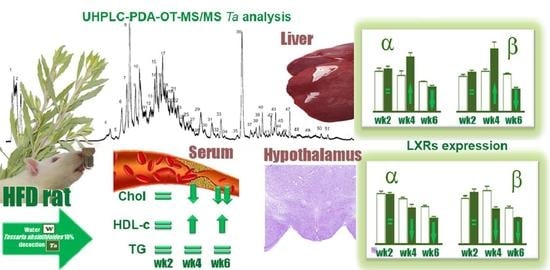
Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) Decoction Improves the Hypercholesterolemia and Alters the Expression of LXRs in Rat Liver and Hypothalamus
Abstract
:1. Introduction
2. Results
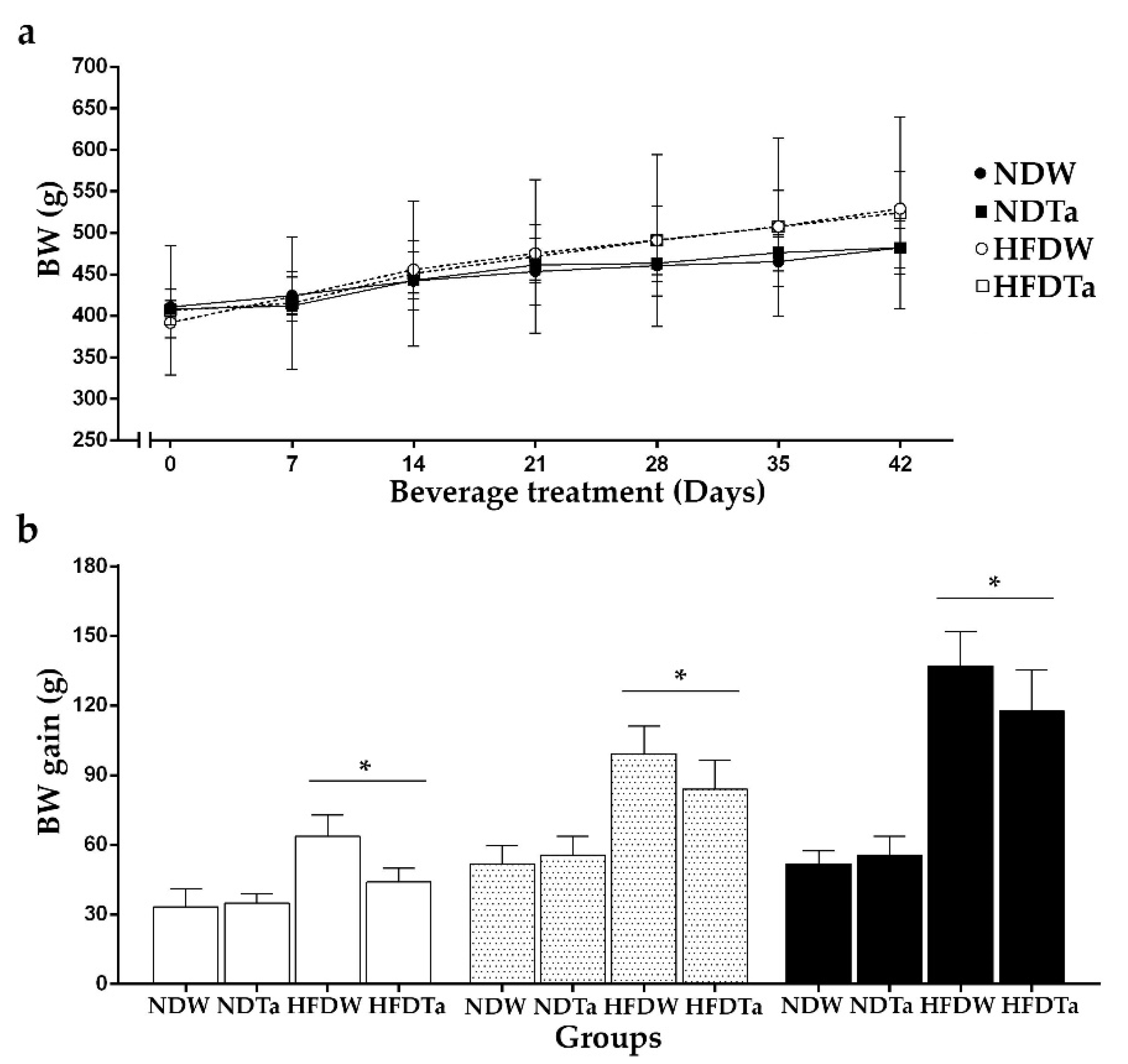
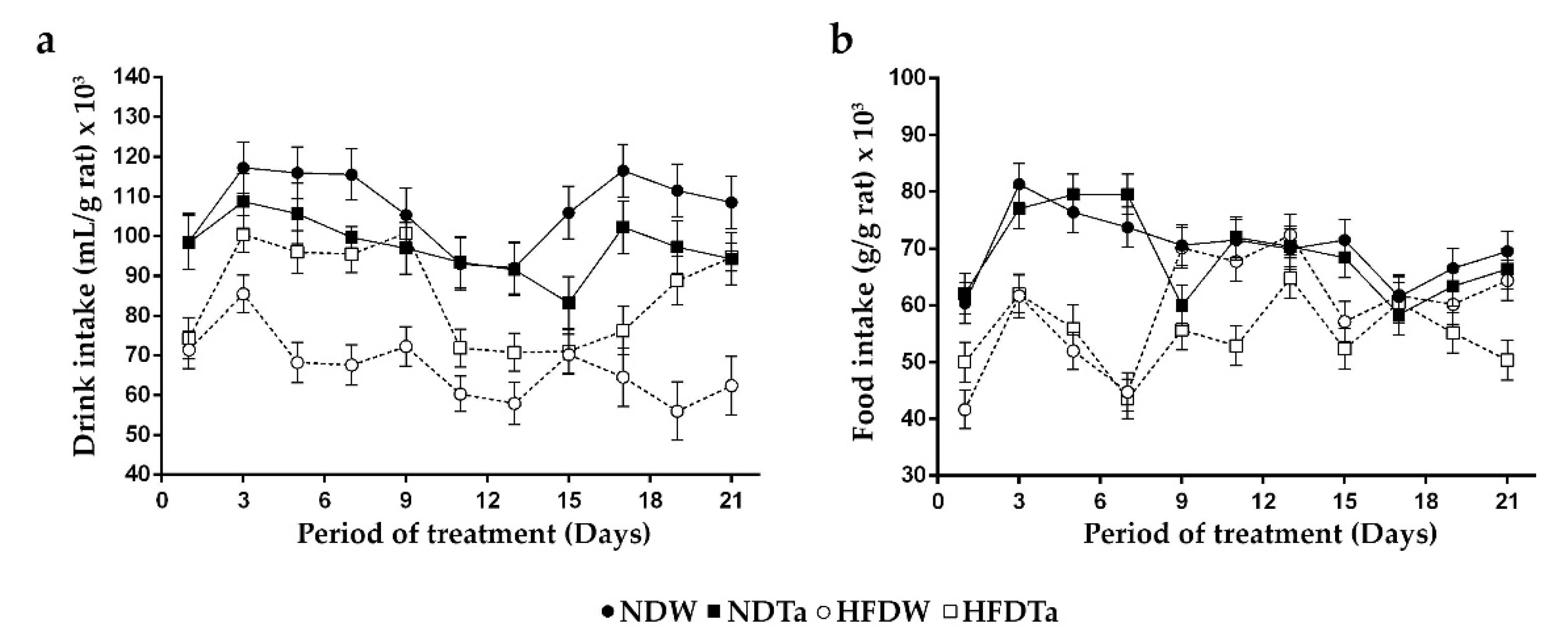
2.1. Body Weight (BW), BW Gain, and the Intake of Beverage and Food
2.2. Levels of TC, HDL-c, and TG
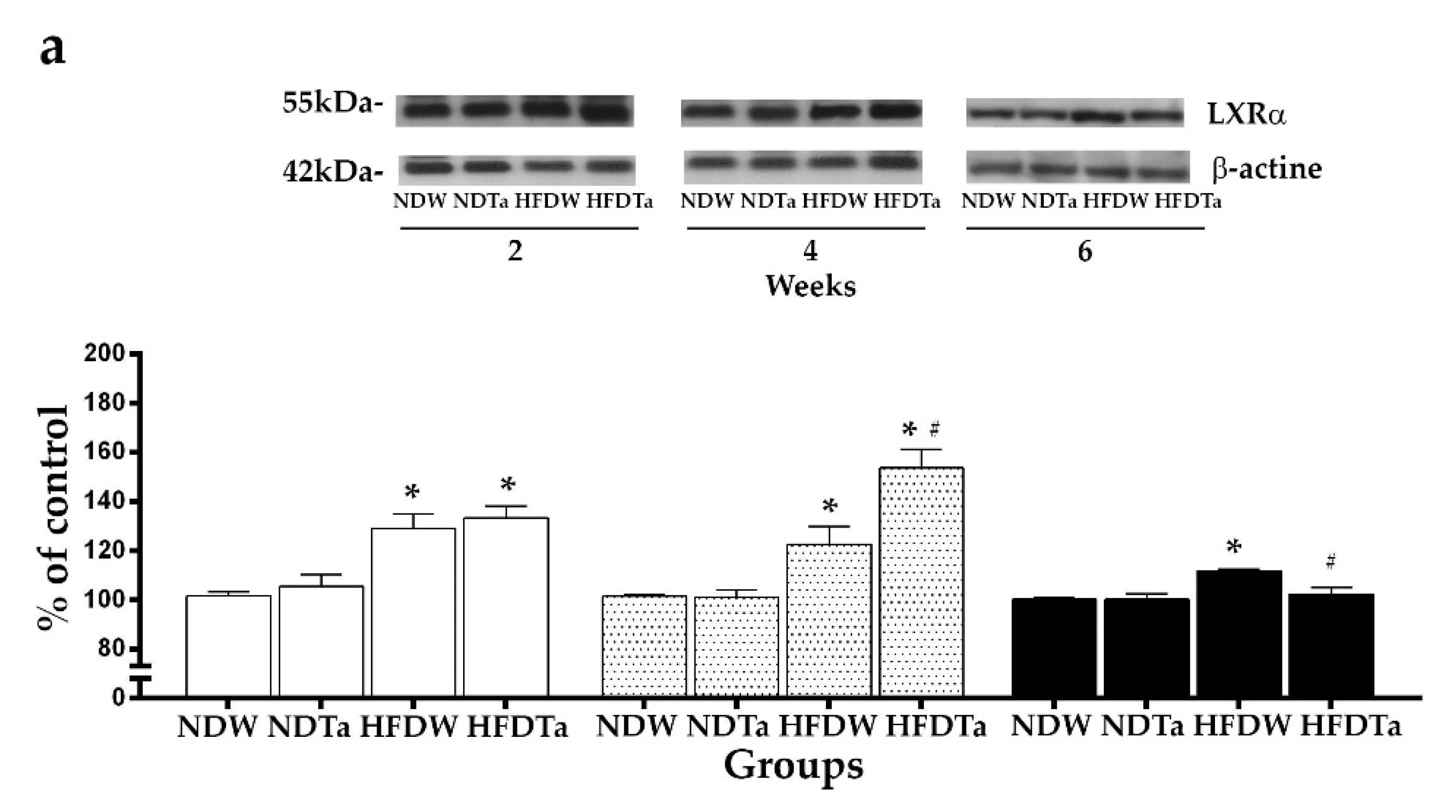
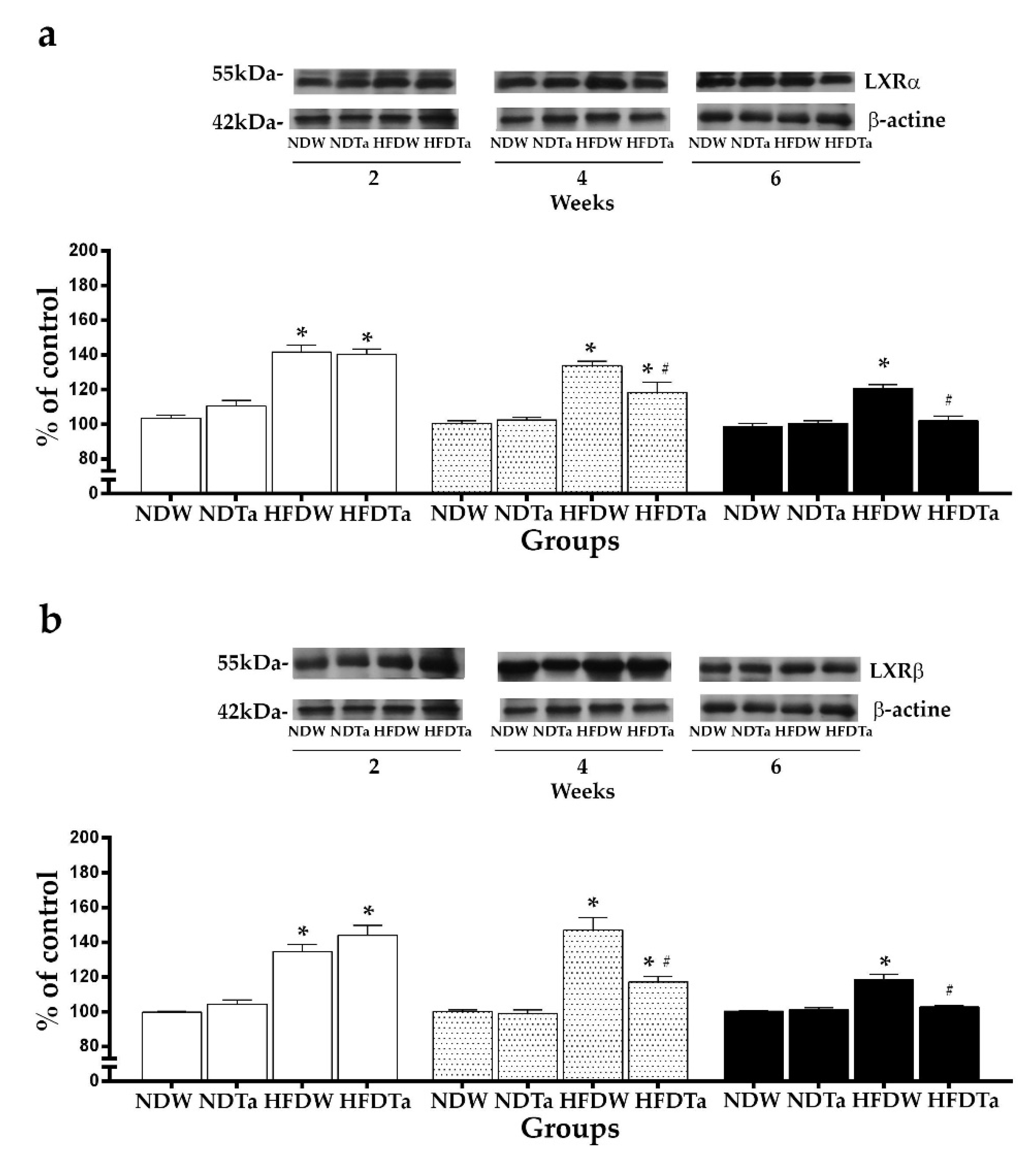
2.3. The LXRs Expression
2.4. UHPLC-OT Analysis of Ta
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of the Decoction of Tessaria absinthioides
4.3. Ultrahigh Resolution Liquid Chromatography Orbitrap MS Analysis UHPLC–PDA–OT-MS/MS of Ta
4.3.1. UHPLC-DAD-MS Instrument
4.3.2. Determination of Total Phenolics and Flavonoids Content
4.4. Animals, Diets, and Experimental Procedure
4.5. Lipid Profile
4.6. Determination of Protein Expression
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghibaudi, L.; Cook, J.; Farley, C.; Van Heek, M.; Hwa, J.J. Fat intake affects adiposity, comorbidity factors, and energy metabolism of Sprague-Dawley rats. Obes. Res. 2002, 10, 956–963. [Google Scholar] [CrossRef]
- Rey, M.; Kruse, M.S.; Magrini-Huamán, R.N.; Coirini, H. High-Fat Diets and LXRs Expression in Rat Liver and Hypothalamus. Cell. Mol. Neurobiol. 2019, 39, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, M. Biological role of liver X receptors. J. Physiol. Pharmacol. 2008, 59, 31–55. [Google Scholar] [PubMed]
- Zelcer, N.; Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqdasy, S.; Trousson, A.; Tauveron, I.; Volle, D.H.; Baron, S.; Lobaccaro, J.-M.A. Once and for all, LXRα and LXRβ are gatekeepers of the endocrine system. Mol. Asp. Med. 2016, 49, 31–46. [Google Scholar] [CrossRef]
- Ulven, S.M.; Dalen, K.T.; Gustafsson, J.Å.; Nebb, H.I. LXR is crucial in lipid metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 59–63. [Google Scholar] [CrossRef]
