Analytical Investigation of Forced Oxidized Anti-VEGF IgG Molecules: A Focus on the Alterations in Antigen and Receptor Binding Activities
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Generation of Oxidative Stress
2.3. Cell Culture Studies
2.4. Analyses
2.4.1. Antigen (VEGF) Binding Assays
2.4.2. Receptor (FcRn) Binding Assay
2.5. Peptide Mapping Using LC/MS-MS
2.6. C1q Binding Assays with ELISA
2.7. Statistical Analyses
3. Results
3.1. Peptide Mapping of mAbs Exposed to Various Oxidation Conditions
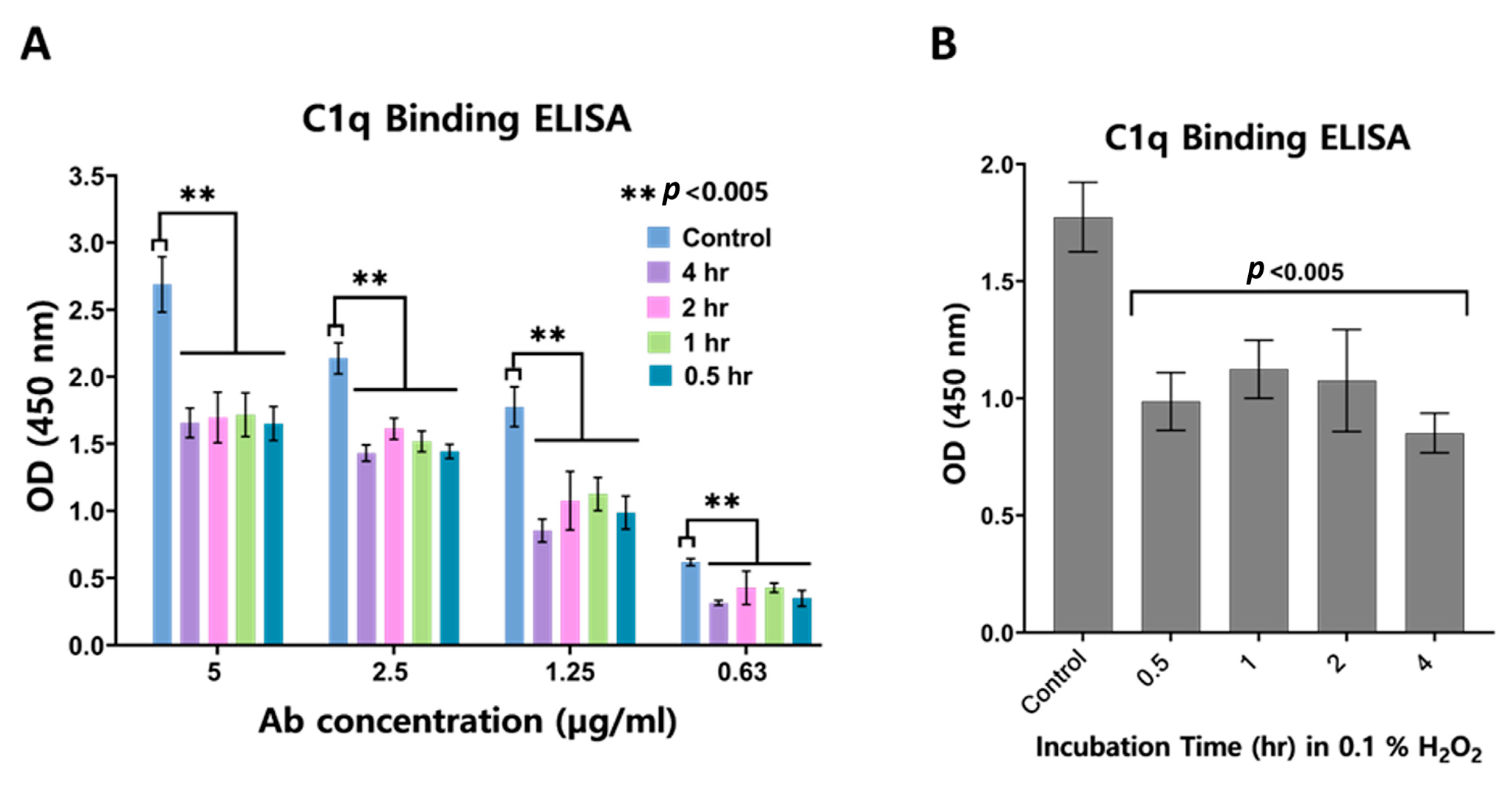
3.2. Impact of Oxidation on C1q Binding
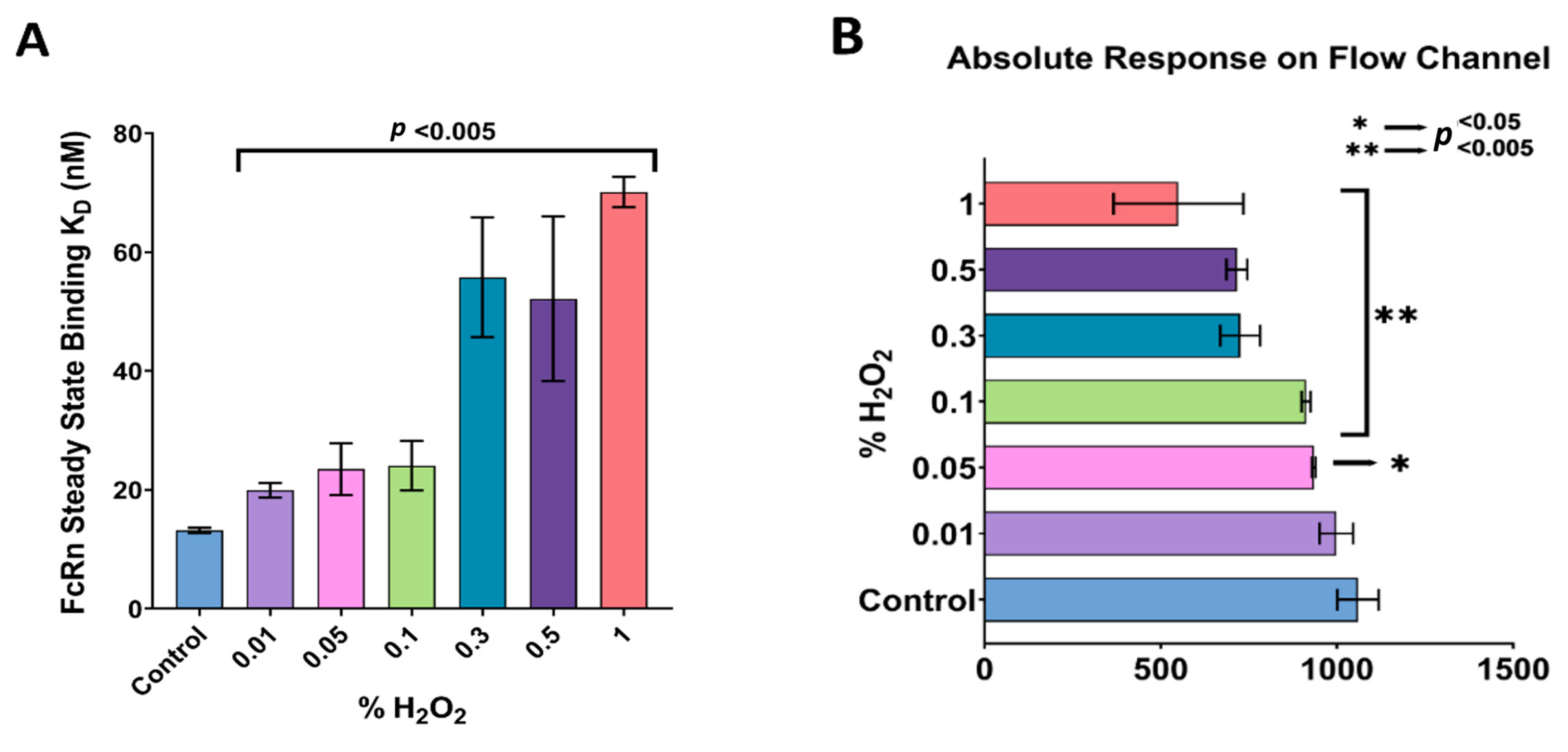
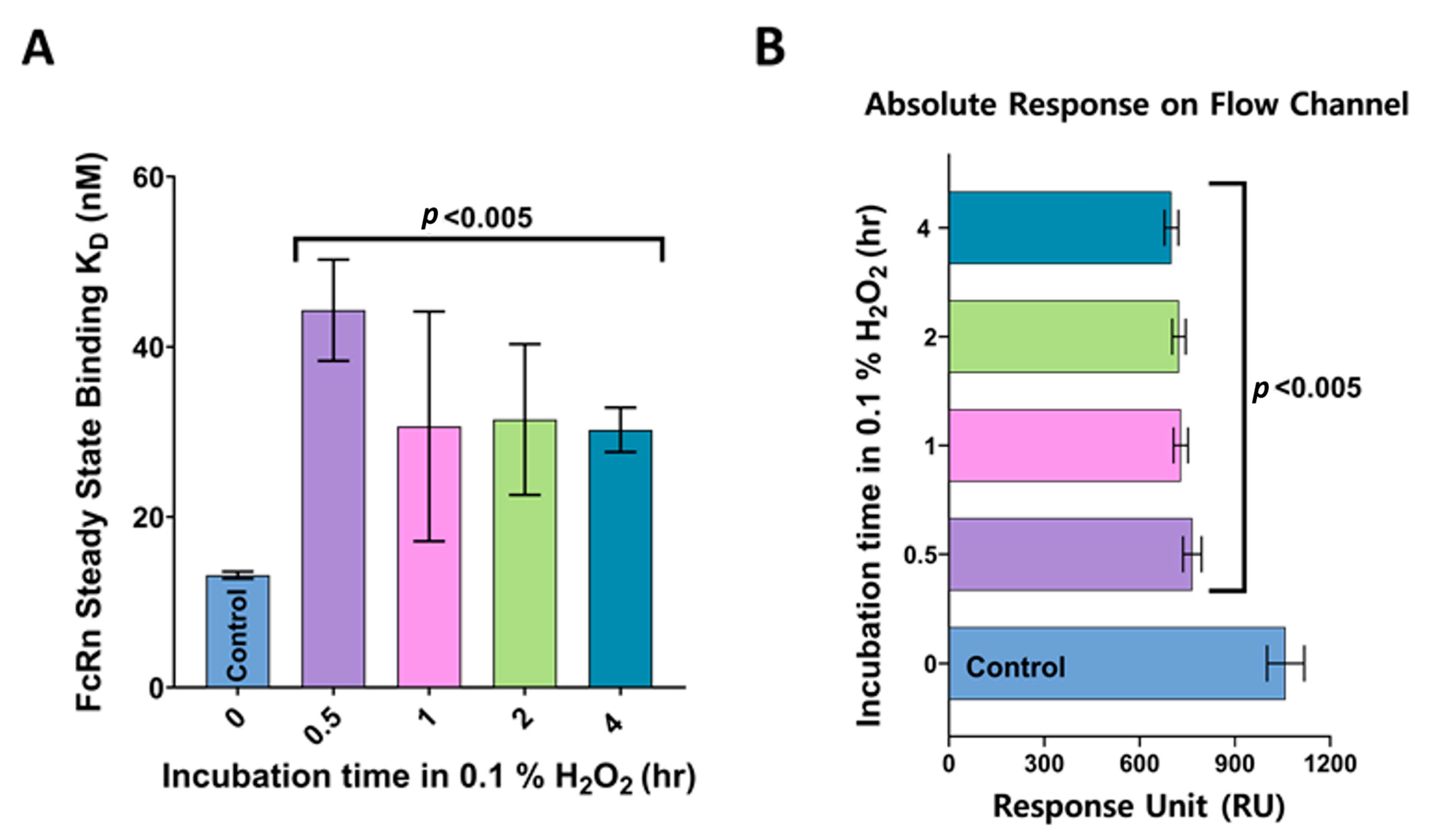
3.3. FcRn Binding Analysis of Anti-VEGF IgGs Exposed to Oxidation
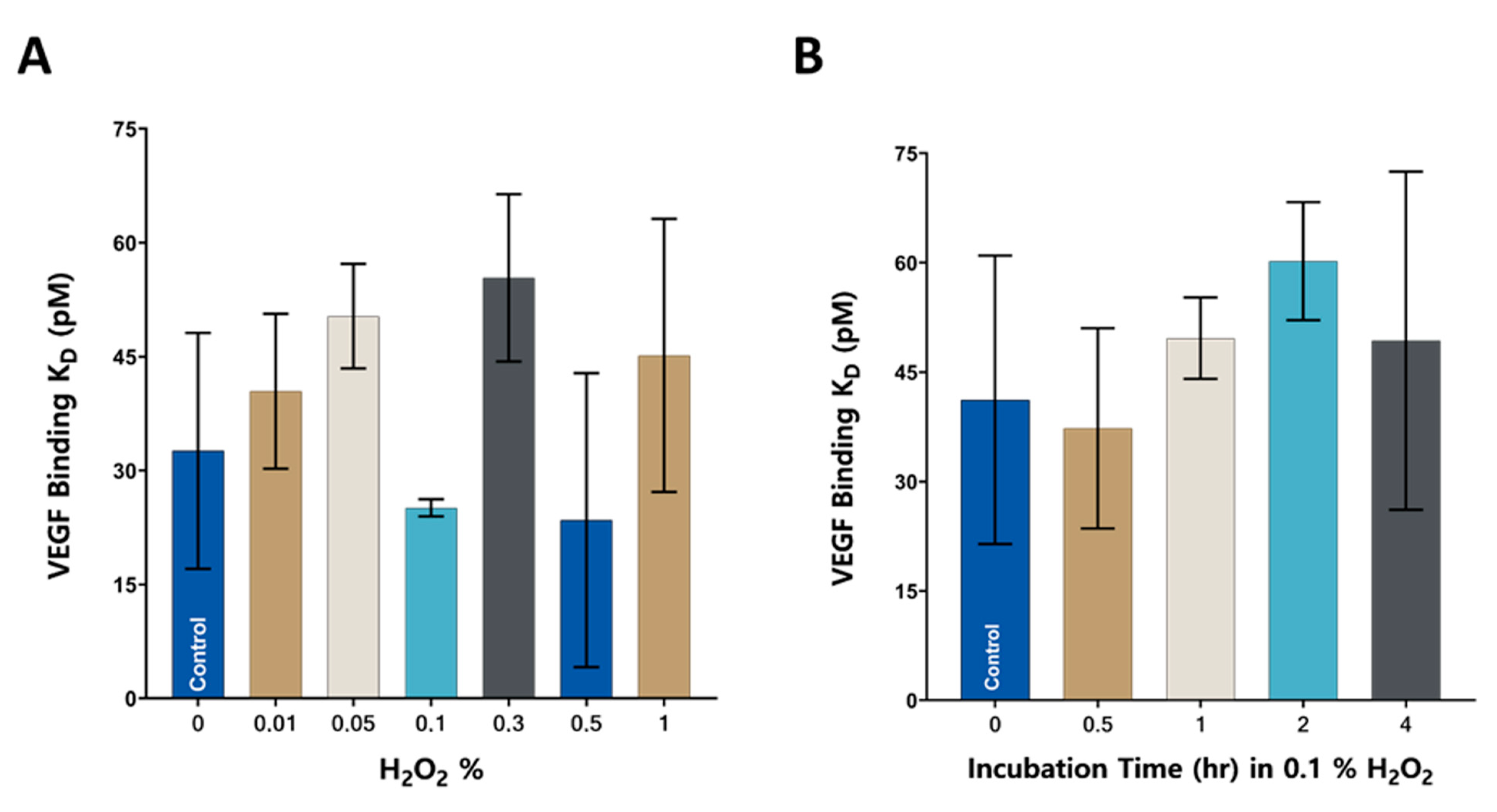
3.4. VEGF Binding Analysis of anti-VEGF IgGs Exposed to Oxidation
3.5. Cell Proliferation Performances of mAbs Exposed to H2O2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, B.F.; Clegg, D.J. Oxygen sensing and metabolic homeostasis. Mol. Cell. Endocrinol. 2014, 397, 51–58. [Google Scholar] [CrossRef]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor Cells Secrete a Vascular Permeability Factor That Promotes Accumulation of Ascites Fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Petrova, T.V. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene 2000, 19, 5598–5605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Jiskoot, W.; Schöneich, C.; Rathore, A.S. Oxidation and Deamidation of Monoclonal Antibody Products: Potential Impact on Stability, Biological Activity, and Efficacy. J. Pharm. Sci. 2022, 111, 903–918. [Google Scholar] [CrossRef]
