Association between Smoking and Urine Indole Levels Measured by a Commercialized Test
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics
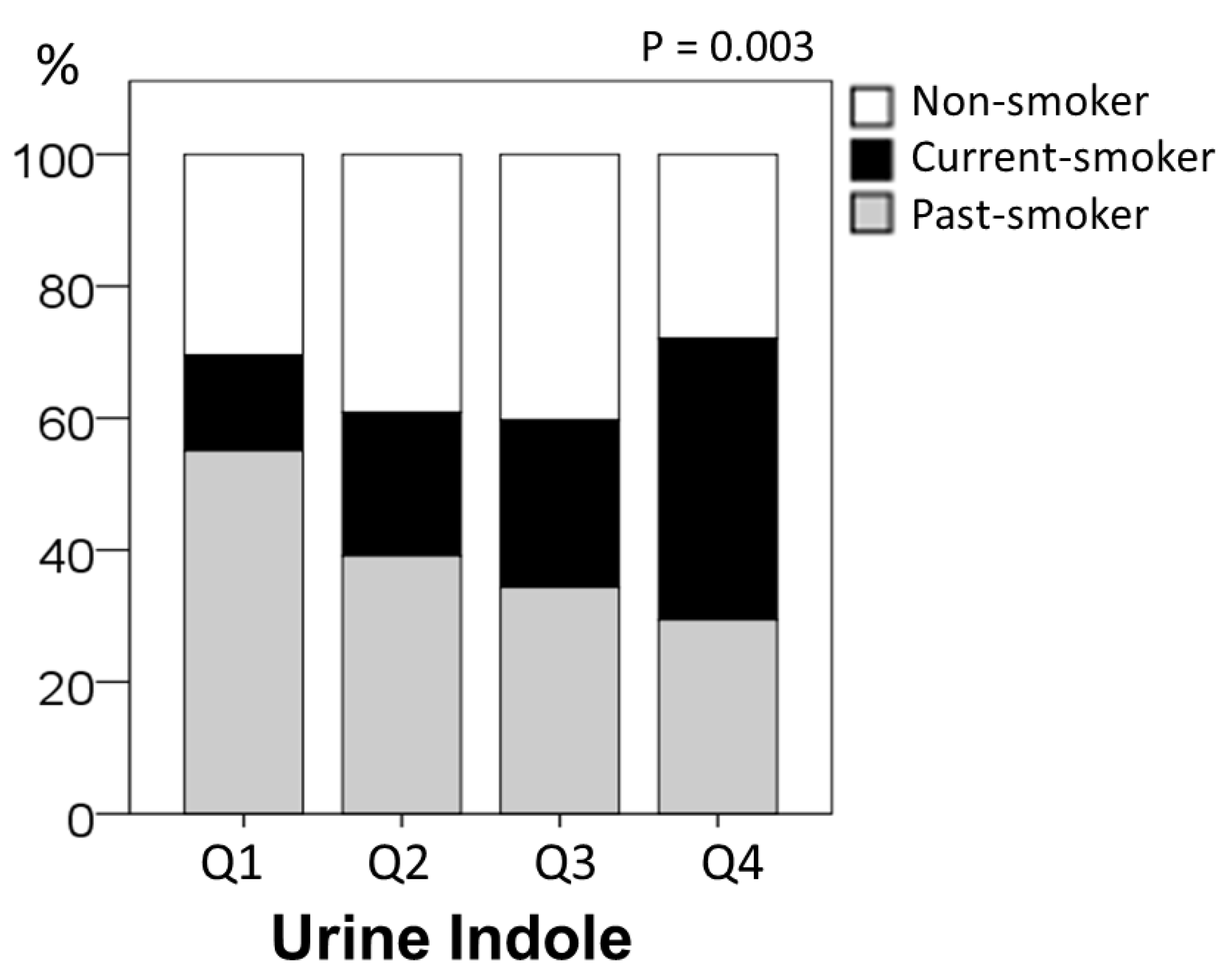
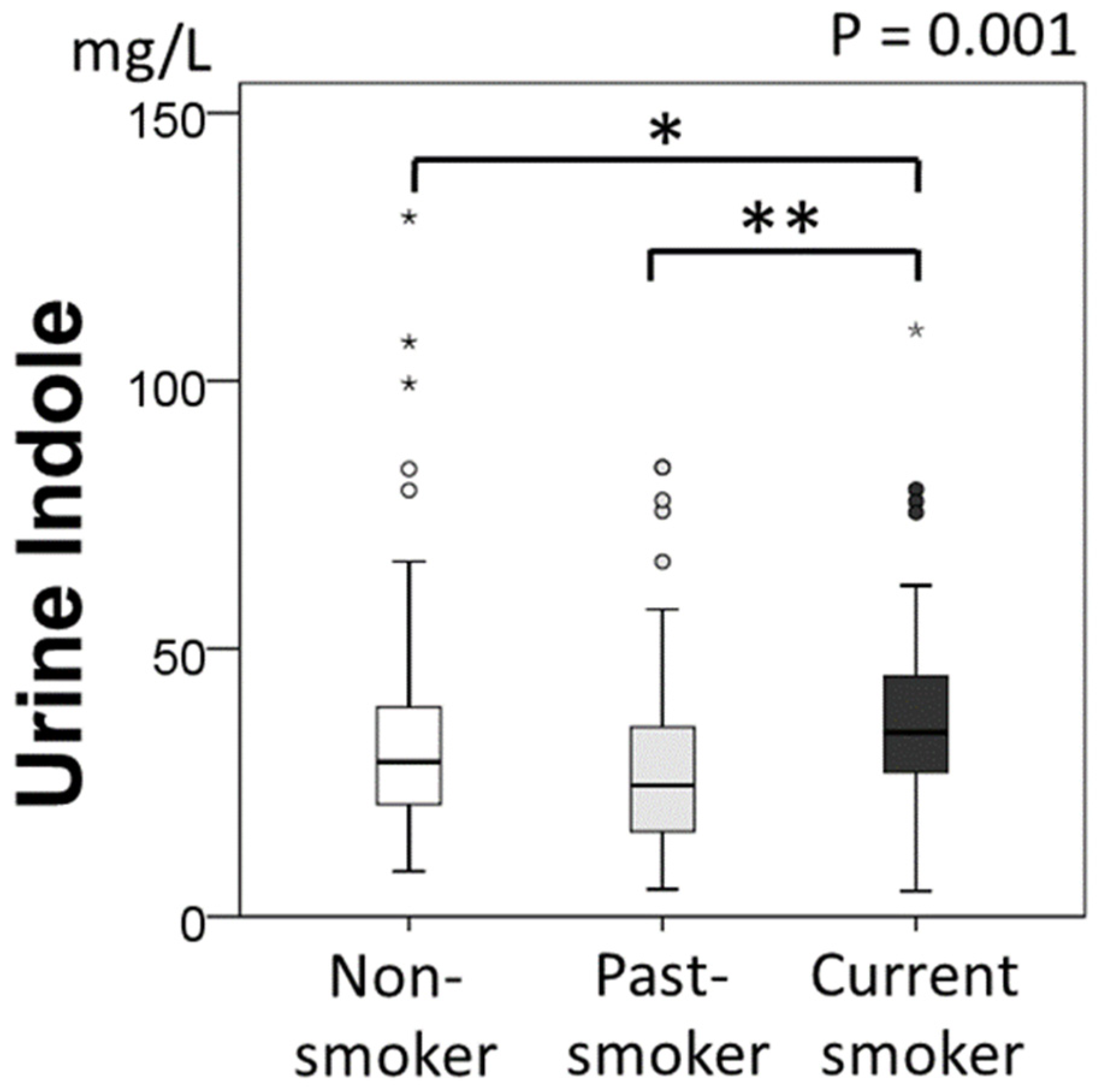
2.2. Urine Indole Levels and Smoking Status
2.3. Univariate and Multivariate Regression Analyses for Urine Indole Concentration
2.4. Diurnal Variations and Effect of Smoking
3. Discussion
4. Material and Methods
4.1. Study Design and Population
4.2. Anthropometric and Biochemical Data
4.3. Diurnal Variations and Effect of Smoking
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.S.; Marcobal, A.; Dodd, D.; Nayfach, S.; Plummer, N.; Meyer, T.; Pollard, K.S.; Sonnenburg, J.L.; Fischbach, M.A. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe 2016, 20, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riveles, K.; Roza, R.; Talbot, P. Phenols, quinolines, indoles, benzene, and 2-cyclopenten-1-ones are oviductal toxicants in cigarette smoke. Toxicol. Sci. 2005, 86, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.R. Smoke Chemistry. In Tobacco Production, Chemistry and Technology; Davis, L., Nielsen, M., Eds.; Blackwell Science Ltd.: Oxford, UK, 1999. [Google Scholar]
- Draper, H.H. Advances in Nutritional Research; Springer Science + Business media LCC: Boston, MA, USA, 1983; Volume 5, pp. 45–47. [Google Scholar]
- Donia, M.S.; Fischbach, M.A. Human microbiota. Small molecules from the human microbiota. Science 2015, 349, 1254766. [Google Scholar] [CrossRef] [Green Version]
- Wagenstaller, M.; Buettner, A. Quantitative Determination of Common Urinary Odorants and Their Glucuronide Conjugates in Human Urine. Metabolites 2013, 3, 637–657. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.L.; Passos, M.; Câmara, J.S. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers-a powerful strategy for breast cancer diagnosis. Talanta 2012, 89, 360–368. [Google Scholar] [CrossRef]