- Steffensen, K.R.; Nilsson, M.; Schuster, G.U.; Stulnig, T.M.; Dahlman-Wright, K.; Gustafsson, J.Å. Gene expression profiling in adipose tissue indicates different transcriptional mechanisms of liver X receptors α and β, respectively. Biochem. Biophys. Res. Commun. 2003, 310, 589–593. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fan, X.; Gabbi, C.; Yakimchuk, K.; Parini, P.; Warner, M.; Gustafsson, J.-Å. Liver X receptor (LXR): A link between-sitosterol and amyotrophic lateral sclerosis-Parkinson’s dementia. Proc. Natl. Acad. Sci. USA 2008, 105, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Whitney, K.D.; Watson, M.A.; Collins, J.L.; Benson, W.G.; Stone, T.M.; Numerick, M.J.; Tippin, T.K.; Wilson, J.G.; Winegar, D.A.; Kliewer, S.A. Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Mol. Endocrinol. 2002, 16, 1378–1385. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clín. 2018, 150, 398–402. [Google Scholar]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef] [PubMed]
- Rideout, T.C.; Harding, S.V.; Marinangeli, C.P.F.; Jones, P.J.H. Combination drug-diet therapies for dyslipidemia. Transl. Res. 2010, 155, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, C. Natural modulators of liver X receptors. J. Integr. Med. 2014, 12, 76–85. [Google Scholar] [CrossRef]
- Komati, R.; Spadoni, D.; Zheng, S.; Sridhar, J.; Riley, K.E.; Wang, G. Ligands of therapeutic utility for the liver X Receptors. Molecules 2017, 22, 88. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, C.; Wu, H.; Sheng, L.; Su, Y.; Zhang, X.; Luan, H.; Sun, G.; Sun, X.; Tian, Y.; et al. Anti-Hyperlipidemic Effects and Potential Mechanisms of Action of the Caffeoylquinic Acid-Rich Pandanus tectorius Fruit Extract in Hamsters Fed a High Fat-Diet. PLoS ONE 2013, 8, e61922. [Google Scholar] [CrossRef] [Green Version]
- Valle, I.F.D.; Roweth, H.G.; Malloy, M.W.; Moco, S.; Barron, D.; Battinelli, E.; Loscalzo, J.; Barabási, A.-L. Network medicine framework shows that proximity of polyphenol targets and disease proteins predicts therapeutic effects of polyphenols. Nat. Food 2021, 2, 143–155. [Google Scholar] [CrossRef]