- Jenkins, N.; Murphy, L.; Tyther, R. Post-translational modifications of recombinant proteins: Significance for biopharmaceuticals. Mol. Biotechnol. 2008, 39, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Wang, Y.; Zhao, J.; Liu, Y.H.; Richardson, D.; Li, H. High throughput peptide mapping method for analysis of site-specific monoclonal antibody oxidation. J. Chromatogr. A 2016, 1460, 51–60. [Google Scholar] [CrossRef]
- Regl, C.; Wohlschlager, T.; Holzmann, J.; Huber, C.G. A Generic HPLC Method for Absolute Quantification of Oxidation in Monoclonal Antibodies and Fc-Fusion Proteins Using UV and MS Detection. Anal. Chem. 2017, 89, 8391–8398. [Google Scholar] [CrossRef]
- Nowak, C.; KCheung, J.; MDellatore, S.; Katiyar, A.; Bhat, R.; Sun, J.; Ponniah, G.; Neill, A.; Mason, B.; Beck, A.; et al. Forced degradation of recombinant monoclonal antibodies: A practical guide. mAbs 2017, 9, 1217–1230. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.P. Forced degradation studies: Current trends and future perspectives for protein-based therapeutics. Expert Rev. Proteom. 2016, 13, 651–658. [Google Scholar] [CrossRef]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Hawe, A.; Wiggenhorn, M.; van de Weert, M.; Garbe, J.H.O.; Mahler, H.C.; Jiskoot, W. Forced degradation of therapeutic proteins. J. Pharm. Sci. 2012, 101, 895–913. [Google Scholar] [CrossRef]
