13C-Metabolic Flux Analysis in Developing Flax (Linum usitatissinum L.) Embryos to Understand Storage Lipid Biosynthesis
Abstract
1. Introduction
2. Results
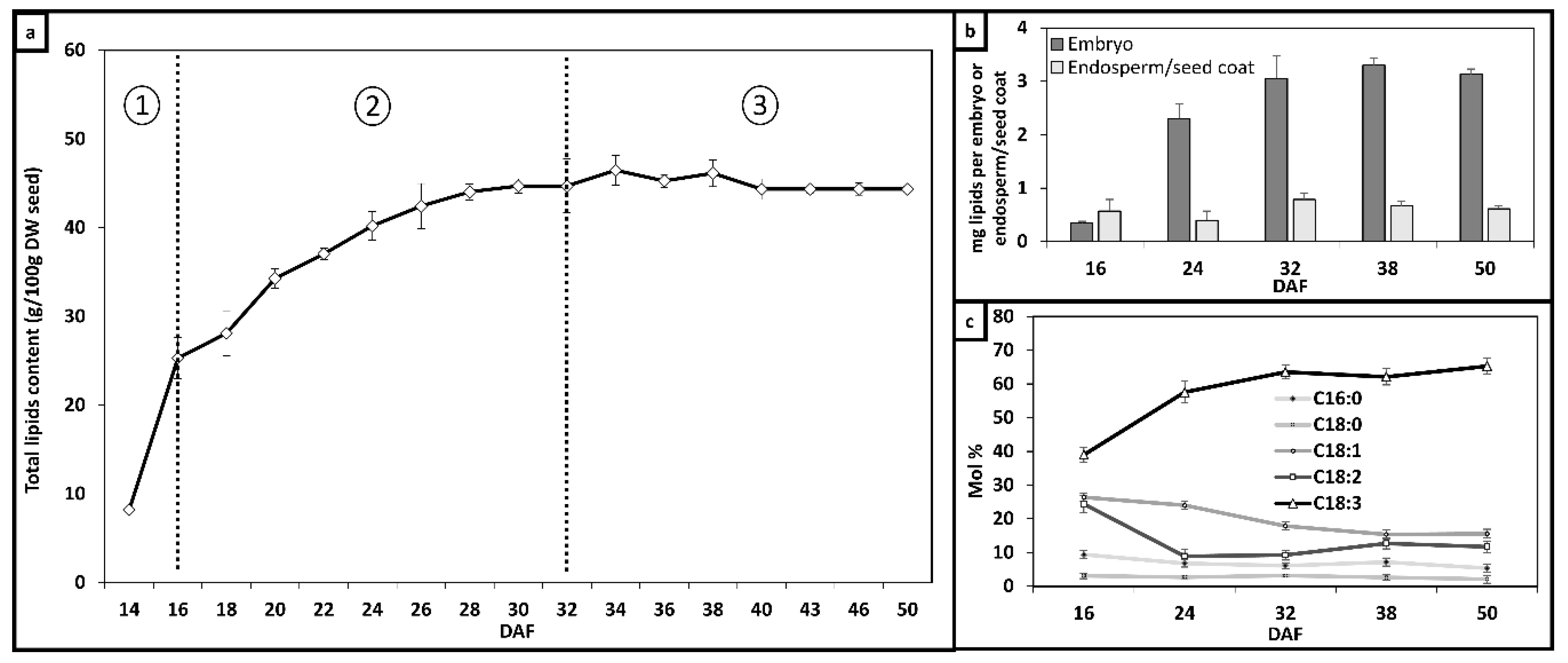
2.1. Optimal Growth Conditions
2.2. In Vitro Culture of Flax Embryos
2.3. Metabolic and Isotopic Steady State
2.4. Metabolic Model Construction
2.5. 13C Metabolic Flux Analysis
2.5.1. Source of Carbon for Fatty Acids Synthesis
2.5.2. Source of Cofactors for Storage Lipid Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Plant Material
4.3. In Vitro Embryo Culture
4.4. Biomass Extraction and Quantification
4.4.1. Lipids and Fatty Acid Analysis
4.4.2. Proteins and Amino Acid Concentration
4.4.3. Starch and Cell Wall
4.5. Metabolites Extraction
4.6. Isotopic Enrichment of Metabolites
4.7. Modeling Metabolic Pathways
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Woodfield, H.K.; Pan, X.; Harwood, J.L.; Weselake, R.J. Acyl-trafficking during plant oil accumulation. Lipids 2015, 50, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Ponce, M.A.; Kilaru, A.; Cao, X.; Durrett, T.P.; Fan, J.; Jensen, J.K.; Thrower, N.A.; Pauly, M.; Wilkerson, C.; Ohlrogge, J.B. Comparative deep transcriptional profiling of four developing oilseeds. Plant J. 2011, 68, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- McKeon, T.A.; Hayes, D.G.; Hildebrand, D.F.; Weselake, R.J. Introduction to industrial oil crops. In Industrial Oil Crops; AOCS Press: Urbana, IL, USA, 2016; Volume 1, pp. 1–13. [Google Scholar]
- Zafar, S.; Li, Y.L.; Li, N.N.; Zhu, K.E.; Tan, X.L. Recent advances in enhancement of oil content in oilseed crops. J. Biotechnol. 2019, 10, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.M.; Mullen, R.T. Engineering plant oils as high-value industrial feedstocks for biorefining: The need for underpinning cell biology research. Physiol. Plant 2008, 132, 11–22. [Google Scholar] [CrossRef]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Thrower, N.; Mhaske, V.; Stymne, S.; Baxter, M.; Yang, W.; Liu, J.; Shaw, K.; Shorrosh, B.; Zhang, M.; et al. PlantFAdb: A resource for exploring hundreds of plant fatty acid structures synthesized by thousands of plants and their phylogenetic relationships. Plant J. 2018, 96, 1299–1308. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Spencer, S.J.; Koroi, A.; Layé, S.; Shukitt-Hale, B.; Barrientos, R.M. Food for thought: How nutrition impacts cognition and emotion. NPJ Sci. Food 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Jhala, J.A.; Hall, M.L. Flax (Linum usitatissimum L.): Current Uses and Future Applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Venglat, P.; Xiang, D.; Qiu, Q.; Stone, L.S.; Tibiche, C.; Cram, D.; Alting-Mees, M.; Nowak, J.; Cloutier, S.; Deyholos, M.; et al. Gene expression analysis of flax seed development. BMC Plant Biol. 2011, 11, 11–17. [Google Scholar] [CrossRef]
