Protein Synthesis Determined from Non-Radioactive Phenylalanine Incorporated by Antarctic Fish
Abstract
:1. Introduction
- “The intracellular free-pool specific radioactivities are elevated and stable during the protein synthesis measurement”
- “Incorporation of radiolabeled” (or isotopically labeled) “amino acid into the bound protein pool should be linear and significant over the time course of protein synthesis”
- “The injected amino acid should flood the plasma, intracellular, extracellular and aminoacyl tRNA pools. Typically, successful flooding doses will elevate intracellular concentrations of the amino acid by about four- to tenfold.”
- “Injecting the amino acid should not result in an elevation of the rate of protein synthesis”
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Intraperitoneal Injection and Tissue Collection
2.3. Methanol Chloroform Extraction
2.4. Phenylalanine Quantification
2.5. Calculation and Statistics
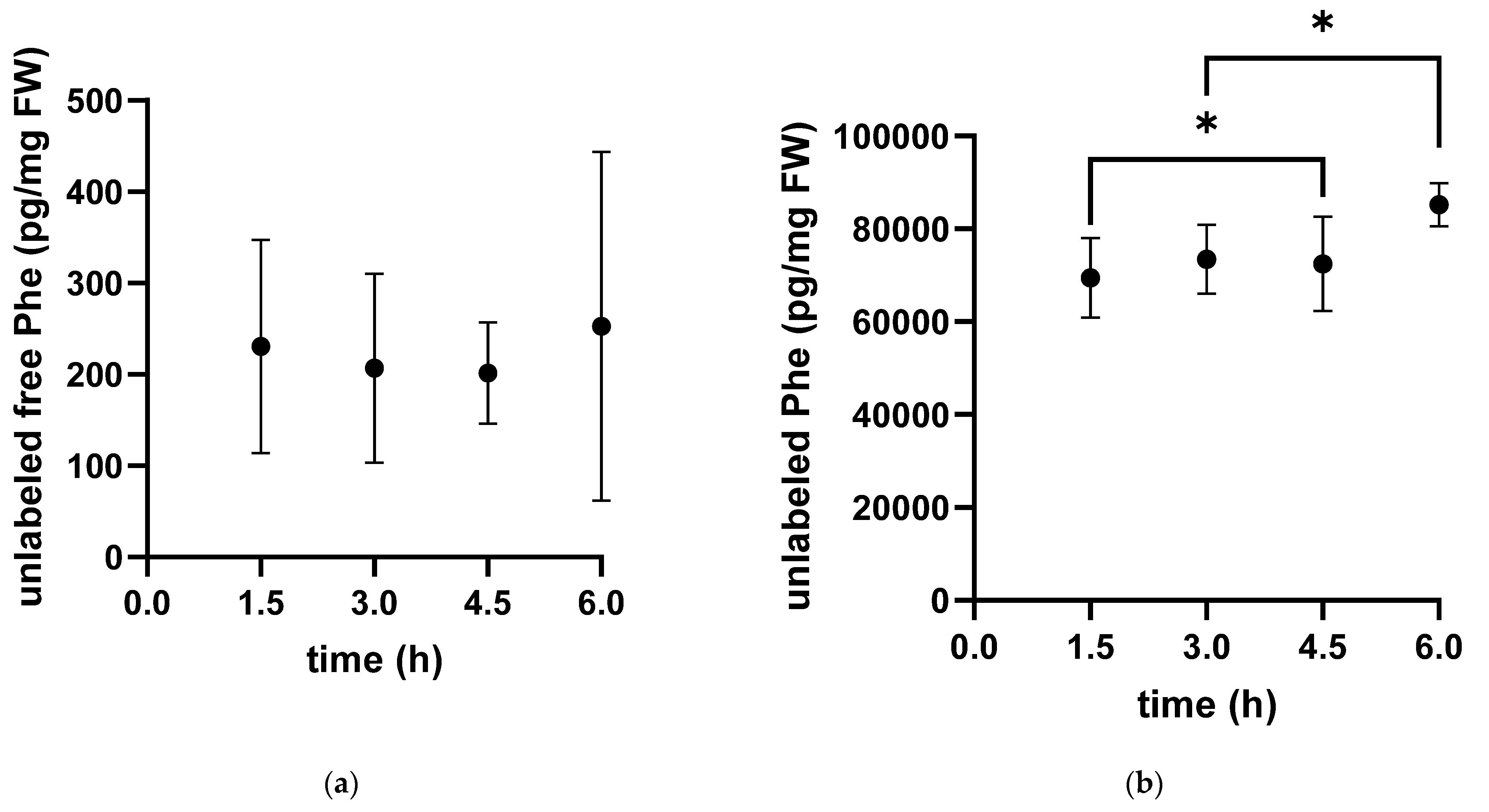
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, S.; Bandiera, R.; Popis, M.; Hussain, S.; Lombard, P.; Aleksic, J.; Sajini, A.; Tanna, H.; Cortés-Garrido, R.; Gkatza, N.; et al. Stem Cell Function and Stress Response Are Controlled by Protein Synthesis. Nature 2016, 534, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, K.P.P.; Rogers, A.D. Protein Metabolism in Marine Animals: The Underlying Mechanism of Growth. Adv. Mar. Biol. 2007, 52, 267–362. [Google Scholar] [CrossRef]
- Houlihan, D.F.; McMillan, D.N.; Laurent, P. Growth Rates, Protein Synthesis, and Protein Degradation Rates in Rainbow Trout: Effects of Body Size. Physiol. Zool. 1986, 59, 482–493. [Google Scholar] [CrossRef]
- Lewis, J.M.; Driedzic, W.R. Tissue-Specific Changes in Protein Synthesis Associated with Seasonal Metabolic Depression and Recovery in the North Temperate Labrid, Tautogolabrus Adspersus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, 474–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassidy, A.A.; Saulnier, R.J.; Lamarre, S.G. Adjustments of Protein Metabolism in Fasting Arctic Charr, Salvelinus Alpinus. PLoS ONE 2016, 11, e0153364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, I.D.; Moksness, E.; Pavlov, D.A.; Houlihan, D.F. Effects of Water Temperature on Protein Synthesis and Protein Growth in Juvenile Atlantic Wolffish (Anarhichas Lupus). Can. J. Fish. Aquat. Sci. 1999, 56, 231–241. [Google Scholar] [CrossRef]
- Katersky, R.S.; Carter, C.G. A Preliminary Study on Growth and Protein Synthesis of Juvenile Barramundi, Lates Calcarifer at Different Temperatures. Aquaculture 2007, 267, 157–164. [Google Scholar] [CrossRef]