- Vermeer, A.W.P.; Norde, W. The Thermal Stability of Immunoglobulin: Unfolding and Aggregation of a Multi-Domain Protein. Biophys. J. 2000, 78, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.L.; Ala-Nissila, T.; Karttunen, M.; Vattulainen, I.; Grant, M. Microscopic mechanism for cold denaturation. Phys. Rev. Lett. 2008, 100, 118101. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Joubert, M.K.; Stevenson, R.; Ketchem, R.R.; Narhi, L.O.; Wypych, J. Chemical Modifications in Therapeutic Protein Aggregates Generated under Different Stress Conditions. J. Biol. Chem. 2011, 286, 25134–25144. [Google Scholar] [CrossRef] [Green Version]
- Dyck, Y.F.K.; Rehm, D.; Joseph, J.F.; Winkler, K.; Sandig, V.; Jabs, W.; Parr, M.K. Forced Degradation Testing as Complementary Tool for Biosimilarity Assessment. Bioengineering 2019, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Jiskoot, W.; Beuvery, E.C.; de Koning, A.A.M.; Herron, J.N.; Crommelin, D.J.A. Analytical Approaches to the Study of Monoclonal Antibody Stability. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1990, 7, 1234–1241. [Google Scholar] [CrossRef]
- Sharma, D.K.; Oma, P.; Pollo, M.J.; Sukumar, M. Quantification and Characterization of Subvisible Proteinaceous Particles in Opalescent mAb Formulations Using Micro-Flow Imaging. J. Pharm. Sci. 2010, 99, 2628–2642. [Google Scholar] [CrossRef]
- Arosio, P.; Rima, S.; Morbidelli, M. Aggregation Mechanism of an IgG2 and two IgG1 Monoclonal Antibodies at low pH: From Oligomers to Larger Aggregates. Pharm. Res. 2013, 30, 641–654. [Google Scholar] [CrossRef]
