Impact of Compressional Force, Croscarmellose Sodium, and Microcrystalline Cellulose on Black Pepper Extract Tablet Properties Based on Design of Experiments Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Black Pepper Extract Tablet and Screening of Level of the Factors Using the OFAT Technique
2.3. Box-Behnken Design for Optimization of Black Pepper Extract Tablet
2.4. Evaluation of Black Pepper Extract Tablet Properties
2.4.1. Weight and Weight Variation
2.4.2. Thickness and Diameter
2.4.3. Hardness
2.4.4. Friability
2.4.5. Disintegration Time
2.5. Analysis of Piperine Content
2.6. Statistical Analysis
3. Results and Discussion
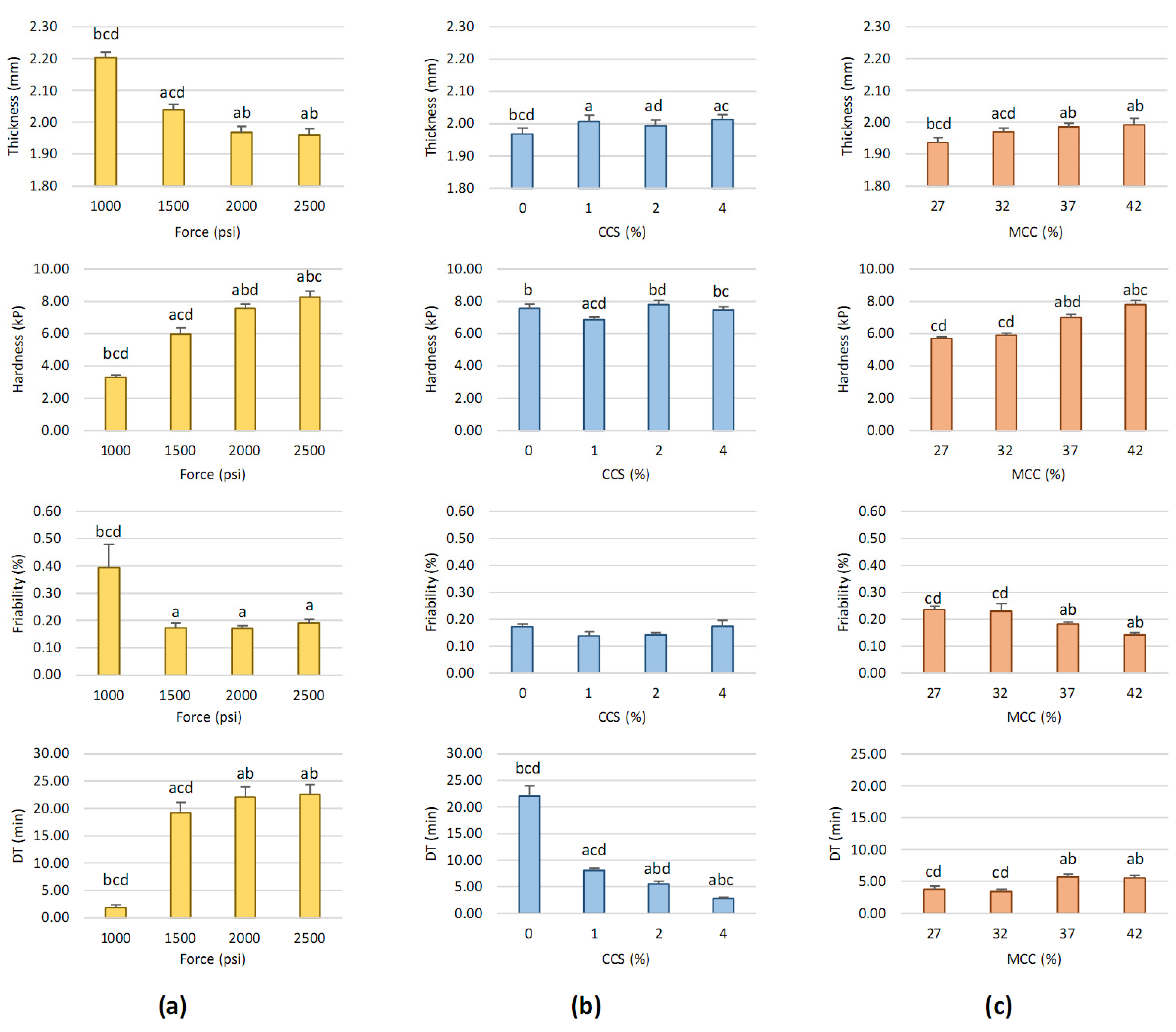
3.1. Impact of Factors on Black Pepper Extract Tablet Properties Obtained from Screening Step by OFAT Technique
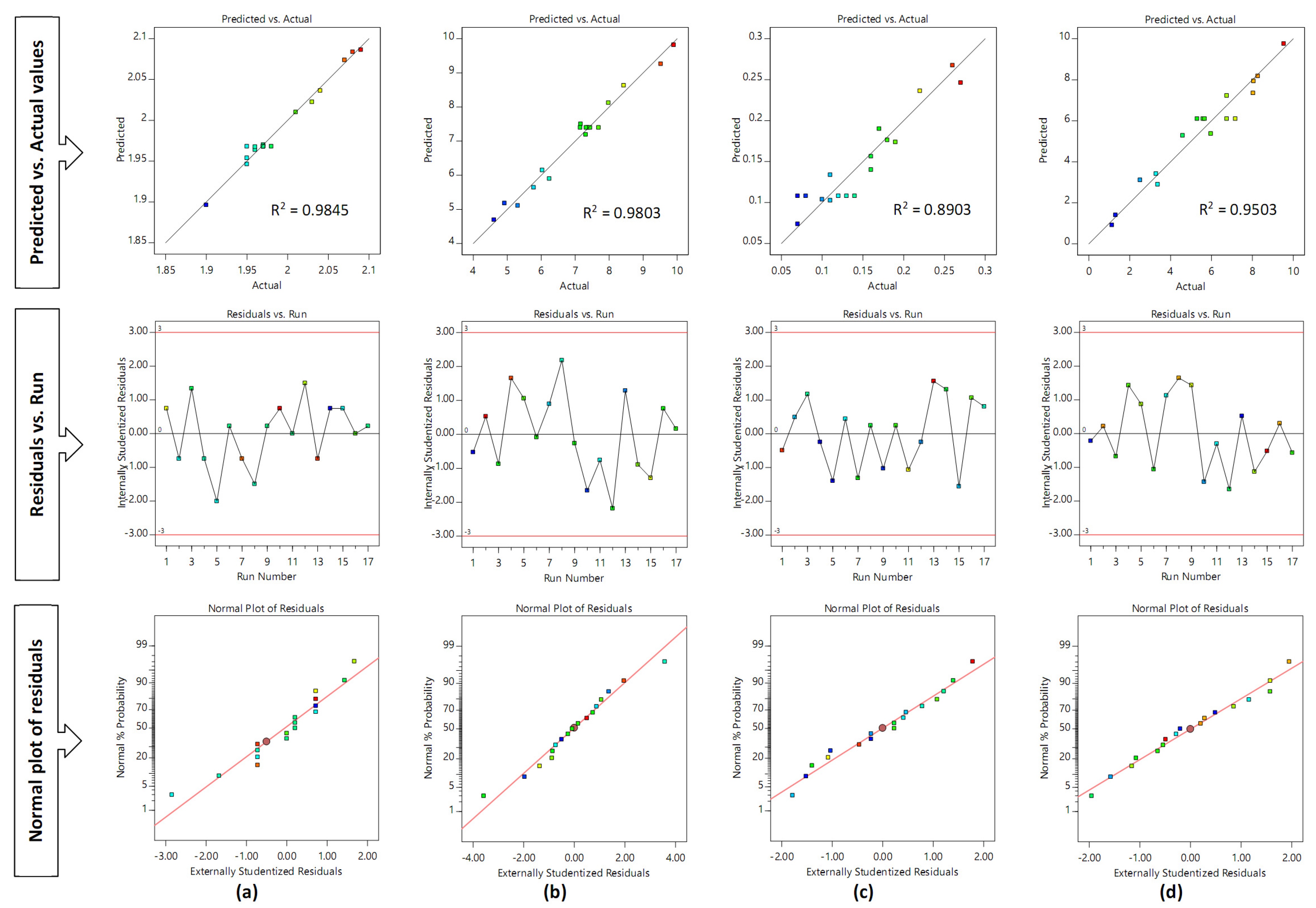
3.2. Impact of Factors on Black Pepper Extract Tablet Properties Obtained from Box–Behnken Design
3.3. Design Spaces and Optimal Condition for Preparation of Black Pepper Extract Tablets
3.4. Piperine Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Haq, I.-U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Pradeep, C.R.; Kuttan, G. Effect of piperine on the inhibition of nitric oxide (NO) and TNF-α production. Immunopharmacol. Immunotoxicol. 2003, 25, 337–346. [Google Scholar] [CrossRef]