- Fouache, A.; Zabaiou, N.; De Joussineau, C.; Morel, L.; Silvente-Poirot, S.; Namsi, A.; Lizard, G.; Poirot, M.; Makishima, M.; Baron, S.; et al. Flavonoids differentially modulate liver X receptors activity-Structure-function relationship analysis. J. Steroid Biochem. Mol. Biol. 2019, 190, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Krečman, V.; Škottová, N.; Walterová, D.; Ulrichová, J.; Šimének, V. Silymarin inhibits the development of diet-induced hypercholesterolemia in rats. Planta Med. 1998, 64, 138–142. [Google Scholar] [CrossRef]
- Campos-Navarro, R.; Scarpa, G.F. The cultural-bound disease “empacho” in Argentina. A comprehensive botanico-historical and ethnopharmacological review. J. Ethnopharmacol. 2013, 148, 349–360. [Google Scholar] [CrossRef]
- Kurina Sanz, M.B.; Donadel, O.J.; Rossomando, P.C.; Reyes, P.R.; Tonn, C.E.; Guerreiro, E. Sesquiterpenes from Tessaria absinthioides. Phytochemistry 1997, 44, 897–900. [Google Scholar] [CrossRef]
- Barboza, G.E.; Cantero, J.J.; Núñez, C.; Pacciaroni, A.; Ariza Espinar, L. Medicinal plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora. Kurtziana 2009, 34, 7–365. [Google Scholar]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Gamarra-Luques, C.; Bórquez, J.; Caballero, D.; Feresin, G.E.; Tapia, A. UHPLC–Q/Orbitrap/MS/MS fingerprinting, free radical scavenging, and antimicrobial activity of tessaria absinthiodes (Hook. & arn.) DC. (asteraceae) lyophilized decoction from Argentina and Chile. Antioxidants 2019, 8, 593. [Google Scholar]
- Ono, M.; Masuoka, C.; Odake, Y.; Ikegashira, S.; Ito, Y.; Nohara, T. Antioxidative Constituents from Tessaria integrifolia. J. Food Sci. Technol. 2000, 6, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Peluso, G.; De Feo, V.; De Simone, F.; Bresciano, E.; Vuotto, M.L. Studies on the inhibitory effects of caffeoylquinic acids on monocyte migration and superoxide ion production. J. Nat. Prod. 1995, 58, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Romio, E.; Gurni, A. Estudio micrográfico preliminar de las estructuras foliares de dos especies palustres americanas con pontencil actividad antiviral. Lat. Am. Caribb. Bull. Med. Aromat. Plants 2007, 6, 219–220. [Google Scholar]