- Gaza-Bulseco, G.; Liu, H. Fragmentation of a Recombinant Monoclonal Antibody at Various pH. Pharm. Res. 2008, 25, 1881–1890. [Google Scholar] [CrossRef]
- Cohen, S.L.; Price, C.; Vlasak, J. β-Elimination and Peptide Bond Hydrolysis: Two Distinct Mechanisms of Human IgG1 Hinge Fragmentation upon Storage. J. Am. Chem. Soc. 2007, 129, 6976–6977. [Google Scholar] [CrossRef]
- Cordoba, A.J.; Shyong, B.-J.; Breen, D.; Harris, R.J. Non-enzymatic hinge region fragmentation of antibodies in solution. J. Chromatogr. B 2005, 818, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Lundell, E.; Sun, Z.; Liu, H. Structural effect of a recombinant monoclonal antibody on hinge region peptide bond hydrolysis. J. Chromatogr. B 2007, 858, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Keck, R.G. The Use of t-Butyl Hydroperoxide as a Probe for Methionine Oxidation in Proteins. Anal. Biochem. 1996, 236, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Folzer, E.; Diepold, K.; Bomans, K.; Finkler, C.; Schmidt, R.; Bulau, P.; Huwyler, J.; Mahler, H.C.; Koulov, A.V. Selective Oxidation of Methionine and Tryptophan Residues in a Therapeutic IgG1 Molecule. J. Pharm. Sci. 2015, 104, 2824–2831. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti-Ciarlet, A.; Wang, W.; Lownes, R.; Pristatsky, P.; Fang, Y.; McKelvey, T.; Li, Y.; Li, Y.; Drummond, J.; Prueksaritanont, T.; et al. Impact of methionine oxidation on the binding of human IgG1 to FcRn and Fcγ receptors. Mol. Immunol. 2009, 46, 1878–1882. [Google Scholar] [CrossRef] [PubMed]