- Ying, X.; Yu, K.; Chen, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell. Immunol. 2013, 285, 49–54. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; Anil Kumar, N.V.; Salehi, B.; Cho, W.C.; Sharifi-Rad, J. Piperine-A major principle of black pepper: A review of its bioactivity and studies. Appl. Sci. 2019, 9, 4270. [Google Scholar]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Peterson, B.; Weyers, M.; Steenekamp, J.H.; Steyn, J.D.; Gouws, C.; Hamman, J.H. Drug bioavailability enhancing agents of natural origin (bioenhancers) that modulate drug membrane permeation and pre-systemic metabolism. Pharmaceutics 2019, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kim, H.Y.; Back, S.Y.; Han, H.-K. Piperine-mediated drug interactions and formulation strategy for piperine: Recent advances and future perspectives. Expert Opin. Drug Metab. Toxicol. 2018, 14, 43–57. [Google Scholar] [CrossRef]
- Mura, P.; Valleri, M.; Baldanzi, S.; Mennini, N. Characterization and evaluation of the performance of different calcium and magnesium salts as excipients for direct compression. Int. J. Pharm. 2019, 567, 118454. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Emadzadeh, B.; Shahidi-Noghabi, M.; Abbaspour, M.; Akbari, E. Physico–chemical properties of powder and compressed tablets based on barberry fruit pulp. J. Food Meas. Charact. 2021, 15, 2469–2480. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Yousefi, A. Effect of different excipients on physicochemical properties of the functional rice bran tablet: Univariate and multivariate studies on a novel food supplement. J. Food Meas. Charact. 2021, 15, 1359–1369. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, Y.; Yin, J.; Hu, H.; Pei, H.; Li, W.; Zhang, X. Characteristics of effervescent tablets of Aronia melanocarpa: Response surface design and antioxidant activity evaluation. J. Food Meas. Charact. 2022, 16, 2969–2977. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST/SEMATECH e-Handbook of Statistical Methods. Available online: http://www.itl.nist.gov/div898/handbook/ (accessed on 27 August 2022).
- Eldin, A.B. General introduction to Design of Experiments (DOE). In Wide Spectra of Quality Control; Akyar, I., Ed.; InTech: Shanghai, China, 2011; pp. 21–26. [Google Scholar]
- Durakovic, B. Design of experiments application, concepts, examples: State of the art. Period. Eng. Nat. Sci. 2017, 5, 421–439. [Google Scholar]
- JMP Statistical Discovery LLC. Design of Experiments. Available online: https://www.jmp.com/en_ph/statistics-knowledge-portal/what-is-design-of-experiments.html (accessed on 27 August 2022).