- King, L.J.; Parke, D.V.; Williams, R.T. The Metabolism of [2-14C]Indole in the Rat. Biochem. J. 1966, 98, 266–277. [Google Scholar] [CrossRef]
- Darkoh, C.; Chappell, C.; Gonzales, C.; Okhuysen, P. A rapid and specific method for the detection of indole in complex biological samples. Appl. Environ. Microbiol. 2015, 81, 8093–8097. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, T.; Vidova, V.; Bienertova-Vasku, J.; Janku, P.; Almasi, M.; Klanova, J.; Spacil, Z. Urinary intermediates of tryptophan as indicators of the gut microbial metabolism. Anal. Chim. Acta 2017, 987, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Smith, T. A modification of the method for determining the production of indol by bacteria. J. Exp. Med. 1987, 2, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Suyama, Y.; Hirayama, C. Serum indole and skatole in patients with various liver diseases. Clin. Chim. Acta 1988, 176, 203–206. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [Green Version]
- Kraal, L.; Abubucker, S.; Kota, K.; Fischbach, M.A.; Mitreva, M. The prevalence of species and strains in the human microbiome: A resource for experimental efforts. PLoS ONE 2014, 9, e97279. [Google Scholar] [CrossRef] [Green Version]
- Skye, S.M.; Hazen, S.L. Microbial Modulation of a Uremic Toxin. Cell Host Microbe 2016, 20, 691–692. [Google Scholar] [CrossRef]
- Capuro, G.; Lahner, E. The interaction between smoking, alcohol and the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 579–588. [Google Scholar] [CrossRef]
