecular and Crystal Structure Study
3.3. Evaluation of the Analgesic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choudhary, M.I.; Naheed, N.; Abbaskhan, A.; Musharraf, S.G.; Siddiqui, H.; Atta-ur-Rahman. Phenolic and other constituents of fresh water fern Salvinia molesta. Phytochemistry 2008, 69, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.; Moore, R.A.; Edwards, J.E.; McQuay, H.J.; Derry, S.; Wiffen, P.J. Systematic review of efficacy of topical rubefacients containing salicylates for the treatment of acute and chronic pain. BMJ 2004, 328, 995–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tramèr, M.R. It’s not just about rubbing—Topical capsaicin and topical salicylates may be useful as adjuvants to conventional pain treatment. BMJ 2004, 328, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negwer, M. Organische Arzneimittel und Ihre Synonyma; Akademie: Berlin, Germany, 1978. [Google Scholar]
- Dwyer, C.; Sowerby, L.; Rotenberg, B.W. Is cocaine a safe topical agent for use during endoscopic sinus surgery? Laryngoscope 2016, 126, 1721–1723. [Google Scholar] [CrossRef] [Green Version]
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications of the Most Relevant APIs, 5th ed.; Thieme: Stuttgart, Germany, 2008. [Google Scholar]
- O’Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed.; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2006. [Google Scholar]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P. 2,1-Benzothiazine 2,2-dioxides. 1. Synthesis, structure, and analgesic activity of 1-R-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid esters. Chem. Heterocycl. Compd. 2013, 49, 1378–1383. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Bereznyakova, N.L. Effect of bromination on the pharmacological properties of methyl 1-allyl-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate. Pharm. Chem. J. 2015, 49, 519–522. [Google Scholar] [CrossRef]
- Azotla-Cruz, L.; Shishkina, S.; Ukrainets, I.; Lijanova, I.; Likhanova, N. Crystal structure of methyl 1-allyl-4-methyl-1H-benzo[c][1,2]thiazine-3-carboxylate 2,2-dioxide. Acta Crystallogr. E Crystallogr. Commun. 2016, 72, 1574–1576. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Sim, G.; Grinevich, L.A. Synthesis and molecular structure of ethyl-4-hydroxy-1-phenyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate. Pharm. Chem. J. 2017, 51, 482–485. [Google Scholar] [CrossRef]
- Azotla-Cruz, l.; Lijanova, I.V.; Ukrainets, I.V.; Likhanova, N.V.; Olivares-Xometl, O.; Bereznyakova, N.L. New synthesis, structure and analgesic properties of methyl 1-R-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates. Sci. Pharm. 2017, 85, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC1981599. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 3 February 2020).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC1981600. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 3 February 2020).
- Ukrainian Law No. 3447-IV. On Protection of Animals from Severe Treatment. Available online: http://zakon2.rada.gov.ua/laws/show/3447-15 (accessed on 4 August 2017).