- Suárez, I.; Salmerón-García, A.; Cabeza, J.; Capitán-Vallvey, L.F.; Navas, N. Development and use of specific ELISA methods for quantifying the biological activity of bevacizumab, cetuximab and trastuzumab in stability studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1032, 155–164. [Google Scholar] [CrossRef]
- Martínez-Ortega, A.; Herrera, A.; Salmerón-García, A.; Cabeza, J.; Cuadros-Rodríguez, L.; Navas, N. Validated reverse phase HPLC diode array method for the quantification of intact bevacizumab, infliximab and trastuzumab for long-term stability study. Int. J. Biol. Macromol. 2018, 116, 993–1003. [Google Scholar] [CrossRef]
- Burkitt, W.; Domann, P.; O’Connor, G. Conformational changes in oxidatively stressed monoclonal antibodies studied by hydrogen exchange mass spectrometry. Protein Sci. 2010, 19, 826–835. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-J.; Zhang, Y.; Georgescu, S.P.; Johnson, K.L.; Kong, D.; Galper, J.B. Human Umbilical Vein Endothelial Cells and Human Dermal Microvascular Endothelial Cells Offer New Insights Into the Relationship Between Lipid Metabolism and Angiogenesis. Stem Cell Rev. 2006, 2, 93–102. [Google Scholar] [CrossRef]
- Kim, T.K.; Park, C.S.; Jang, J.; Kim, M.R.; Na, H.J.; Lee, K.; Kim, H.J.; Heo, K.; Yoo, B.C.; Kim, Y.M.; et al. Inhibition of VEGF-dependent angiogenesis and tumor angiogenesis by an optimized antibody targeting CLEC14a. Mol. Oncol. 2018, 12, 356–372. [Google Scholar] [CrossRef] [Green Version]