- Basch, E.; Mphil, D.; Bent, S.; Collins, J.; Dacey, C.; Hammerness, P.; Harrisson, M.; Smith, M.; Szapary, P.; Ulbritcht, C.; et al. Flax and flaxseed oil (Linum usitatissimum): A review by the Natural Standard Research Collaboration. J. Soc. Int. Oncol. 2007, 5, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Martin, T.M.; Shachar-Hill, Y. Lipid analysis of developing Camelina sativa seeds and cultured embryos. Phytochemistry 2015, 118, 23–32. [Google Scholar] [PubMed]
- Ramli, U.S.; Salas, J.J.; Quant, P.A.; Harwood, J.L. Use of metabolic control analysis to give quantitative information on control of lipid biosynthesis in the important oil crop, Elaeis guineensis (oilpalm). New Phytol. 2009, 184, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.; Lauterbach, C.; Sauer, N. Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol. 2005, 139, 701–712. [Google Scholar]
- Yadav, U.P.; Ayre, B.G.; Bush, D.R. Transgenic approaches to altering carbon and nitrogen partitioning in whole plants: Assessing the potential to improve crop yields and nutritional quality. Front. Plant Sci. 2005, 6, 1–13. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Baud, S.; Lepiniec, L. Physiological and developmental regulation of seed oil production. Prog. Lipid Res. 2010, 49, 235–249. [Google Scholar] [CrossRef]
- Baud, S.; Boutin, J.P.; Miquel, M.; Lepiniec, L.; Rochat, C. An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol. Biochem. 2002, 40, 151–160. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. In Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2013; Volume 2013, pp. 1–65. [Google Scholar]
- Barvkar, V.T.; Pardeshi, V.C.; Kale, S.M.; Kadoo, N.Y.; Giri, A.P.; Gupta, V.S. Proteome profiling of flax (Linum usitatissimum) seed: Characterization of functional metabolic pathways operating during seed development. J. Proteome Res. 2012, 11, 6264–7626. [Google Scholar] [CrossRef]
- Klubicová, K.; Danchenko, M.; Skultety, L.; Berezhna, V.V.; Rashydov, N.M.; Hajduch, M. Radioactive Chernobyl environment has produced high-oil flax seeds that show proteome alterations related to carbon metabolism during seed development. J. Proteome Res. 2013, 12, 4799–4806. [Google Scholar] [CrossRef]
- Schwender, J.; Ohlrogge, J.B. Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos. Plant Physiol. 2002, 130, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Flores-Tornero, M.; Anoman, A.D.; Rosa-Tellez, S.; Toujani, W.; Weber, A.P.; Eisenhut, M.; Kurz, S.; Alseekh, S.; Fernie, A.R.; Munoz-Bertomeu, J.; et al. Overexpression of the triose phosphate translocator (TPT) complements the abnormal metabolism and development of plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase mutants. Plant J. 2017, 89, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Schwender, J.; Ohlrogge, J.B.; Shachar-Hill, Y. A flux model of glycolysis and the oxidative pentosephosphate pathway in developing Brassica napus embryos. J. Biol. Chem. 2003, 278, 29442–29453. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.P.; Dale, V.L.; Shachar-Hill, Y. Understanding fatty acid synthesis in developing maize embryos using metabolic flux analysis. Metab. Eng. 2010, 12, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.P.; Goffman, F.D.; Ohlrogge, J.B.; Shachar-Hill, Y. Carbon conversion efficiency and central metabolic fluxes in developing sunflower (Helianthus annuus L.) embryos. Plant J. 2007, 52, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Schwender, J.; Goffman, F.; Ohlrogge, J.B.; Shachar-Hill, Y. Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds. Nature 2004, 432, 779–782. [Google Scholar] [CrossRef]