- Gibson, M. (Ed.) Pharmaceutical Preformulation and Formulation: A Practical Guide from Candidate Drug Selection to Commercial Dosage Form, 2nd ed.; Informa Healthcare: New York, NY, USA, 2016; Volume 199. [Google Scholar]
- Steele, G. Quality by Design (QbD) and the development and manufacture of drug substance. In Pharmaceutical Quality by Design: A Practical Approach; Schlindwein, W.S., Gibson, M., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 61–95. [Google Scholar]
- Manley, L.; Hilden, J.; Valero, P.; Kramer, T. Tablet compression force as a process analytical technology (PAT): 100% inspection and control of tablet weight uniformity. J. Pharm. Sci. 2019, 108, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Marais, A.F.; Song, M.; Villiers, M.M.d. Effect of compression force, humidity and disintegrant concentration on the disintegration and dissolution of directly compressed furosemide tablets using croscarmellose sodium as disintegrant. Trop. J. Pharm. Res. 2003, 2, 125–135. [Google Scholar]
- Pimhataivoot, P.; Tipduangta, P.; Sirithunyalug, B.; Sirithunyalug, J. Versatile compression force measuring system for rotary tablet presses. CMU J. Nat. Sci. 2011, 10, 243–244. [Google Scholar]
- Desai, P.M.; Er, P.X.; Liew, C.V.; Heng, P.W. Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach. AAPS PharmSciTech 2014, 15, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Suksaeree, J.; Monton, C.; Chankana, N.; Charoenchai, L. Application of microwave-assisted drying to shorten granules drying process for the preparation of Thunbergia laurifolia Lindl. leaf tablets. Trends Sci. 2023, 20, 4993. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Hiremath, P.; Nuguru, K.; Agrahari, V. Chapter 8—Material Attributes and Their Impact on Wet Granulation Process Performance. In Handbook of Pharmaceutical Wet Granulation; Narang, A.S., Badawy, S.I.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 263–315. [Google Scholar]
- Rojas, J.; Guisao, S.; Ruge, V. Functional assessment of four types of disintegrants and their effect on the spironolactone release properties. AAPS PharmSciTech 2012, 13, 1054–1062. [Google Scholar] [CrossRef] [Green Version]
- Apeji, Y.E.; Zechariah, F.D.; Anyebe, S.N.; Tytler, B.; Olowosulu, A.K.; Oyi, A.R. Effect of mode of superdisintegrant incorporation on tableting properties of metronidazole granules. Pharm. Sci. Asia 2019, 46, 25–32. [Google Scholar] [CrossRef]
- Chaerunisaa, A.Y.; Sriwidodo, S.; Abdassah, M. Microcrystalline cellulose as pharmaceutical excipient. In Pharmaceutical Formulation Design—Recent Practices; Ahmad, U., Akhtar, J., Eds.; IntechOpen: London, UK, 2019; pp. 1–21. [Google Scholar]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Suksaeree, J.; Wunnakup, T.; Monton, C. Synergistic antioxidant activity of plant compositions contained in Chatuphalathika herbal recipe: Terminalia chebula Retz. var. chebula, Terminalia arjuna Wight and Arn., Terminalia bellirica (Gaertn.) Roxb., and Phyllanthus emblica L. Adv. Tradit. Med. 2022, 22, 547–556. [Google Scholar] [CrossRef]
