High-Fat-Diet Suppressed Ketone Body Utilization for Lipogenic Pathway in Brown Adipose Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Fat and High-Sucrose Diet in Mice
2.2. Measurement of Plasma Glucose and Total Ketone Bodies
2.3. Cellular Differentiation Induction of Primary Cultured Brown Adipocytes
2.4. Isoproterenol Treatment of Primary Cultured Brown Adipocytes
2.5. Introduction of siRNA against Acetoacetyl-CoA Synthetase in Primary Cultured Cells
2.6. RNA Extraction
2.7. Lipid Staining with Oil Red O
2.8. Statistical Analysis
3. Results
3.1. Lipid and Ketone Body Utilization in BAT Was Decreased by High-Fat-Diet-Induced Obesity
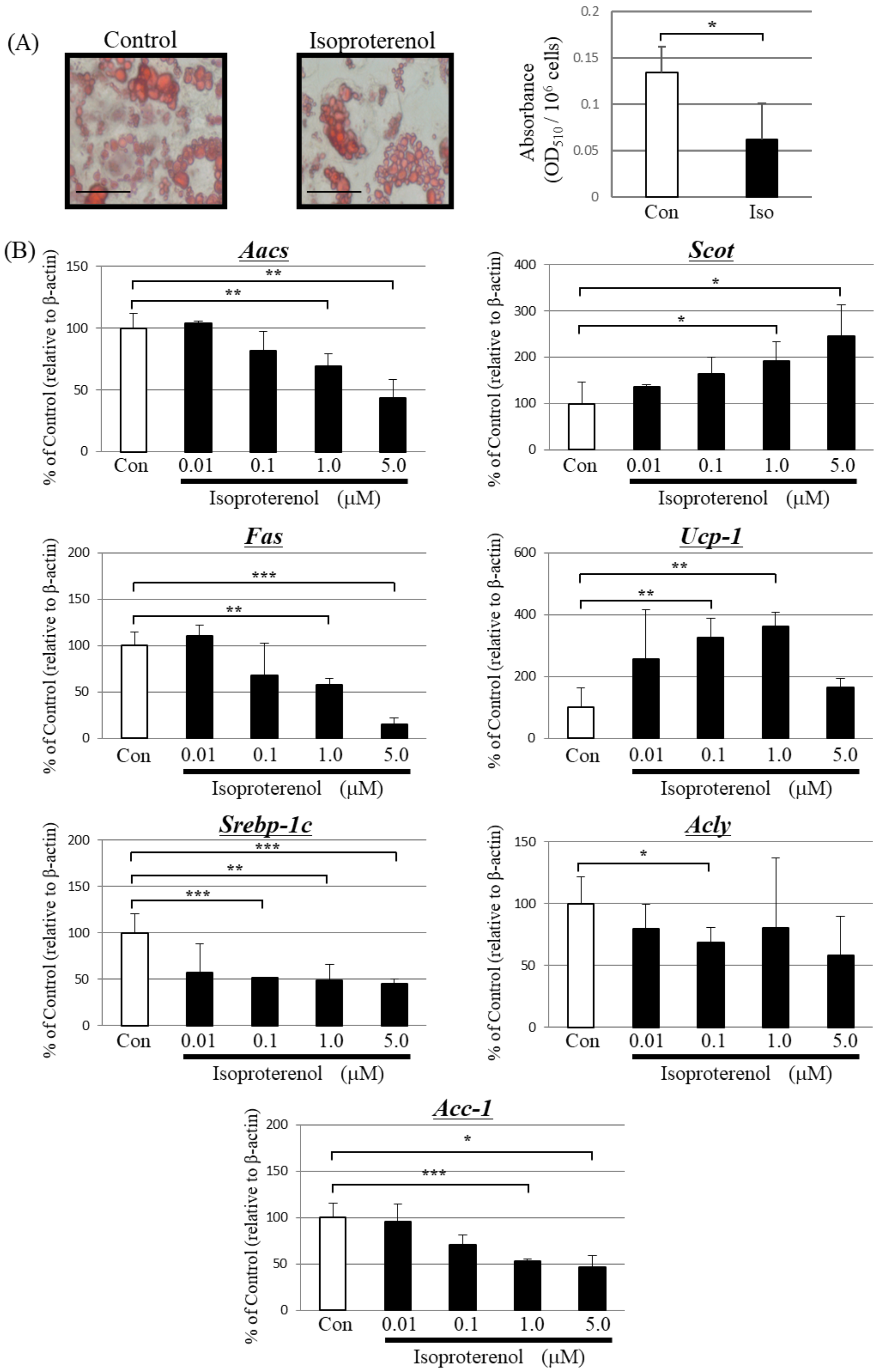
3.2. β-Adrenergic Agonists Suppress Ketone Body Utilization for Lipid Synthesis in BAT
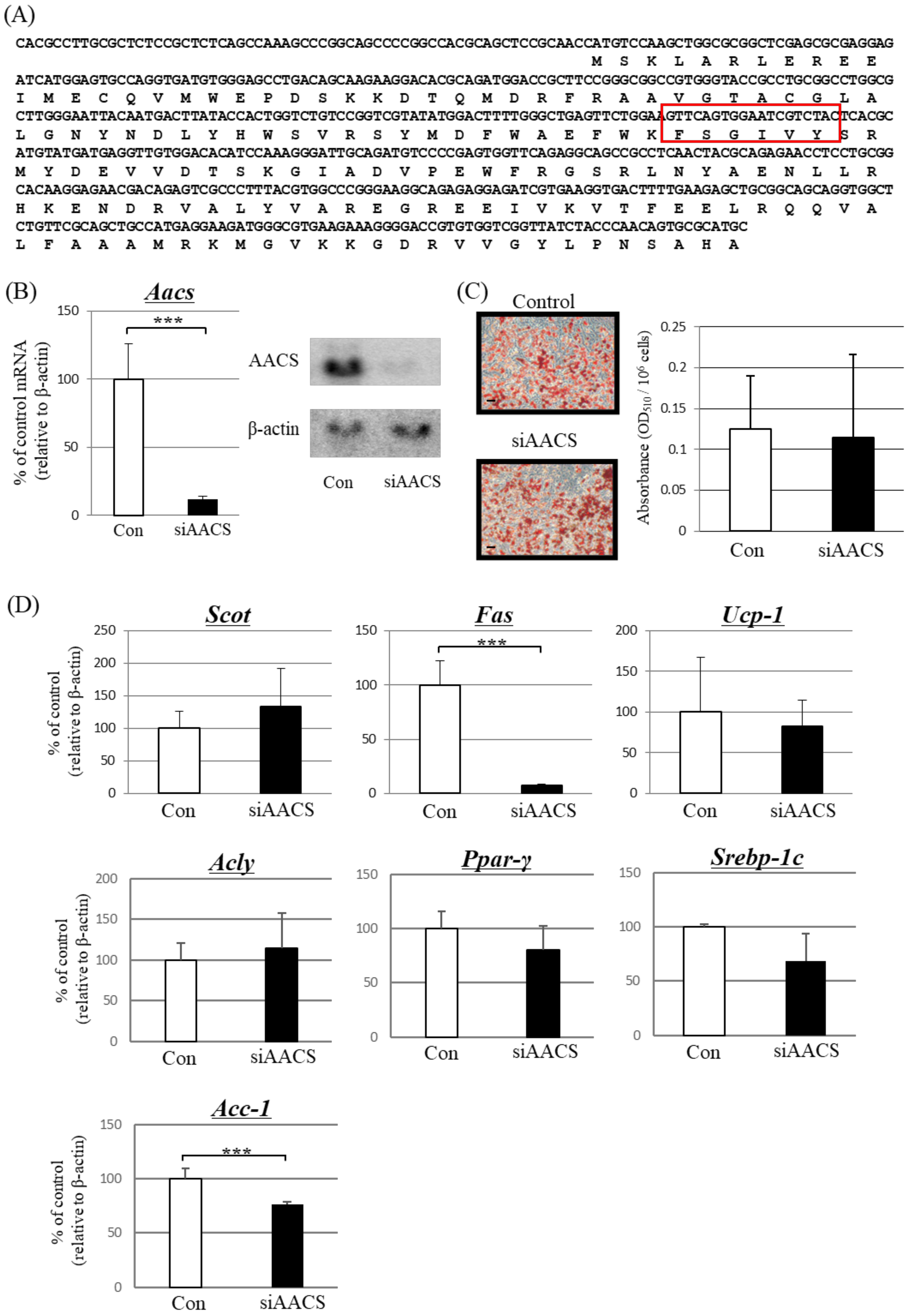
3.3. Suppression of AACS Expression Significantly Reduced FAS Expression in Primary Cultured Brown Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, R.; Dugré, N.; Allan, G.M.; Lindblad, A.J. Ketogenic diet for weight loss. Can. Fam. Physician 2018, 64, 906. [Google Scholar] [PubMed]
- Dabek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of Cellular Biochemistry, Epigenetics and Metabolomics by Ketone Bodies. Implications of the Ketogenic Diet in the Physiology of the Organism and Pathological States. Nutrients 2020, 12, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, E.R.; Warren, C.E.; Hanegan, C.M.; Larsen, J.G.; du Randt, J.D.; Cannon, M.; Saito, J.Y.; Campbell, R.J.; Kemberling, C.M.; Miller, G.S.; et al. A Novel Ketone-Supplemented Diet Improves Recognition Memory and Hippocampal Mitochondrial Efficiency in Healthy Adult Mice. Metabolites 2022, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sohn, I.; Ahn, J.I.; Lee, K.H.; Lee, Y.S.; Lee, Y.S. Hepatic gene expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene 2004, 340, 99–109. [Google Scholar] [CrossRef]
- Chen, Y.T.; Hsu, A.H.; Chiou, S.Y.; Lin, Y.C.; Lin, J.S. AB-Kefir Reduced Body Weight and Ameliorated Inflammation in Adipose Tissue of Obese Mice Fed a High-Fat Diet, but Not a High-Sucrose Diet. Nutrients 2021, 13, 2182. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Robinson, A.M.; Williamson, D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980, 60, 143–187. [Google Scholar] [CrossRef]