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1014–1016. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Shishkina, S.V.; Petrushova, L.A.; Sim, G. 2,1-Benzothiazine 2,2-dioxides. 9. Alkylation of methyl 4-hydroxy-1-methyl-2,2-dioxo-1Н-2λ6,1-benzothiazine-3-carboxylate with ethyl iodide. Chem. Heterocycl. Compd. 2014, 50, 1741–1747. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Taran, S.G.; Evtifeeva, O.A.; Gorokhova, O.V.; Filimonova, N.I.; Turov, A.V. 4-Hydroxy-2-quinolones. 26. Bromination of 3-substituted 2-oxo-4-hydroxyquinolones. Chem. Heterocycl. Compd. 1995, 31, 176–179. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Jaradat, N.A.; Bevz, O.V.; Turov, A.V. 4-Hydroxy-2-quinolones. 204. Synthesis, bromination, and analgetic properties of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid arylalkylamides. Chem. Heterocycl. Compd. 2012, 48, 1347–1356. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Golik, N.Y.; Chernenok, I.N.; Shishkina, S.V.; Parshikov, V.A. 4-Hydroxy-2-quinolones. 220. Bromination of ethyl 7-hydroxy-5-oxo-2,3-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-6-carboxylate. Chem. Heterocycl. Compd. 2013, 49, 1665–1669. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Shishkina, S.V.; Baumer, V.N.; Gorokhova, O.V.; Petrushova, L.A.; Sim, G. The structure of two pseudo-enantiomeric forms of N-benzyl-4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide and their analgesic properties. Acta Crystallogr. C Struct. Chem. 2016, 72, 411–415. [Google Scholar] [CrossRef]
- Shishkina, S.V.; Levandovskiy, I.A.; Ukrainets, I.V.; Sidorenko, L.V.; Grinevich, L.A.; Yanchuk, I.B. Polymorphic modifications of a 1H-pyrrolo[3,2,1-ij]quinoline-5-carboxamide possessing strong diuretic properties. Acta Crystallogr. C Struct. Chem. 2018, 74, 1759–1767. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Hamza, G.M.; Burian, A.A.; Voloshchuk, N.I.; Malchenko, O.V.; Shishkina, S.V.; Grinevich, L.A.; Grynenko, V.V.; Sim, G. Molecular conformations and biological activity of N-hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Sci. Pharm. 2018, 86, 50. [Google Scholar] [CrossRef] [Green Version]
- Ukrainets, I.V.; Burian, A.A.; Baumer, V.N.; Shishkina, S.V.; Sidorenko, L.V.; Tugaibei, I.A.; Voloshchuk, N.I.; Bondarenko, P.S. Synthesis, crystal structure and biological activity of ethyl 4-methyl-2,2-dioxo- 1H-2λ6,1-benzothiazine-3-carboxylate polymorphic modifications. Sci. Pharm. 2018, 86, 21. [Google Scholar] [CrossRef] [Green Version]
- Shishkina, S.V.; Ukrainets, I.V.; Vashchenko, O.V.; Voloshchuk, N.I.; Bondarenko, P.S.; Petrushova, L.A.; Sim, G. Biological properties of two enantiomorphic forms of N-(2,6-dimethylphenyl)-4-hydroxy- 2,2-dioxo-1H -2λ6,1benzothiazine-3-carboxamide, a structural analogue of piroxicam. Acta Crystallogr. C Struct. Chem. 2020, 76, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Grinevich, L.A.; Sim, G. Synthesis, spatial structure and analgesic activity of sodium 3-benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate solvates. Sci. Pharm. 2016, 84, 705. [Google Scholar] [CrossRef] [Green Version]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Zefirov, Y.V. Reduced intermolecular contacts and specific interactions in molecular crystals. Crystallogr. Rep. 1997, 42, 865–886. [Google Scholar]