- Rogers, M.A.; Greene, M.T.; Saint, S.; Chenoweth, C.E.; Malani, P.N.; Trivedi, I.; Aronoff, D.M. Higher Rates of Clostridium difficile Infection among Smokers. PLoS ONE 2012, 7, e42091. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, Y.; Kim, S.J.; Lee, E.J.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.; Kim, H.N.; Lee, J.H. Association between Cigarette Smoking Status and Composition of Gut Microbiota: Population-Based Cross-Sectional Study. J. Clin. Med. 2018, 7, 282. [Google Scholar] [CrossRef] [Green Version]
- Biedermann, L.; Zeitz, J.; Mwinyi, J.; Sutter-Minder, E.; Rehman, A.; Ott, S.J.; Steurer-Stey, C.; Frei, A.; Frei, P.; Scharl, M.; et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE 2013, 8, e59260. [Google Scholar] [CrossRef]
- Biedermann, L.; Brülisauer, K.; Zeitz, J.; Frei, P.; Scharl, M.; Vavricka, S.R.; Fried, M.; Loessner, M.J.; Rogler, G.; Schuppler, M. Smoking Cessation Alters Intestinal Microbiota: Insights from Quantitative Investigations on Human Fecal Samples Using FISH. Inflamm. Bowel Dis. 2014, 20, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Knarreborg, A.; Beck, J.; Jensen, M.T.; Laue, A.; Agergaard, N.; Jensen, B.B. Effect of non-starch polysaccharides on production and absorption of indolic compounds in entire male pigs. Anim. Sci. 2002, 74, 445–453. [Google Scholar] [CrossRef]
- Li, G.; Young, K.D. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 2013, 159, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Terahara, M.; Takahashi, M.; Iwata, M.; Sato, H.; Kaneko, T. Inhibitory Effect of Yogurt on Production of Indole, Phenol and Ammonia by Intestinal Bacteria. J. Jpn. Soc. Nutr. Food Sci. 1993, 46, 139–145. [Google Scholar] [CrossRef]
- Farowski, F.; Els, G.; Tsakmaklis, A.; Higgins, P.G.; Kahlert, C.R.; Stein-Thoeringer, C.K.; Bobardt, J.S.; Dettmer-Wilde, K.; Oefner, P.J.; Vehreschild, J.J.; et al. Assessment of urinary 3-indoxyl sulfate as a marker for gut microbiota diversity and abundance of Clostridiales. Gut Microbes 2019, 10, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.; Oefner, P.J.; Hiergeist, A.; Koestler, J.; Gessner, A.; Weber, M.; Hahn, J.; Wolff, D.; Stammler, F.; Spang, R.; et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood 2015, 126, 1723–1728. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jensen, B.B.; Højberg, O.; Noel, S.J.; Canibe, N. Development of a species-specific TaqMan-MGB real-time PCR assay to quantify Olsenella scatoligenes in pigs offered a chicory root-based diet. AMB Express 2018, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jensen, B.B.; Canibe, N. The Mode of Action of Chicory Roots on Skatole Production in Entire Male Pigs Is neither via Reducing the Population of Skatole-Producing Bacteria nor via Increased Butyrate Production in the Hindgut. Appl. Environ. Microbiol. 2019, 85, e02327-18. [Google Scholar] [CrossRef] [Green Version]
- Weems, J.M.; Cutler, N.S.; Moore, C.; Nichols, W.K.; Martin, D.; Makin, E.; Lamb, J.G.; Yost, G.S. 3-Methylindole is mutagenic and a possible pulmonary carcinogen. Toxicol. Sci. 2009, 112, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Turesky, R.J.; Yuan, J.M.; Wang, R.; Peterson, S.; Yu, M.C. Tobacco smoking and urinary levels of 2-amino-9H-pyrido[2,3-b]indole in men of Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1554–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, H.; Kijima, K.; Maruo, G. Determination of mutagenic heterocyclic amines in combustion smoke samples. Bull. Environ. Contam. Toxicol. 1998, 60, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Deptarment of Health and Human Services, Public Health Service, Office of Surgeon General. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Deptarment of Health and Human Services, Public Health Service, Office of Surgeon General: Rockville, MD, USA, 2010. Available online: http://www.surgeongeneral.gov/library (accessed on 22 February 2022).