- Ito, M.; Fukui, T.; Kamokari, M.; Saito, T.; Tomita, K. Purification and characterization of acetoacetyl-CoA synthetase from rat liver. Biochim. Biophys. Acta 1984, 794, 183–193. [Google Scholar]
- Nakamoto, M.; Takahashi, N.; Iwahori, A.; Sato, H.; Fukui, T. Effects of development on acetoacetyl-CoA synthetase biosynthesis in rat liver. Biol. Pharm. Bull. 1999, 22, 981–983. [Google Scholar] [CrossRef] [Green Version]
- Ohnuki, M.; Takahashi, N.; Yamasaki, M.; Fukui, T. Different localization in rat brain of the novel cytosolic ketone body-utilizing enzyme, acetoacetyl-CoA synthetase, as compared to succinyl-CoA:3-oxoacid CoA-transferase. Biochim. Biophys. Acta 2005, 1729, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Hasegawa, S.; Suzuki, H.; Hidai, K.; Saitoh, Y.; Fukui, T. Acetoacetyl-CoA synthetase gene is abundant in rat adipose, and related with fatty acid synthesis in mature adipocytes. Biochem. Biophys. Res. Commun. 2005, 335, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Noda, K.; Maeda, A.; Matsuoka, M.; Yamasaki, M.; Fukui, T. Acetoacetyl-CoA synthetase, a ketone body-utilizing enzyme, is controlled by SREBP-2 and affects serum cholesterol levels. Mol. Genet. Metab. 2012, 107, 553–560. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ikeda, Y.; Yamasaki, M.; Fukui, T. The role of acetoacetyl-CoA synthetase, a ketone body-utilizing enzyme, in 3T3-L1 adipocyte differentiation. Biol. Pharm. Bull. 2012, 35, 1980–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, M.; Hasegawa, S.; Kitani, T.; Hidai, K.; Fukui, T. Differential effects of obesity on acetoacetyl-CoA synthetase gene in rat adipose tissues. Eur. J. Lipid Sci. Technol. 2007, 109, 617–622. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hasegawa, S.; Imai, M.; Takahashi, N.; Fukui, T. High-fat diet-induced obesity stimulates ketone body utilization in osteoclasts of the mouse bone. Biochem. Biophys. Res. Commun. 2016, 473, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Von Bank, H.; Hurtado-Thiele, M.; Oshimura, N.; Simcox, J. Mitochondrial Lipid Signaling and Adaptive Thermogenesis. Metabolites 2021, 11, 124. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.J.; Inokuchi, T.; Stern, J.S.; Horwitz, B.A. Brown adipose tissue lipectomy leads to increased fat deposition in Osborne-Mendel rats. Am. J. Physiol. 1985, 248, R231–R235. [Google Scholar] [CrossRef]