- Vivot, E.; Massa, R.; Cruañes, M.J.; Muñoz, J.D.D.; Ferraro, G.; Gutkind, G.; Martino, V. Actividad antimicrobiana in vitro de seis especies autóctonas de la Flora de Entre Ríos (Argentina). Lat. Am. J. Pharm. 2007, 26, 563–566. [Google Scholar]
- Persia, F.A.; Rinaldini, E.; Carrión, A.; Hapon, M.B.; Gamarra-Luques, C.D. Evaluation of cytotoxic and antitumoral properties of Tessaria absinthioides (hook & arn) dc, “pájaro bobo”, aqueous extract. Medicina 2017, 77, 283–290. [Google Scholar]
- Torres Carro, R.; Isla, M.I.; Ríos, J.L.; Giner, R.M.; Alberto, M.R. Anti-inflammatory properties of hydroalcoholic extracts of Argentine Puna plants. Food Res. Int. 2015, 67, 230–237. [Google Scholar] [CrossRef]
- Ahmad, U.; Ahmad, R.S.; Arshad, M.S.; Mushtaq, Z.; Hussain, S.M.; Hameed, A. Antihyperlipidemic efficacy of aqueous extract of Stevia rebaudiana Bertoni in albino rats. Lipids Health Dis. 2018, 17, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Al-Jubouri, H.H.F.; Al-Jalil, B.H.; Farid, I.; Jasim, F.; Wehbi, S. The effect of chamomile on hyperlipidemias in rats. J. Fac. Med. 1990, 32, 5–11. [Google Scholar]
- Weggemans, R.M.; Trautwein, E.A. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: A meta-analysis. Eur. J. Clin. Nutr. 2003, 57, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mech-anisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Ose, L.; Marais, A.D. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: A multicenter, randomized, placebo-controlled trial. J. Pediatr. 2003, 143, 74–80. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Thomson, P.D. Phytosterols and vascular disease. Atherosclerosis 2006, 186, 12–19. [Google Scholar] [CrossRef]
- Kruse, M.S.; Rey, M.; Vega, M.C.; Coirini, H. Alterations of LXRa and LXRb expression in the hypothalamus of glucose-intolerant rats. J. Endocrinol. 2012, 215, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plat, J.; Nichols, J.A.; Mensink, R.P. Plant sterols and stanols: Effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 2005, 46, 2468–2476. [Google Scholar] [CrossRef] [Green Version]