- Monton, C.; Suksaeree, J. Interaction of plant ingredients contained in Chatuphalathika herbal remedy based on chemical analysis aspect: Four-component simplex lattice design. Adv. Tradit. Med. 2021, 21, 535–544. [Google Scholar] [CrossRef]
- Duangjit, S.; Mehr, L.M.; Kumpugdee-Vollrath, M.; Ngawhirunpat, T. Role of simplex lattice statistical design in the formulation and optimization of microemulsions for transdermal delivery. Biol. Pharm. Bull. 2014, 37, 1948–1957. [Google Scholar] [CrossRef] [Green Version]
- Suksaeree, J.; Monton, C.; Charoenchai, L.; Chankana, N.; Wunnakup, T. Optimization of process and formulation variables for Semha–Pinas extract effervescent tablets using the Box–Behnken design. AAPS PharmSciTech 2023, 24, 52. [Google Scholar] [CrossRef]
- Monton, C.; Keawchay, P.; Pokkrong, C.; Kamnoedthapaya, P.; Navabhatra, A.; Suksaeree, J.; Wunnakup, T.; Chankana, N.; Songsak, T. Fabrication of direct compressible tablets containing Chatuphalathika extract obtained through microwave-assisted extraction: An optimization approach. Sci. Pharm. 2023, 91, 17. [Google Scholar] [CrossRef]
- Suksaeree, J.; Monton, C.; Charoenchai, L.; Chankana, N. Microwave-assisted drying of Prasakanphlu herbal granules and formulation development of Prasakanphlu tablets: Design of Experiments approach. Adv. Tradit. Med. 2023, in press. [CrossRef]
- Suksaeree, J.; Monton, C.; Chankana, N.; Charoenchai, L. Microcrystalline cellulose promotes superior direct compressed Boesenbergia rotunda (L.) Mansf. extract tablet properties to spray-dried rice starch and spray-dried lactose. Arab J. Basic Appl. Sci. 2023, 30, 13–25. [Google Scholar] [CrossRef]
- Tak, J.W.; Gupta, B.; Thapa, R.K.; Woo, K.B.; Kim, S.Y.; Go, T.G.; Choi, Y.; Choi, J.Y.; Jeong, J.H.; Choi, H.G.; et al. Preparation and optimization of immediate release/sustained release bilayered tablets of loxoprofen using Box-Behnken design. AAPS PharmSciTech 2017, 18, 1125–1134. [Google Scholar] [CrossRef]
- Narendra, C.; Srinath, M.S.; Babu, G. Optimization of bilayer floating tablet containing metoprolol tartrate as a model drug for gastric retention. AAPS PharmSciTech 2006, 7, E23–E29. [Google Scholar] [CrossRef] [Green Version]
- Soares, L.A.; Ortega, G.G.; Petrovick, P.R.; Schmidt, P.C. Optimization of tablets containing a high dose of spray-dried plant extract: A technical note. AAPS PharmSciTech 2005, 6, E367–E371. [Google Scholar] [CrossRef] [Green Version]
- Samala, M.L.; Janga, R.B. Design, statistical optimization of Nizatidine floating tablets using natural polymer. Future J. Pharm. Sci. 2021, 7, 2. [Google Scholar] [CrossRef]
- Zakowiecki, D.; Edinger, P.; Papaioannou, M.; Hess, T.; Kubiak, B.; Terlecka, A. Exploiting synergistic effects of brittle and plastic excipients in directly compressible formulations of sitagliptin phosphate and sitagliptin hydrochloride. Pharm. Dev. Technol. 2022, 27, 702–713. [Google Scholar] [CrossRef]
- Mostafa, H.F.; Ibrahim, M.A.; Sakr, A. Development and optimization of dextromethorphan hydrobromide oral disintegrating tablets: Effect of formulation and process variables. Pharm. Dev. Technol. 2013, 18, 454–463. [Google Scholar] [CrossRef]
- Eyjolfsson, R. Chapter 1—Introduction. In Design and Manufacture of Pharmaceutical Tablets; Eyjolfsson, R., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 1–28. [Google Scholar]