| Entry | Hydrogen Bond | The Symmetry Operation | Geometric Characteristics | ||
|---|---|---|---|---|---|
| H … А, Å | D … A, Å | D–H … A, Degrees | |||
| 1 | N(1Sa)‒H(1Ns) … O(2w) | x, y, z | 2.03 | 2.916 (8) | 170 |
| 2 | N(1Sa)‒H(2NS) … O(5b) | −0.5 + x, 1 − y, z | 2.03 | 2.912 (7) | 173 |
| 3 | N(1Sa)‒H(3NS) … N(1b) | −0.5 + x, 2 − y, z | 2.05 | 2.937 (8) | 174 |
| 4 | N(1Sb)‒H(4NS) … O(5a) | x, y, z | 2.04 | 2.931 (7) | 178 |
| 5 | N(1Sb)‒H(5NS) … N(1a) | x, 1 + y, z | 2.02 | 2.910 (8) | 173 |
| 6 | N(1Sb)‒H(6NS) …O(1w) | −0.5 + x, 2 − y, z | 2.07 | 2.955 (8) | 171 |
| 7 | O(2Sa)‒H(2OS) … O(1w) | −0.5 + x, 1 − y, z | 2.07 | 2.774 (7) | 144 |
| 8 | O(3Sa)‒H(3OS) … O(5a) | −0.5 + x, 1 − y, z | 2.13 | 2.915 (7) | 160 |
| 9 | O(2Sb)‒H(5OS) … O(2w) | x, 1 + y, z | 1.94 | 2.775 (8) | 171 |
| 10 | O(3Sb)‒H(6OS) … O(5b) | x, y, z | 2.11 | 2.915 (7) | 168 |
| 11 | O(1w)‒H(1wa) … O(4b) | x, y, z | 2.00 | 2.751 (8) | 147 |
| 12 | O(1w)‒H(1wb) … O(1Sa) | 0.5 + x, 2 − y, z | 1.83 | 2.730 (8) | 175 |
| 13 | O(2w)‒H(2wa) … O(1Sb) | x, y, z | 1.90 | 2.749 (8) | 177 |
| 14 | O(2w)‒H(2wb) … O(4a) | x, y, z | 1.91 | 2.759 (8) | 172 |
| Entry | Product | R | Lengthening of the Latent Period Compared to the Initial Level (%) | ||
|---|---|---|---|---|---|
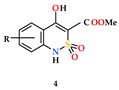 |  |  | |||
| 1 | a | H | 3.7 | 101.2 | 4.1 |
| 2 | b | 6-F | 60.0 | 24.6 | 37.2 |
| 3 | c | 7-F | 61.5 | 49.8 | 47.6 |
| 4 | d | 6,7-F2 | 5.4 | 4.2 | 3.7 |
| 5 | e | 5-Cl | 58.7 | 31.5 | 7.4 |
| 6 | f | 6-Cl | 8.4 | 64.1 | 88.8 |
| 7 | g | 7-Cl | 77.3 | 109.5 | 67.6 |
| 8 | h | 8-Cl | 19.4 | 29.7 | 10.4 |
| 9 | i | 6-Br | 8.6 | 106.3 | 2.9 |
| 10 | j | 7-Br | 88.8 | 25.6 | 3.2 |
| 11 | k | 6,8-Br2 | 28.5 | 33.2 | 23.4 |
| 12 | l | 6-I | 3.4 | 4.8 | 1.9 |
| 13 | m | 6-Me | 1.3 | 7.5 | 5.3 |
| 14 | n | 6-OMe | 27.0 | 21.4 | 0.8 |
| 15 | o | 7-OMe | 63.8 | 19.6 | 2.9 |
| 16 | p | 6,7-(OMe)2 | 8.3 | 11.7 | 14.3 |
| 17 | Meloxicam | – | 63.9 | – | – |
| 18 | Xefocam | – | – | 55.6 | 36.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Sidorenko, L.V.; Alekseeva, T.V.; Torianyk, I.I.; Davidenko, A.A. Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate and Its Analogs Modified in the Benzene Moiety of the Molecule as New Analgesics. Sci. Pharm. 2020, 88, 10. https://doi.org/10.3390/scipharm88010010
Ukrainets IV, Petrushova LA, Shishkina SV, Sidorenko LV, Alekseeva TV, Torianyk II, Davidenko AA. Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate and Its Analogs Modified in the Benzene Moiety of the Molecule as New Analgesics. Scientia Pharmaceutica. 2020; 88(1):10. https://doi.org/10.3390/scipharm88010010
Chicago/Turabian StyleUkrainets, Igor V., Lidiya A. Petrushova, Svitlana V. Shishkina, Lyudmila V. Sidorenko, Tatiana V. Alekseeva, Inna I. Torianyk, and Alexandra A. Davidenko. 2020. "Methyl 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxylate and Its Analogs Modified in the Benzene Moiety of the Molecule as New Analgesics" Scientia Pharmaceutica 88, no. 1: 10. https://doi.org/10.3390/scipharm88010010