- Gürel, B.; Çapkın, E.; Parlar, A.; Özkan, A.; Çorbacıoğlu, M.; Dağlikoca, D.E.; Yüce, M. Optimized Methods for Analytical and Functional Comparison of Biosimilar mAb Drugs: A Case Study for Avastin, Mvasi, and Zirabev. Sci. Pharm. 2022, 90, 36. [Google Scholar] [CrossRef]
- Maghni, K.; Nicolescu, O.M.; Martin, J.G. Suitability of cell metabolic colorimetric assays for assessment of CD4+ T cell proliferation: Comparison to 5-bromo-2-deoxyuridine (BrdU) ELISA. J. Immunol. Methods 1999, 223, 185–194. [Google Scholar] [CrossRef] [PubMed]
- GE Healthcare Life Sciences. BiacoreTM T200 Instrument Handbook 28-9768-63 Edition AC; GE Healthcare Life Sciences: Uppsala, Sweden, 2013. [Google Scholar]
- de Mol, N.J.; Fischer, M.J.E. Surface Plasmon Resonance. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 627. [Google Scholar] [CrossRef]
- Gurel, B.; Berksoz, M.; Capkin, E.; Parlar, A.; Pala, M.C.; Ozkan, A.; Capan, Y.; Daglikoca, D.E.; Yuce, M. Structural and Functional Analysis of CEX Fractions Collected from a Novel Avastin® Biosimilar Candidate and Its Innovator: A Comparative Study. Pharmaceutics 2022, 14, 1571. [Google Scholar] [CrossRef] [PubMed]
- GE Healthcare. Biacore T200—Getting Started (28-9840-98). cmi.hms.harvard.edu. 2013. Available online: https://cmi.hms.harvard.edu/files/cmi/files/biacoret200_gettingstarted_handbook.pdf (accessed on 30 April 2023).