- Monton, C.; Luprasong, C. Effect of temperature and duration time of maceration on nitrate content of Vernonia cinerea (L.) Less.: Circumscribed central composite design and method validation. Int. J. Food Sci. 2019, 2019, 1281635. [Google Scholar] [CrossRef] [Green Version]
- Thai Food and Drug Administration. List of Plants That Can Be Used in Food Supplement. Available online: https://food.fda.moph.go.th/law/data/announ_fda/600810_name.pdf (accessed on 20 October 2022).


| Formulas | Factors | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Force (psi) |
CCS (%) |
MCC (%) | Thickness | Hardness | |||||
|
Actual (mm) |
Predicted (mm) |
Error (%) * |
Actual (kP) |
Predicted (kP) |
Error (%) * | ||||
| 1 | 1500 | 1 | 37 | 2.09 ± 0.02 | 2.09 | 0.00 | 4.92 ± 0.15 | 5.18 | −5.28 |
| 2 | 2500 | 1 | 37 | 1.95 ± 0.02 | 1.95 | 0.00 | 8.43 ± 0.17 | 8.63 | −2.37 |
| 3 | 1500 | 3 | 37 | 2.08 ± 0.03 | 2.08 | 0.00 | 5.31 ± 0.32 | 5.11 | 3.77 |
| 4 | 2500 | 3 | 37 | 1.96 ± 0.02 | 1.96 | 0.00 | 9.52 ± 0.27 | 9.26 | 2.73 |
| 5 | 1500 | 2 | 32 | 2.04 ± 0.02 | 2.04 | 0.00 | 4.61 ± 0.19 | 4.69 | −1.74 |
| 6 | 2500 | 2 | 32 | 1.90 ± 0.02 | 1.90 | 0.00 | 7.98 ± 0.32 | 8.12 | −1.75 |
| 7 | 1500 | 2 | 42 | 2.07 ± 0.02 | 2.07 | 0.00 | 5.78 ± 0.33 | 5.64 | 2.42 |
| 8 | 2500 | 2 | 42 | 1.95 ± 0.01 | 1.95 | 0.00 | 9.90 ± 0.25 | 9.82 | 0.81 |
| 9 | 2000 | 1 | 32 | 1.96 ± 0.01 | 1.97 | −0.51 | 6.24 ± 0.32 | 5.90 | 5.45 |
| 10 | 2000 | 3 | 32 | 1.97 ± 0.01 | 1.97 | 0.00 | 6.03 ± 0.32 | 6.15 | −1.99 |
| 11 | 2000 | 1 | 42 | 2.01 ± 0.02 | 2.01 | 0.00 | 7.31 ± 0.27 | 7.19 | 1.64 |
| 12 | 2000 | 3 | 42 | 2.03 ± 0.02 | 2.02 | 0.49 | 7.16 ± 0.09 | 7.50 | −4.75 |
| 13 | 2000 | 2 | 37 | 1.97 ± 0.01 | 1.97 | 0.00 | 7.37 ± 0.22 | 7.39 | −0.27 |
| 14 | 2000 | 2 | 37 | 1.98 ± 0.01 | 1.97 | 0.51 | 7.15 ± 0.33 | 7.39 | −3.36 |
| 15 | 2000 | 2 | 37 | 1.97 ± 0.01 | 1.97 | 0.00 | 7.32 ± 0.33 | 7.39 | −0.96 |
| 16 | 2000 | 2 | 37 | 1.95 ± 0.02 | 1.97 | −1.03 | 7.69 ± 0.33 | 7.39 | 3.90 |
| 17 | 2000 | 2 | 37 | 1.97 ± 0.01 | 1.97 | 0.00 | 7.44 ± 0.15 | 7.39 | 0.67 |
| Formulas | Factors | Responses | |||||||
| Force (psi) | CCS (%) | MCC (%) | Friability | DT | |||||
| Actual (%) | Predicted (%) | Error (%) * | Actual (min) | Predicted (min) | Error (%) * | ||||
| 1 | 1500 | 1 | 37 | 0.18 ± 0.02 | 0.18 | 0.00 | 2.51 ± 0.51 | 3.11 | −23.90 |
| 2 | 2500 | 1 | 37 | 0.11 ± 0.02 | 0.13 | −18.18 | 9.54 ± 0.35 | 9.76 | −2.31 |
| 3 | 1500 | 3 | 37 | 0.27 ± 0.02 | 0.25 | 7.41 | 1.13 ± 0.29 | 0.91 | 19.47 |
| 4 | 2500 | 3 | 37 | 0.07 ± 0.01 | 0.07 | 0.00 | 5.97 ± 0.45 | 5.37 | 10.05 |
| 5 | 1500 | 2 | 32 | 0.26 ± 0.03 | 0.27 | −3.85 | 1.31 ± 0.41 | 1.40 | −6.87 |
| 6 | 2500 | 2 | 32 | 0.16 ± 0.02 | 0.14 | 12.50 | 6.75 ± 0.32 | 7.22 | −6.96 |
| 7 | 1500 | 2 | 42 | 0.17 ± 0.07 | 0.19 | −11.76 | 3.37 ± 0.28 | 2.90 | 13.95 |
| 8 | 2500 | 2 | 42 | 0.11 ± 0.04 | 0.10 | 9.09 | 8.27 ± 0.13 | 8.18 | 1.09 |
| 9 | 2000 | 1 | 32 | 0.16 ± 0.02 | 0.16 | 0.00 | 8.04 ± 0.13 | 7.35 | 8.58 |
| 10 | 2000 | 3 | 32 | 0.22 ± 0.01 | 0.24 | −9.09 | 3.29 ± 0.27 | 3.42 | −3.95 |
| 11 | 2000 | 1 | 42 | 0.19 ±0.02 | 0.17 | 10.53 | 8.06 ± 0.48 | 7.93 | 1.61 |