- Traves, P.G.; Hortelano, S.; Zeini, M.; Chao, T.-H.; Lam, T.; Neuteboom, S.T.; Theodorakis, E.A.; Palladino, M.A.; Castrillo, A.; Bosca, L. Selective Activation of Liver X Receptors by Acanthoic Acid-related Diterpenes. Mol. Pharmacol. 2007, 71, 1545–1553. [Google Scholar] [CrossRef] [Green Version]
- Coirini, H.; Marusic, E.T.; de Nicola, A.F.; Rainbow, T.C.; McEwen, B.S. Identification of mineralocorticoid binding sites in rat brain by competition studies and density gradient centrifugation. Neuroendocrinology 1983, 37, 354–360. [Google Scholar] [CrossRef]
- Rey, M.; Kruse, M.S.; Alvarez, L.D.; Ghini, A.A.; Veleiro, A.S.; Burton, G.; Coirini, H. Neuroprotective action of synthetic steroids with oxygen bridge. Activity on GABAA receptor. Exp. Neurol. 2013, 249, 49–58. [Google Scholar] [CrossRef]


| Lipid Parameter | Group | Weeks | ||
|---|---|---|---|---|
| 2 | 4 | 6 | ||
| TC (mg/dL) | NDW | 48.80 ± 2.90 | 48.20 ± 1.70 | 49.20 ± 0.60 |
| NDTa | 47.60 ± 5.30 | 46.10 ± 2.30 | 47.60 ± 1.90 | |
| HFDW | 74.70 ± 5.50 * | 69.10 ± 6.30 * | 70.50 ± 5.00 * | |
| HFDTa | 75.00 ± 5.60 * | 51.90 ± 3.40 # | 46.40 ± 13.2 # | |
| HDL-c (mg/dL) | NDW | 22.30 ± 2.90 | 20.00 ± 3.10 | 24.90 ± 3.20 |
| NDTa | 23.10 ± 3.40 | 22.60 ± 2.70 | 23.40 ± 2.90 | |
| HFDW | 13.10 ± 2.30 * | 13.80 ± 2.50 * | 16.60 ± 2.90 * | |
| HFDTa | 12.60 ± 2.50 * | 22.70 ± 2.90 # | 21.60 ± 3.20 # | |
| TG (mg/dL) | NDW | 141.60 ± 2.19 | 141.00 ± 2.91 | 147.50 ± 3.10 |
| NDTa | 126.90 ± 1.76 | 161.40 ± 3.78 | 139.60 ± 3.10 | |
| HFDW | 184.50 ± 2.13 * | 223.90 ± 9.20 * | 248.80 ± 19.8 * | |
| HFDTa | 193.20 ± 1.04 * | 238.30 ± 5.70 * | 238.30 ± 2.13 * | |
| Peak Number | Retention Time (min) | UV Max | Tentative Identification | Elemental Composition [M-H] | Measured Mass (m/z) | Theoretical Mass (m/z) | Ac Curacy (δppm) | MSn Ions (δppm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.21 | - | Quinicacid * | C7H11O6− | 191.05579 | 191.05501 | 4.03 | 144.00844 |
| 2 | 1.31 | - | Manoheptulose * | C7H13O7− | 209.06633 | 209.06558 | 3.37 | 153.01857 |
| 3 | 1.82 | - | Citric acid * | C6H7O7− | 191.01863 | 191.01939 | 3.76 | 144.00844 |