- Himms-Hagen, J.; Desautels, M. A mitochondrial defect in brown adipose tissue of the obese (ob/ob) mouse: Reduced binding of purine nucleotides and a failure to respond to cold by an increase in binding. Biochem. Biophys. Res. Commun. 1978, 83, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.H.; Ilic, V. Activities of enzymes of acetoacetate metabolism in rat brown adipose tissue during development. Biochem. J. 1985, 231, 773–775. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.; Agius, L. Fatty acid synthesis and ketone body utilization by brown adipose tissue of the rat. Response to cold or nutritional state? Biochim. Biophys. Acta 1983, 753, 244–248. [Google Scholar] [CrossRef]
- Panic, V.; Pearson, S.; Banks, J.; Tippetts, T.S.; Velasco-Silva, J.N.; Lee, S.; Simcox, J.; Geoghegan, G.; Bensard, C.L.; van Ry, T.; et al. Mitochondrial pyruvate carrier is required for optimal brown fat thermogenesis. eLife 2020, 9, e52558. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kashiwaya, Y.; King, M.T.; Baxa, U.; Tam, J.; Niu, G.; Chen, X.; Clarke, K.; Veech, R.L. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 2012, 26, 2351–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, M.; Hasegawa, S.; Imai, M.; Fukui, T.; Takahashi, N. Browning Effect of Brominated Flame Retardant, TBBP-A, on Undifferentiated Adipocytes. BPB Rep. 2021, 4, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, K.; Takayanagi, S.; Sasaki, S.; Akita, H.; Harashima, H. RNA interference-based silencing reveals the regulatory role of fatty acid-binding protein 4 in the production of IL-6 and vascular endothelial growth factor in 3T3-L1 adipocytes. Endocrinology 2012, 153, 5629–5636. [Google Scholar] [CrossRef] [Green Version]
- Sim, C.K.; Kim, S.Y.; Brunmeir, R.; Zhang, Q.; Li, H.; Dharmasegaran, D.; Leong, C.; Lim, Y.Y.; Han, W.; Xu, F. Regulation of white and brown adipocyte differentiation by RhoGAP DLC1. PLoS ONE 2017, 12, e0174761. [Google Scholar] [CrossRef] [Green Version]