- Foster, A.R.; Houlihan, D.F.; Gray, C.; Medale, F.; Fauconneau, B.; Kaushikj, S.J.; Le Bail, P.Y. The Effects of Ovine Growth Hormone on Protein Turnover in Rainbow Trout. Gen. Comp. Endocrinol. 1991, 82, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.A.; Driedzic, W.R.; Campos, D.; Heinrichs-Caldas, W.; Almeida-Val, V.M.F.; Val, A.L.; Lamarre, S.G. Protein Synthesis Is Lowered by 4EBP1 and EIF2-a Signaling While Protein Degradation May Be Maintained in Fasting, Hypoxic Amazonian Cichlids Astronotus Ocellatus. J. Exp. Biol. 2018, 221, jeb167601. [Google Scholar] [CrossRef] [Green Version]
- Fraser, K.P.P.; Peck, L.S.; Clark, M.S.; Clarke, A.; Hill, S.L. Life in the Freezer: Protein Metabolism in Antarctic Fish. R. Soc. Open Sci. 2022, 9, 211272. [Google Scholar] [CrossRef]
- Smith, M.A.K.; Haschemeyer, A.E.V. Protein Metabolism and Cold Adaptation in Antarctic Fish. Physiol. Zool. 1980, 53, 373–382. [Google Scholar] [CrossRef]
- Smith, R.W.; Houlihan, D.F. Protein Synthesis and Oxygen Consumption in Fish Cells. J. Comp. Physiol. B 1995, 165, 93–101. [Google Scholar] [CrossRef]
- Storch, D.; Lannig, G.; Pörtner, H.O. Temperature-Dependent Protein Synthesis Capacities in Antarctic and Temperate (North Sea) Fish (Zoarcidae). J. Exp. Biol. 2005, 208, 2409–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlick, P.J.; Millward, D.J.; James, W.P.T. The Diurnal Response of Muscle and Liver Protein Synthesis in Vivo in Meal Fed Rats. Biochem. J. 1973, 136, 935–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyst, S.D.; Norton, A.C.; Dundas, C.R.; Eremin, O.; Ferguson, K.; Garlick, P.J. Anaesthetic Agents and Their Effect on Tissue Protein Synthesis in the Rat. Clin. Sci. 1989, 77, 651–655. [Google Scholar] [CrossRef]
- Fraser, K.P.P.; Lyndon, A.R.; Houlihan, D.F. Protein Synthesis and Growth in Juvenile Atlantic Halibut, Hippoglossus Hippoglossus (L.): Application of 15N Stable Isotope Tracer. Aquac. Res. 1998, 29, 289–298. [Google Scholar] [CrossRef]
- McCarthy, I.D.; Owen, S.F.; Watt, P.W.; Houlihan, D.F. Individuals Maintain Similar Rates of Protein Synthesis over Time on the Same Plane of Nutrition under Controlled Environmental Conditions. PLoS ONE 2016, 11, e0152239. [Google Scholar] [CrossRef] [Green Version]
- Garlick, P.J.; McNurlan, M.A.; Preedy, V.R. A Rapid and Convenient Technique for Measuring the Rate of Protein Synthesis in Tissues by Injection of [3H]Phenylalanine. Biochem. J. 1980, 192, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Lamarre, S.G.; Saulnier, R.J.; Blier, P.U.; Driedzic, W.R. A Rapid and Convenient Method for Measuring the Fractional Rate of Protein Synthesis in Ectothermic Animal Tissues Using a Stable Isotope Tracer. Comp. Biochem. Physiol. Part-B Biochem. Mol. Biol. 2015, 182, 1–5. [Google Scholar] [CrossRef]
- Lewis, J.M.; Grove, T.J.; O’Brien, K.M. Energetic Costs of Protein Synthesis Do Not Differ between Red- and White-Blooded Antarctic Notothenioid Fishes. Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2015, 187, 177–183. [Google Scholar] [CrossRef]