- Schwender, J.; Shachar-Hill, Y.; Ohlrogge, J.B. Mitochondrial metabolism in developing embryos of Brassica napus. J. Biol. Chem. 2006, 281, 34040–34047. [Google Scholar] [CrossRef]
- Lonien, J.; Schwender, J. Analysis of metabolic flux phenotypes for two Arabidopsis mutants with severe impairment in seed storage lipid synthesis. Plant Physiol. 2009, 151, 1617–1634. [Google Scholar] [CrossRef]
- Benard, C.; Acket, S.; Rossez, Y.; Fernandez, O.; Berton, T.; Gibon, Y.; Cabasson, C. Untargeted analysis of semipolar compounds by LC-MS and targeted analysis of fatty acids by GC-MS/GC-FID: From plant cultivation to extract preparation. In Plant Metabolomics; Antonio, C., Ed.; Method in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1778, pp. 101–124. [Google Scholar]
- Acket, S.; Degournay, A.; Gosset, M.; Merlier, F.; Troncoso-Ponce, M.A.; Thomasset, B. Analysis of 13C labeling amino acids by capillary electrophoresis—High resolution mass spectrometry in developing flaxseed. Anal. Biochem. 2018, 547, 14–18. [Google Scholar] [CrossRef]
- Jazmin, L.J.; O’Grady, J.P.; Ma, F.; Allen, D.K.; Morgan, J.A.; Young, J.D. Isotopically Nonstationary MFA (INST-MFA) of Autotrophic Metabolism. In Plant Metabolic Flux Analysis; Dieuaide-Noubhani, M., Alonso, A., Eds.; Humana Press: Totowa, NJ, USA, 2014; Volume 1090, pp. 181–210. [Google Scholar]
- Acket, S.; Degournay, A.; Merlier, F.; Thomasset, B. 13C labeling analysis of sugars by high resolution-mass spectrometry for Metabolic Flux Analysis. Anal. Biochem. 2017, 527, 45–48. [Google Scholar] [CrossRef]
- Wang, Z.; Hobson, N.; Galindo, L.; Zhu, S.; Shi, D.; McDill, J.; Yang, L.; Hawkins, S.; Neutelings, G.; Datla, R.; et al. The genome of flax (Linum usitatissimum) assembled de novo from short shotgun sequence reads. Plant J. 2012, 72, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Fenart, S.; Assoumou Ndong, Y.-P.; Duarte, J.; Rivière, N.; Wilmer, J.; Van Wuytswinkel, O.; Lucau, A.; Cariou, E.; Neutelings, G.; Gutierrez, L.; et al. Development and validation of a flax (Linum usitatissimum L.) gene expression oligo microarray. BMC Genom. 2010, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fenart, S.; Chabi, M.; Gallina, S.; Huis, R.; Neutelings, G.; Riviere, N.; Thomasset, B.; Hawkins, S.; Lucau-Danila, A. Intra-platform comparison of 25-mer and 60-mer oligonucleotide Nimblegen DNA microarrays. BMC Res. Notes 2013, 6, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Troufflard, S. Study of Carbon Metabolism in Linseed Developing Embryos during Lipid Storage Accumulation. Ph.D. Thesis, University of Picardie Jules Verne, Amiens, France, 2004. Available online: http://www.theses.fr/2004AMIE0419 (accessed on 1 January 2014).