- Abdiche, Y.N.; Yeung, Y.A.; Chaparro-Riggers, J.; Barman, I.; Strop, P.; Chin, S.M.; Pham, A.; Bolton, G.; McDonough, D.; Lindquist, K.; et al. The Neonatal Fc Receptor (FcRn) Binds Independently to Both Sites of the IgG Homodimer with Identical Affinity. MAbs 2015, 7, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Neuber, T.; Frese, K.; Jaehrling, J.; Jäger, S.; Daubert, D.; Felderer, K.; Linnemann, M.; Höhne, A.; Kaden, S.; Kölln, J.; et al. Characterization and screening of IgG binding to the neonatal Fc receptor. mAbs 2014, 6, 928–942. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; McKay, P.; Yee, L.T.; Dutina, G.; Hass, P.E.; Nijem, I.; Allison, D.; Cowan, K.J.; Lin, K.; Quarmby, V.; et al. Impact of SPR biosensor assay configuration on antibody: Neonatal Fc receptor binding data. mAbs 2017, 9, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Gundry, R.L.; White, M.Y.; Murray, C.I.; Kane, L.A.; Fu, Q.; Stanley, B.A.; Van Eyk, J.E. Preparation of Proteins and Peptides for Mass Spectrometry Analysis in a Bottom-Up Proteomics Workflow. In Current Protocols in Molecular Biology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 1–23. [Google Scholar] [CrossRef] [Green Version]
- Chumsae, C.; Gaza-Bulseco, G.; Sun, J.; Liu, H. Comparison of methionine oxidation in thermal stability and chemically stressed samples of a fully human monoclonal antibody. J. Chromatogr. B 2007, 850, 285–294. [Google Scholar] [CrossRef]
- Liu, H.; Gaza-Bulseco, G.; Xiang, T.; Chumsae, C. Structural effect of deglycosylation and methionine oxidation on a recombinant monoclonal antibody. Mol. Immunol. 2008, 45, 701–708. [Google Scholar] [CrossRef]
- Roberts, G.D.; Johnson, W.P.; Burman, S.; Anumula, K.; Carr, S.A. An Integrated Strategy for Structural Characterization of the Protein and Carbohydrate Components of Monoclonal Antibodies: Application to Anti-Respiratory Syncytial Virus MAb. Anal. Chem. 1995, 67, 3613–3625. [Google Scholar] [CrossRef]
- Shen, F.J.; Kwong, M.Y.; Keck, R.G.; Harris, R.J. The application of tert-butylhydroperoxide oxidation to study sites of potential methionine oxidation in a recombinant antibody. In Techniques in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 1996; pp. 275–284. [Google Scholar] [CrossRef]
- Kroon, D.J.; Baldwin-Ferro, A.; Lalan, P. Identification of Sites of Degradation in a Therapeutic Monoclonal Antibody by Peptide Mapping. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1992, 9, 1386–1393. [Google Scholar] [CrossRef]
- Lam, X.M.; Yang, J.Y.; Cleland, J.L. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J. Pharm. Sci. 1997, 86, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Ambrogelly, A.; Gozo, S.; Katiyar, A.; Dellatore, S.; Kune, Y.; Bhat, R.; Sun, J.; Li, N.; Wang, D.; Nowak, C.; et al. Analytical comparability study of recombinant monoclonal antibody therapeutics. mAbs 2018, 10, 513–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ionescu, R.; Peekhaus, N.; Leung, J.; Ha, S.; Vlasak, J. Separation of post-translational modifications in monoclonal antibodies by exploiting subtle conformational changes under mildly acidic conditions. J. Chromatogr. A 2010, 1217, 6496–6502. [Google Scholar] [CrossRef]
- Martin, W.L.; West, A.P.; Gan, L.; Bjorkman, P.J. Crystal structure at 2.8 Å of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Mol. Cell 2001, 7, 867–877. [Google Scholar] [CrossRef]
- Lam, X.M.; Lai, W.G.; Chan, E.K.; Ling, V.; Hsu, C.C. Site-Specific Tryptophan Oxidation Induced by Autocatalytic Reaction of Polysorbate 20 in Protein Formulation. Pharm. Res. 2011, 28, 2543–2555. [Google Scholar] [CrossRef]