- Manabe, S.; Wada, O. Carcinogenic tryptophan pyrolysis products in cigarette smoke condensate and cigarette smoke-polluted indoor air. Environ. Pollut. 1990, 64, 121–132. [Google Scholar] [CrossRef]
- Hoffmann, D.; Hoffmann, I.; El-Bayoumy, K. The less harmful cigarette: A controversial issue. A tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001, 14, 767–790. [Google Scholar] [CrossRef]
- Franzen, K.F.; Willig, J.; Cayo Talavera, S.; Meusel, M.; Sayk, F.; Reppel, M.; Dalhoff, K.; Mortensen, K.; Droemann, D. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: A randomized, double-blinded pilot study. Vasc. Med. 2018, 23, 419–425. [Google Scholar] [CrossRef]
- Espinoza-Derout, J.; Hasan, K.M.; Shao, X.M.; Jordan, M.C.; Sims, C.; Lee, D.L.; Sinha, S.; Simmons, Z.; Mtume, N.; Liu, Y.; et al. Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H445–H459. [Google Scholar] [CrossRef]
- Stewart, C.J.; Auchtung, T.A.; Ajami, N.J.; Velasquez, K.; Smith, D.P.; De La Garza, R., 2nd; Salas, R.; Petrosino, J.F. Effects of Tobacco Smoke and Electronic Cigarette Vapor Exposure on the Oral and Gut Microbiota in Humans: A Pilot Study. PeerJ 2018, 6, e4693. [Google Scholar] [CrossRef]
- European Confederation of Laboratory Medicine. European Urinalysis Guidelines. Scand. J. Clin. Lab. Investig. Suppl. 2000, 231, 1–86. [Google Scholar]
- National Cholesterol Education Program (US). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Haffner, S.M.; Miettinen, H.; Stern, M.P. The homeostasis model in the San Antonio Heart Study. Diabetes Care 1997, 20, 1087–1092. [Google Scholar] [CrossRef]

| Urine Indole Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| ≤19.73 | 19.74–29.19 | 29.20–40.78 | 40.79+ | |||
| (No. of Subjects) | (273) | (69) | (69) | (67) | (68) | p-Value |
| Age (years) | 45.7 ± 5.9 | 46.1 ± 4.7 | 46.6 ± 5.6 | 45.1 ± 6.6 | 45.1 ± 6.7 | 0.365 |
| BMI (kg/m2) | 23.7 ± 2.2 | 23.9 ± 2.0 | 23.4 ± 2.0 | 23.7 ± 2.3 | 23.7 ± 2.3 | 0.592 |
| Circumference of Chest (cm) | 94.8 ± 5.3 | 94.7 ± 5.1 | 94.3 ± 5.1 | 95.3 ± 5.5 | 94.8 ± 5.7 | 0.752 |
| Circumference of Abdomen (cm) | 82.7 ± 6.1 | 83.6 ± 5.2 | 82.1 ± 5.3 | 82.5 ± 7.2 | 82.7 ± 6.3 | 0.531 |
| Systolic Blood Pressure (mmHg) | 116.3 ± 11.6 | 114.9 ± 10.9 | 115.7 ± 11.6 | 116.7 ± 12.5 | 117.8 ± 11.5 | 0.499 |