- Young, J.D. INCA: A computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics 2014, 30, 1333–1335. [Google Scholar] [CrossRef]
- Leighty, R.W.; Antoniewicz, M.R. COMPLETE-MFA: Complementary parallel labeling experiments technique for metabolic flux analysis. Metab. Eng. 2013, 20, 49–55. [Google Scholar] [CrossRef]
- Droste, P.; Nöh, K.; Wiechert, W. Omix—A visualization tool for metabolic networks with highest usability and customizability in focus. Chemie Ingenieur Technik 2013, 85, 849–862. [Google Scholar] [CrossRef]
- Schwender, J.; Hay, J.O. Predictive modeling of biomass component tradeoffs in Brassica napus developing oilseeds based on in silico manipulation of storage metabolism. Plant Physiol. 2012, 160, 1218–1236. [Google Scholar] [CrossRef]
- Cocuron, J.C.; Koubaa, M.; Kimmelfield, R.; Ross, Z.; Alonso, A.P. A combined metabolomics and fluxomics analysis identifies steps limiting oil synthesis in maize embryos. Plant Physiol. 2019, 181, 961–975. [Google Scholar] [CrossRef]
- O’Grady, J.; Schwender, J.; Shachar-Hill, Y.; Morgan, J.A. Metabolic cartography: Experimental quantification of metabolic fluxes from isotopic labeling studies. J. Exp. Bot. 2012, 63, 2293–2308. [Google Scholar] [CrossRef]
- Goffman, F.D.; Alonso, A.P.; Schwender, J.; Shachar-Hill, Y.; Ohlrogge, J.B. Light enables a very high efficiency of carbon storage in developing embryos of rapeseed. Plant Physiol. 2005, 138, 2269–2279. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Acket, S.; Degournay, A.; Merlier, F.; Thomasset, B. Data documenting the comparison between the theoretically expected values of free sugars mass isotopomer composition with standards using GC-MS and LC-HRMS for Metabolic Flux Analysis. Data Brief 2017, 12, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Young, J.D.; Walther, J.L.; Antoniewicz, M.R.; Yoo, H.; Stephanopoulos, G. An Elementary Metabolite Unit (EMU) based method of isotopically nonstationary flux analysis. Biotechnol. Bioeng. 2008, 99, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Antoniewicz, M.R.; Kelleher, J.K.; Stephanopoulos, G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab. Eng. 2006, 8, 324–337. [Google Scholar] [CrossRef]




| Reactions | Flax Embryos Astral | Rapeseed Embryos Reston [25,28,29] | A. thaliana Embryos ws, pkp, col, wri [30] | Sunflower Embryos Ames 7576 [27] | Maïze Embryos LH 59 [28] | Maïze Embryos Alex [43] | |
|---|---|---|---|---|---|---|---|
| Carbon source for PYR.p (mol%) | PEP -> PYR.p | 99.50% | 74% | 73% to 88% | 93% | 70% | 54% |
| MAL -> PYR.p | 0.50% | NC | 2% | 7% | 30% | 46% | |
| PYR.c -> PYR.p | NC | 26% | 10% to 25% | NC | NC | NC | |
| Source of NADPH relative to demand for fatty acids biosynthesis (mol%) | by OPPP | 187% | 38% | - | 106% | 76% | 74% |
| by malic enzyme | 0.55% | NC | - | 7% | 30% | 56% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acket, S.; Degournay, A.; Rossez, Y.; Mottelet, S.; Villon, P.; Troncoso-Ponce, A.; Thomasset, B. 13C-Metabolic Flux Analysis in Developing Flax (Linum usitatissinum L.) Embryos to Understand Storage Lipid Biosynthesis. Metabolites 2020, 10, 14. https://doi.org/10.3390/metabo10010014
Acket S, Degournay A, Rossez Y, Mottelet S, Villon P, Troncoso-Ponce A, Thomasset B. 13C-Metabolic Flux Analysis in Developing Flax (Linum usitatissinum L.) Embryos to Understand Storage Lipid Biosynthesis. Metabolites. 2020; 10(1):14. https://doi.org/10.3390/metabo10010014
Chicago/Turabian StyleAcket, Sébastien, Anthony Degournay, Yannick Rossez, Stéphane Mottelet, Pierre Villon, Adrian Troncoso-Ponce, and Brigitte Thomasset. 2020. "13C-Metabolic Flux Analysis in Developing Flax (Linum usitatissinum L.) Embryos to Understand Storage Lipid Biosynthesis" Metabolites 10, no. 1: 14. https://doi.org/10.3390/metabo10010014
APA StyleAcket, S., Degournay, A., Rossez, Y., Mottelet, S., Villon, P., Troncoso-Ponce, A., & Thomasset, B. (2020). 13C-Metabolic Flux Analysis in Developing Flax (Linum usitatissinum L.) Embryos to Understand Storage Lipid Biosynthesis. Metabolites, 10(1), 14. https://doi.org/10.3390/metabo10010014