| 4 | 7.26 | - | Unknown | C16H11O15N3− | 365.01859 | 365.01847 | 0.23 | |
| 5 | 8.47 | 330 | Unknown | C4H7O12− | 246.99167 | 246.99320 | −6.2 | 152.01080 |
| 6 | 8.73 | 239–320 | Caffeoylquinicacid (chlorogenicacid) * | C16H17O9− | 353.08671 | 353.08786 | 3.86 | 275.0235, 191.05481 (quinicacid), 707.18115 (2M-H) |
| 7 | 9.55 | 223 | 3-Hydroxysuberic acid | C8H13O5− | 189.07645 | 189.07575 | 3.69 | |
| 8 | 10.03 | 330 | Vanillic acid * | C9H7O4− | 179.03465 | 179.03389 | 4.28 | 135.04436 |
| 9 | 10.07 | 283 | 5,3,4,7 tetrahydroxypentosyltessaric acid | C20H31O10− | 431.19241 | 431.19117 | 2.87 | 311.11367, 135.04433 |
| 10 | 10.36 | 330 | Unknown | C10H9O15N3− | 411.00293 | 411.00282 | 0.32 | |
| 11 | 10.43 | 288–346 | Hymenoxynin | C21H34O9− | 429.21313 | 429.21191 | 2.85 | 267.21184 (M-hexosemoiety) |
| 12 | 11.12 | 330 | Unknown | C15H7O11N− | 377.00092 | 377.00136 | −1.18 | |
| 13 | 11.48 | 330 | Unknown | C10H9O15N3− | 411.00296 | 411.00282 | 0.34 | |
| 14 | 11.62 | 239–320 | 1′,5′ Di-caffeoyl quinic acid (cynarin) | C25H23O12− | 515.11945 | 515.11840 | 2.02 | 191.05551 (quinic acid), 179.03429 |
| 15 | 11.82 | 239–320 | 3′,5′ Di-caffeoyl quinic acid * | C25H23O12− | 515.11951 | 515.11840 | 2.14 | 191.05562 (quinic acid), 179.03429 |
| 16 | 11.90 | 255–354 | Ilicic acid * | C15H23O3− | 251.16516 | 251.16417 | 4.5 | 233.15470 (M-H2O), 207.17544 (M-CO2),171.95076 |
| 17 | 12.00 | 239–320 | 4′,5′ Di-caffeoyl quinic acid | C25H23O12− | 515.11840 | 515.11945 | 2.14 | 191.05551 (quinic acid), 179.03429 |
| 18 | 12.20 | 335 | Bruceantin | C28H36O11− | 547.21739 | 547.21832 | 1.70 | |
| 19 | 12.21 | 255–365 | Eudesmane 4(15), 11(13)-dien-12, 5βolide * | C15H19O3− | 247.13380 | 247.13287 | 3.77 | 205.15968 (M-CO2),149.09645 |
| 20 | 12.57 | 289–329 | 3′,4′,5′ Tri-caffeoylquinic acid | C34H39O15− | 677.15033 | 677.15119 | 14.30 | 515.11963 (Di-CQA), 191.05561(quinic acid) |
| 21 | 12.81 | 278 | Unknown | C30H33O15N10− | 773.13715 | 773.21214 | −0.08 | |
| 22 | 12.96 | 278 | Unknown | C26H8ON10− | 476.08734 | 476.08771 | −0.76 | |
| 23 | 13.21 | 278 | Unknown | C12HO16N14− | 953.17645 | 953.17632 | −0.98 | 476.08722 |