- Wang, W.; Lu, P.; Fang, Y.; Hamuro, L.; Pittman, T.; Carr, B.; Hochman, J.; Prueksaritanont, T. Monoclonal Antibodies with Identical Fc Sequences Can Bind to FcRn Differentially with Pharmacokinetic Consequences. Drug Metab. Dispos. 2011, 39, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vlasak, J.; Li, Y.; Pristatsky, P.; Fang, Y.; Pittman, T.; Roman, J.; Wang, Y.; Prueksaritanont, T.; Ionescu, R. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol. Immunol. 2011, 48, 860–866. [Google Scholar] [CrossRef]
- Haberger, M.; Bomans, K.; Diepold, K.; Hook, M.; Gassner, J.; Schlothauer, T.; Zwick, A.; Spick, C.; Kepert, J.F.; Hienz, B.; et al. Assessment of chemical modifications of sites in the CDRs of recombinant antibodies. mAbs 2014, 6, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Feng, J.; Lin, H.Y.; Mullapudi, S.; Bishop, E.; Tous, G.I.; Casas-Finet, J.; Hakki, F.; Strouse, R.; Schenerman, M.A. Identification of a Single Tryptophan Residue as Critical for Binding Activity in a Humanized Monoclonal Antibody against Respiratory Syncytial Virus. Anal. Chem. 2007, 79, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Hensel, M.; Steurer, R.; Fichtl, J.; Elger, C.; Wedekind, F.; Petzold, A.; Schlothauer, T.; Molhoj, M.; Reusch, D.; Bulau, P. Identification of Potential Sites for Tryptophan Oxidation in Recombinant Antibodies Using tert-Butylhydroperoxide and Quantitative LC-MS. PLoS ONE 2011, 6, e17708. [Google Scholar] [CrossRef] [Green Version]
- Boyd, D.; Kaschak, T.; Yan, B. HIC resolution of an IgG1 with an oxidized Trp in a complementarity determining region. J. Chromatogr. B 2011, 879, 955–960. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, H.; Fu, Y.; Jusuf, S.; Zeng, M.; Ludwig, R.; Krystek, S.R., Jr.; Chen, G.; Tao, L.; Das, T.K. Isomerization and Oxidation in the Complementarity-Determining Regions of a Monoclonal Antibody: A Study of the Modification-Structure-Function Correlations by Hydrogen-Deuterium Exchange Mass Spectrometry. Anal. Chem. 2016, 88, 2041–2050. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.; Yun, J.; Park, S.J. Evaluation of the Physico-Chemical and Biological Stability of SB8 (Aybintio), a Proposed Biosimilar to Bevacizumab, Under Ambient and In-Use Conditions. Adv. Ther. 2020, 37, 4308–4324. [Google Scholar] [CrossRef]
- Gaza-Bulseco, G.; Faldu, S.; Hurkmans, K.; Chumsae, C.; Liu, H. Effect of methionine oxidation of a recombinant monoclonal antibody on the binding affinity to protein A and protein G. J. Chromatogr. B 2008, 870, 55–62. [Google Scholar] [CrossRef]
- Hong, J.; Lee, Y.; Lee, C.; Eo, S.; Kim, S.; Lee, N.; Park, J.; Park, S.; Seo, D.; Jeong, M.; et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade®(infliximab). mAbs 2017, 9, 364–382. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parlar, A.; Gurel, B.; Sönmez, M.R.; Yüce, M. Analytical Investigation of Forced Oxidized Anti-VEGF IgG Molecules: A Focus on the Alterations in Antigen and Receptor Binding Activities. Sci. Pharm. 2023, 91, 31. https://doi.org/10.3390/scipharm91030031
Parlar A, Gurel B, Sönmez MR, Yüce M. Analytical Investigation of Forced Oxidized Anti-VEGF IgG Molecules: A Focus on the Alterations in Antigen and Receptor Binding Activities. Scientia Pharmaceutica. 2023; 91(3):31. https://doi.org/10.3390/scipharm91030031
Chicago/Turabian StyleParlar, Ayhan, Busra Gurel, Mehmet Reşit Sönmez, and Meral Yüce. 2023. "Analytical Investigation of Forced Oxidized Anti-VEGF IgG Molecules: A Focus on the Alterations in Antigen and Receptor Binding Activities" Scientia Pharmaceutica 91, no. 3: 31. https://doi.org/10.3390/scipharm91030031