| 12 | 2000 | 3 | 42 | 0.10 ± 0.03 | 0.10 | 0.00 | 4.59 ± 0.29 | 5.28 | −15.03 |
| 13 | 2000 | 2 | 37 | 0.12 ± 0.04 | 0.11 | 8.33 | 5.30 ± 0.37 | 6.10 | −15.09 |
| 14 | 2000 | 2 | 37 | 0.14 ± 0.03 | 0.11 | 21.43 | 5.59 ± 0.60 | 6.10 | −9.12 |
| 15 | 2000 | 2 | 37 | 0.08 ± 0.03 | 0.11 | −37.50 | 7.17 ± 0.31 | 6.10 | 14.92 |
| 16 | 2000 | 2 | 37 | 0.07 ± 0.00 | 0.11 | −57.14 | 6.75 ± 0.41 | 6.10 | 9.63 |
| 17 | 2000 | 2 | 37 | 0.13 ± 0.01 | 0.11 | 15.38 | 5.67 ± 0.51 | 6.10 | −7.58 |
| Source | Thickness | Hardness | Friability | DT | |||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient a | p-Value | Coefficient a | p-Value | Coefficient a | p-Value | Coefficient a | p-Value | ||
| Model | - | <0.0001 * | - | < 0.0001 * | - | 0.0119 * | - | 0.0009 * | |
| Intercept | 1.9680 | - | 7.3940 | - | 0.1080 | - | 6.0960 | - | |
| Linear | X1-Force | −0.0650 | <0.0001 * | 1.9013 | <0.0001 * | −0.0538 | 0.0016 * | 2.7763 | <0.0001 * |
| X2-CCS | 0.0038 | 0.3257 | 0.1400 | 0.2458 | 0.0025 | 0.8232 | −1.6463 | 0.0009 * | |
| X3-MCC | 0.0238 | 0.0003 * | 0.6613 | 0.0006 * | −0.0288 | 0.0321 * | 0.6125 | 0.0776 | |
| Interaction | X1X2 | 0.0050 | 0.3522 | 0.1750 | 0.2998 | −0.0325 | 0.0704 | −0.5475 | 0.2328 |
| X1X3 | 0.0050 | 0.3522 | 0.1875 | 0.2693 | 0.0100 | 0.5327 | −0.1350 | 0.7568 | |
| X2X3 | 0.0025 | 0.6336 | 0.0150 | 0.9262 | −0.0375 | 0.0434 * | 0.3200 | 0.4702 | |
| Quadratic | X1² | 0.0248 | 0.0015 * | 0.0168 | 0.9155 | 0.0285 | 0.0966 | −1.1893 | 0.0226 * |
| X2² | 0.0273 | 0.0008 * | −0.3658 | 0.0474 * | 0.0210 | 0.2004 | −0.1193 | 0.7788 | |
| X3² | −0.0028 | 0.5915 | −0.3433 | 0.0589 | 0.0385 | 0.0359 * | 0.01825 | 0.9656 | |
| Lack of Fit | - | 0.6356 | - | 0.0890 | - | 0.5143 | - | 0.4341 | |
| Responses | Predicted Values | Actual Values | Error (%) * | 95% CI (Lower to Upper) |
|---|---|---|---|---|
| Thickness (mm) (n = 20) | 1.97 | 1.98 ± 0.02 | 0.51 | 1.94–2.00 |
| Hardness (kP) (n = 10) | 7.41 | 7.36 ± 0.24 | −0.68 | 6.60–8.22 |
| Friability (%) (n = 3, each 11) | 0.11 | 0.11 ± 0.02 | 0.00 | 0.03–0.19 |
| DT (min) (n = 6) | 5.76 | 5.59 ± 0.39 | −3.04 | 3.59–7.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monton, C.; Wunnakup, T.; Suksaeree, J.; Charoenchai, L.; Chankana, N. Impact of Compressional Force, Croscarmellose Sodium, and Microcrystalline Cellulose on Black Pepper Extract Tablet Properties Based on Design of Experiments Approach. Sci. Pharm. 2023, 91, 30. https://doi.org/10.3390/scipharm91030030
Monton C, Wunnakup T, Suksaeree J, Charoenchai L, Chankana N. Impact of Compressional Force, Croscarmellose Sodium, and Microcrystalline Cellulose on Black Pepper Extract Tablet Properties Based on Design of Experiments Approach. Scientia Pharmaceutica. 2023; 91(3):30. https://doi.org/10.3390/scipharm91030030
Chicago/Turabian StyleMonton, Chaowalit, Thaniya Wunnakup, Jirapornchai Suksaeree, Laksana Charoenchai, and Natawat Chankana. 2023. "Impact of Compressional Force, Croscarmellose Sodium, and Microcrystalline Cellulose on Black Pepper Extract Tablet Properties Based on Design of Experiments Approach" Scientia Pharmaceutica 91, no. 3: 30. https://doi.org/10.3390/scipharm91030030