| 24 | 13.46 | 278 | 3,4-Dihydroxy-costic acid * | C15H21O4− | 265.14459 | 265.14344 | 4.32 | 247.13395 (M-H2O) |
| 25 | 14.04 | 278 | Unknown | C28H23O12N9− | 677.15039 | 677.14741 | 4.40 | |
| 26 | 14.70 | 323 | Ginnalin A | C20H19O13− | 467.08200 | 467.08202 | −0.03 | 249.08006 |
| 27 | 14.29 | 213, 287, 326 | 1-O-Caffeoyl-5-O-feruloylquinic acid | C26H25O12− | 529.13405 | 529.13519 | 2.15 | 191.05577 (quinicacid), 134.03664 |
| 28 | 16.20 | 278 | 3,5-Dihydroxy-costic acid | C15H21O4− | 265.14456 | 265.14344 | 4.24 | 247.13392(M-H2O) |
| 29 | 16.72 | 225 | 10-Undecenoic acid * | C43H35O18− | 227.12779 | 227.11839 | 2.64 | 183.13847 |
| 30 | 15.55 | 265–329 | Tetra caffeoylquinic acid | C43H35O18− | 839.17999 | 839.18289 | 3.43 | 191.05552 (quinicacid), 179.03423 |
| 31 | 15.93 | 335 | Sambucinol | C15H21O4− | 265.14344 | 265.14453 | 4.13 | |
| 32 | 15.94 | 235 | Scorzonerin | C30H36O11− | 571.21872 | 571.21739 | 1.63 | 467.08185, 327.21765 |
| 33 | 16.45 | 335 | 2,3,4-Trihydroxyoctadeca-2,4-dienoic acid | C18H31O5− | 327.21660 | 327.21783 | 3.77 | 183.13832 |
| 34 | 16.48 | 222 | Trihydroxy-octadecadienoic acid | C18H31O5− | 327.21790 | 327.21660 | 3.95 | 283.22787(M-CO2) |
| 35 | 17.69 | 283 | Tessaric acid * | C15H19O3− | 247.13379 | 247.13287 | 3.71 | 205.15979 (M-CO2), 149.09644 |
| 36 | 18.34 | 222 | Trihydroxy-octadecaenoic acid | C18H33O5− | 329.23225 | 329.23364 | 4.22 | 285.24352(M-CO2) |
| 37 | 18.69 | 335 | Jaceidin | C18H15O8− | 359.07614 | 359.07745 | 3.64 | |
| 38 | 19.09 | 283 | Tessaric acid isomer | C15H19O3− | 247.13383 | 247.13287 | 3.90 | 205.15972 (M-CO2),163.11223 |
| 39 | 19.25 | 218 | 5-Acetyl, 3-hydroxy-4 dihydrocostic acid | C17H25O5− | 309.17090 | 309.16965 | 4.03 | 291.16019(M-H2O), 267.16018(M-acetylmoiety)152.08374 |
| 40 | 19.47 | 283 | Tessaric acid isomer | C15H19O3− | 247.13383 | 247.13287 | 3.90 | 205.15979 (M-CO2),162.01357 |
| 41 | 19.80 | 320 | Artelasticin | C30H33O6− | 489.24282 | 489.24405 | 2.5 | 245.99947 |
| 42 | 19.96 | 283 | 3-oxo-gamma costic acid * | C15H19O3− | 247.13383 | 247.13287 | 3.90 | 231.13903, 233.11812, 219.13902, 215.00955, 149.09644 |