- Owen, S.F.; McCarthy, I.D.; Watt, P.W.; Ladero, V.; Sanchez, J.A.; Houlihan, D.F.; Rennie, M.J. In Vivo Rates of Protein Synthesis in Atlantic Salmon (Salmo Salar L.) Smolts Determined Using a Stable Isotope Flooding Dose Technique. Fish Physiol. Biochem. 1999, 20, 87–94. [Google Scholar] [CrossRef]
- Buse, M.G.; Reid, S.S. Leucine. A Possible Regulator of Protein Turnover in Muscle. J. Clin. Investig. 1975, 56, 1250–1261. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Trakadis, Y.J.; Scriver, C.R. Phenylalanine Hydroxylase Deficiency. Genet. Med. 2011, 13, 697–707. [Google Scholar] [CrossRef]
- Bechshøft, C.L.; Schjerling, P.; Bornø, A.; Holm, L. Existence of Life-Time Stable Proteins in Mature Rats-Dating of Proteins’ Age by Repeated Short-Term Exposure to Labeled Amino Acids throughout Age. PLoS ONE 2017, 12, e0185605. [Google Scholar] [CrossRef] [Green Version]
- Houlihan, D.F.; McCarthy, I.D.; Carter, C.G.; Marttin, F. Protein Turnover and Amino Acid Flux in Fish Larvae. Ices Mar. Sci. Symp. 1995, 201, 87–99. [Google Scholar]
- Berthelot, C.; Clarke, J.; Desvignes, T.; William Detrich, H.; Flicek, P.; Peck, L.S.; Peters, M.; Postlethwait, J.H.; Clark, M.S. Adaptation of Proteins to the Cold in Antarctic Fish: A Role for Methionine? Genome Biol. Evol. 2019, 11, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Brodte, E.; Knust, R.; Pörtner, H.O.; Arntz, W.E. Biology of the Antarctic Eelpout Pachycara Brachycephalum. Deep. Res. Part II Top. Stud. Oceanogr. 2006, 53, 1131–1140. [Google Scholar] [CrossRef]
- Buentello, J.A.; Pohlenz, C.; Margulies, D.; Scholey, V.P.; Wexler, J.B.; Tovar-Ramírez, D.; Neill, W.H.; Hinojosa-Baltazar, P.; Gatlin, D.M. A Preliminary Study of Digestive Enzyme Activities and Amino Acid Composition of Early Juvenile Yellowfin Tuna (Thunnus Albacares). Aquaculture 2011, 312, 205–211. [Google Scholar] [CrossRef]
- Cresswell, T.; Metian, M.; Fisher, N.S.; Charmasson, S.; Hansman, R.L.; Bam, W.; Bock, C.; Swarzenski, P.W. Exploring New Frontiers in Marine Radioisotope Tracing–Adapting to New Opportunities and Challenges. Front. Mar. Sci. 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Langenbuch, M.; Bock, C.; Leibfritz, D.; Pörtner, H.O. Effects of Environmental Hypercapnia on Animal Physiology: A 13C NMR Study of Protein Synthesis Rates in the Marine Invertebrate Sipunculus Nudus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Applebaum, S.L.; Manahan, D.T. Metabolic Cost of Protein Synthesis in Larvae of the Pacific Oyster (Crassostrea Gigas) Is Fixed across Genotype, Phenotype, and Environmental Temperature. Biol. Bull. 2016, 230, 175–187. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, I.D.; Nicholls, R.; Malham, S.K.; Whiteley, N.M. Validation of the Flooding Dose Technique to Determine Fractional Rates of Protein Synthesis in a Model Bivalve Species, the Blue Mussel (Mytilus Edulis L.). Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2016, 191, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeets, J.S.J.; Horstman, A.M.H.; Vles, G.F.; Emans, P.J.; Goessens, J.P.B.; Gijsen, A.P.; van Kranenburg, J.M.X.; van Loon, L.J.C. Protein Synthesis Rates of Muscle, Tendon, Ligament, Cartilage, and Bone Tissue in Vivo in Humans. PLoS ONE 2019, 14, e0224745. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, D.P.J.; Horstman, A.M.H.; Smeets, J.S.J.; den Dulk, M.; Grabsch, H.I.; Dejong, C.H.C.; Rensen, S.S.; Olde Damink, S.W.M.; van Loon, L.J.C. Tumour-Specific and Organ-Specific Protein Synthesis Rates in Patients with Pancreatic Cancer. J. Cachexia. Sarcopenia Muscle 2019, 10, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, A.C.; Schröer, M.; Bock, C.; Steeger, H.U.; Paul, R.J.; Pörtner, H.O. Indicators of Oxygen- and Capacity-Limited Thermal Tolerance in the Lugworm Arenicola Marina. Clim. Res. 2008, 37, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Southam, A.D.; Hines, A.; Viant, M.R. High-Throughput Tissue Extraction Protocol for NMR- and MS-Based Metab-olomics. Anal. Biochem. 2008, 372, 204–212. [Google Scholar] [CrossRef]
- Brodte, E.; Knust, R.; Pörtner, H.O. Temperature-Dependent Energy Allocation to Growth in Antarctic and Boreal Eelpout (Zoarcidae). Polar Biol. 2006, 30, 95–107. [Google Scholar] [CrossRef]
- Pan, F.T.C.; Applebaum, S.L.; Manahan, D.T. Differing Thermal Sensitivities of Physiological Processes Alter ATP Allocation. J. Exp. Biol. 2020, 224, jeb233379. [Google Scholar] [CrossRef]

| Time (h) | Ks (% Day−1) | Labeled, Free Phe (%) |
|---|---|---|
| 1.5 | 0.052 ± 0.029 | 68.54 ± 12.36 |
| 3 | 0.055 ± 0.015 | 75.58 ± 14.14 |
| 4.5 | 0.041 ± 0.015 | 80.64 ± 5.57 |
| Total Mean | 0.049 ± 0.021 | 75.50 ± 11.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krebs, N.; Tebben, J.; Bock, C.; Mark, F.C.; Lucassen, M.; Lannig, G.; Pörtner, H.-O. Protein Synthesis Determined from Non-Radioactive Phenylalanine Incorporated by Antarctic Fish. Metabolites 2023, 13, 338. https://doi.org/10.3390/metabo13030338
Krebs N, Tebben J, Bock C, Mark FC, Lucassen M, Lannig G, Pörtner H-O. Protein Synthesis Determined from Non-Radioactive Phenylalanine Incorporated by Antarctic Fish. Metabolites. 2023; 13(3):338. https://doi.org/10.3390/metabo13030338
Chicago/Turabian StyleKrebs, Nina, Jan Tebben, Christian Bock, Felix C. Mark, Magnus Lucassen, Gisela Lannig, and Hans-Otto Pörtner. 2023. "Protein Synthesis Determined from Non-Radioactive Phenylalanine Incorporated by Antarctic Fish" Metabolites 13, no. 3: 338. https://doi.org/10.3390/metabo13030338