| Diastolic Blood Pressure (mmHg) | 74.3 ± 8.7 | 74.1 ± 7.5 | 73.5 ± 9.7 | 74.1 ± 8.4 | 75.6 ± 9.0 | 0.525 |
| WBC (×103/μL) | 4760 ± 1132 | 4700 ± 1081 | 4701 ± 1094 | 4770 ± 1278 | 4871 ± 1084 | 0.795 |
| RBC (×106/μL) | 496 ± 35 | 501 ± 33 | 490 ± 33 | 496 ± 38 | 497 ± 34 | 0.362 |
| Hb (g/dL) | 15.0 ± 0.8 | 15.1 ± 0.8 | 15.0 ± 0.9 | 15.0 ± 0.9 | 15.1 ± 0.7 | 0.644 |
| Ht (%) | 46.6 ± 2.5 | 46.6 ± 2.4 | 46.3 ± 2.7 | 46.7 ± 2.7 | 46.7 ± 2.4 | 0.789 |
| PLT (/μL) | 22.6 ± 4.6 | 22.3 ± 4.6 | 23.4 ± 4.6 | 21.7 ± 4.8 | 22.8 ± 4.3 | 0.143 |
| T-CHO (mg/dL) | 196 ± 30 | 200 ± 34 | 198 ± 28 | 192 ± 29 | 193 ± 28 | 0.355 |
| HDL-C (mg/dL) | 60 ± 14 | 58 ± 14 | 60 ± 14 | 61 ± 13 | 60 ± 15 | 0.565 |
| LDL-C (mg/dL) | 114 ± 28 | 118 ± 28 | 117 ± 26 | 111 ± 30 | 112 ± 27 | 0.369 |
| TG (mg/dL) | 75 (56, 100) | 89 (57, 119) | 79 (57, 95) | 73 (56, 93) | 66 (51, 89) | 0.050 |
| AST (IU/L) | 24 ± 16 | 27 ± 30 | 22 ± 5 | 23 ± 8 | 22 ± 6 | 0.187 |
| ALT (IU/L) | 22 ± 11 | 25 ± 13 | 20 ± 9 | 22 ± 13 | 20 ± 10 | 0.041 |
| γ-GTP (IU/L) | 36 ± 27 | 42 ± 34 | 30 ± 16 * | 39 ± 28 | 32 ± 27 | 0.027 |
| BUN (mg/dL) | 14 ± 3 | 13 ± 3 | 14 ± 3 | 15 ± 3 | 15 ± 4 * | 0.007 |
| Cr (mg/dL) | 0.97 ± 0.12 | 0.97 ± 0.10 | 0.96 ± 0.12 | 0.95 ± 0.14 | 1.01 ± 0.12 | 0.056 |
| UA (mg/dL) | 6.5 ± 1.3 | 6.6 ± 1.3 | 6.3 ± 1.2 | 6.4 ± 1.3 | 6.7 ± 1.3 | 0.297 |
| FBS (mg/dL) | 97 ± 9 | 98 ± 8 | 97 ± 8 | 98 ± 8 | 97 ± 11 | 0.752 |
| IRI (μU/mL) | 4.9 ± 2.0 | 5.2 ± 2.2 | 4.7 ± 1.8 | 5.1 ± 2.2 | 4.9 ± 2.0 | 0.459 |
| HOMA-IR | 1.2 ± 0.5 | 1.3 ± 0.6 | 1.1 ± 0.5 | 1.2 ± 0.6 | 1.2 ± 0.5 | 0.432 |
| HOMA-beta | 53 ± 21 | 54 ± 20 | 50 ± 17 | 53 ± 22 | 54 ± 24 | 0.652 |
| HbA1C (%) | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.7 ± 0.4 | 0.980 |
| CRP (mg/dL) | 0.09 (0.08, 0.12) | 0.10 (0.09, 0.14) | 0.09 (0.09, 0.11) | 0.10 (0.08, 0.14) | 0.09 (0.08, 0.13) | 0.076 |
| Previous History (Under treatment) | ||||||
| Hypertension, n (%) | 11 (4) | 3 (4) | 3 (4) | 4 (6) | 1 (2) | 0.606 |
| Hyperlipidemia, n (%) | 10 (4) | 4 (6) | 4 (6) | 2 (3) | 0 | 0.216 |
| Hyperuricemia, n (%) | 24 (9) | 9 (13) | 5 (7) | 7 (10) | 3 (4) | 0.306 |
| Metabolic Syndrome, n (%) | 12 (4) | 3 (4) | 6 (9) | 0 | 3 (4) | 0.106 |
| NAFLD, n (%) | 63 (23) | 14 (20) | 15 (22) | 10 (27) | 16 (25) | 0.820 |
| Alcohol intake (g/day) | 1.14 (0.57, 2.29) | 1.14 (0.58, 2.46) | 1.20 (0.57, 2.49) | 1.14 (0.57, 2.00) | 1.03 (0.41, 2.57) | 0.430 |