| 43 | 20.14 | 335 | Chrysosplenetin | C19H17O8− | 373.09313 | 373.09317 | 3.68 | 310.40393 |
| 44 | 20.39 | 335 | 3-Acetyl,5-hydroxy-4 dihydro costic acid | C17H25O5− | 309.16965 | 309.17099 | 4.33 | |
| 45 | 21.12 | 335 | Geranylpropionate | C15H21O2− | 209.15361 | 209.15439 | 3.73 | |
| 46 | 21.53 | 218 | Eupatorin | C18H15O8− | 343.08258 | 343.08123 | −4.11 | 329.06663 (M-CH3),315.0533 (M-2CH3), 313.03531 |
| 47 | 22.53 | 218 | Unknown | C13H27O8− | 311.16876 | 311.17004 | −4.11 | |
| 48 | 23.41 | 335 | Gamma costic acid * | C15H21O2− | 233.15453 | 233.15451 | 3.87 | 215.00955, (M-H2O)205.15973(M-CO) |
| 49 | 23.41 | 335 | Gamma costic acid isomer | C15H21O2− | 233.15453 | 233.15361 | 3.94 | 215.00955, (M-H2O)205.15973(M-CO) |
| 50 | 23.97 | 335 | Alpha Costic acid | C15H21O2− | 233.15451 | 233.15361 | 3.87 | 215.00953,(M-H2O)205.15965 (M-CO) |
| 51 | 24.61 | 320 | 2-Hydroxydocosanoic acid | C22H43O3− | 355.32067 | 355.32175 | 3.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, M.; Kruse, M.S.; Magrini-Huamán, R.N.; Gómez, J.; Simirgiotis, M.J.; Tapia, A.; Feresin, G.E.; Coirini, H. Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) Decoction Improves the Hypercholesterolemia and Alters the Expression of LXRs in Rat Liver and Hypothalamus. Metabolites 2021, 11, 579. https://doi.org/10.3390/metabo11090579
Rey M, Kruse MS, Magrini-Huamán RN, Gómez J, Simirgiotis MJ, Tapia A, Feresin GE, Coirini H. Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) Decoction Improves the Hypercholesterolemia and Alters the Expression of LXRs in Rat Liver and Hypothalamus. Metabolites. 2021; 11(9):579. https://doi.org/10.3390/metabo11090579
Chicago/Turabian StyleRey, Mariana, María S. Kruse, Rocío N. Magrini-Huamán, Jessica Gómez, Mario J. Simirgiotis, Alejandro Tapia, Gabriela E. Feresin, and Héctor Coirini. 2021. "Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) Decoction Improves the Hypercholesterolemia and Alters the Expression of LXRs in Rat Liver and Hypothalamus" Metabolites 11, no. 9: 579. https://doi.org/10.3390/metabo11090579