| Smoking Status | ||||||
| Non-smoker, n (%) | 94 (34) | 21 (30) | 27 (40) | 27 (40) | 19 (28) | 0.003 |
| Current smoker, n (%) | 71 (26) | 10 (15) | 15 (22) | 17 (25) | 29 (43) | |
| Past smoker, n (%) | 108 (40) | 38 (55) | 27 (39) | 23 (34) | 20 (29) | |
| n = 273 | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| β | p | β | p | β | p | |
| Age (years) | −0.061 | 0.315 | −0.063 | 0.307 | ||
| BMI (kg/m2) | 0.002 | 0.974 | −0.007 | 0.918 | ||
| TG (mg/dL) | −0.130 | 0.031 | −0.095 | 0.121 | −0.116 | 0.051 |
| ALT (IU/L) | −0.120 | 0.047 | −0.090 | 0.160 | ||
| BUN (mg/dL) | 0.148 | 0.014 | 0.116 | 0.060 | 0.152 | 0.011 |
| Cr (mg/dL) | 0.121 | 0.045 | 0.096 | 0.120 | ||
| Hypertension, yes 1, no 0 | 0.148 | 0.015 | 0.024 | 0.690 | ||
| Hyperlipidemia, yes 1, no 0 | 0.109 | 0.072 | 0.092 | 0.122 | ||
| NAFLD, yes 1, no 0 | −0.036 | 0.548 | −0.087 | 0.203 | ||
| Alcohol intake (g/day) | −0.087 | 0.158 | −0.085 | 0.156 | ||
| Current-Smoking, yes 1, no 0 | 0.160 | 0.008 | 0.155 | 0.011 | 0.173 | 0.004 |
| Participants | Time 1 | Time 2 | Time 3 |
|---|---|---|---|
| A1 | 22.52 | 22.97 | 23.21 |
| A2 | 39.01 | 37.89 | 38.34 |
| A3 | 12.55 | 40.22 | 28.00 |
| A4 | 29.62 | 35.48 | 44.20 |
| B1 | 28.57 | 33.94 | 33.69 |
| B2 | 46.70 | 34.13 | 30.02 |
| B3 | 44.36 | 58.36 | 60.86 |
| B4 | 37.69 | 28.65 | 14.91 |
| C1 | 51.44 | 51.43 | 65.79 |
| C2 | 52.35 | 46.56 | 29.54 |
| C3 | 42.67 | 42.68 | 45.85 |
| C4 | 35.23 | 36.35 | 28.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mine, M.; Masaki, N.; Toya, T.; Namba, T.; Nagatomo, Y.; Takase, B.; Adachi, T. Association between Smoking and Urine Indole Levels Measured by a Commercialized Test. Metabolites 2022, 12, 234. https://doi.org/10.3390/metabo12030234
Mine M, Masaki N, Toya T, Namba T, Nagatomo Y, Takase B, Adachi T. Association between Smoking and Urine Indole Levels Measured by a Commercialized Test. Metabolites. 2022; 12(3):234. https://doi.org/10.3390/metabo12030234
Chicago/Turabian StyleMine, Masataka, Nobuyuki Masaki, Takumi Toya, Takayuki Namba, Yuji Nagatomo, Bonpei Takase, and Takeshi Adachi. 2022. "Association between Smoking and Urine Indole Levels Measured by a Commercialized Test" Metabolites 12, no. 3: 234. https://doi.org/10.3390/metabo12030234