- Ryu, M.H.; Cha, Y.S. The effects of a high-fat or high-sucrose diet on serum lipid profiles, hepatic acyl-CoA synthetase, carnitine palmitoyltransferase-I, and the acetyl-CoA carboxylase mRNA levels in rats. J. Biochem. Mol. Biol. 2003, 36, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Rothwell, N.J.; Stock, M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979, 281, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.A.; Williams, J.A. Adrenergic receptors mediating depolarization in brown adipose tissue. Am. J. Physiol. 1976, 231, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, M.; Hasegawa, S.; Yamanaka, H.; Narishima, R.; Fukui, T. Ketone body utilization is regulated by male-specific factors in rat subcutaneous adipocytes. Exp. Clin. Endocrinol. Diabetes 2009, 117, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Yu, X.X.; Lewin, D.A.; Forrest, W.; Adams, S.H. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: Prediction from differential gene expression and confirmation in vivo. FASEB J. 2002, 16, 155–168. [Google Scholar] [CrossRef]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-Stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Del Sal, G. Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
- Henriksbo, B.D.; Tamrakar, A.K.; Xu, J.; Duggan, B.M.; Cavallari, J.F.; Phulka, J.; Stampfli, M.R.; Ashkar, A.A.; Schertzer, J.D. Statins Promote Interleukin-1beta-Dependent Adipocyte Insulin Resistance Through Lower Prenylation, Not Cholesterol. Diabetes 2019, 68, 1441–1448. [Google Scholar] [CrossRef]
- Lodhi, I.J.; Yin, L.; Jensen-Urstad, A.P.; Funai, K.; Coleman, T.; Baird, J.H.; El Ramahi, M.K.; Razani, B.; Song, H.; Fu-Hsu, F.; et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab. 2012, 16, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhou, J.H.; Zhang, H.; Canfran-Duque, A.; Singh, A.K.; Perry, R.J.; Shulman, G.I.; Fernandez-Hernando, C.; Min, W. Brown adipose TRX2 deficiency activates mtDNA-NLRP3 to impair thermogenesis and protect against diet-induced insulin resistance. J. Clin. Investig. 2022, 132, e148852. [Google Scholar] [CrossRef]
- Pan, R.; Chen, Y. Latest Advancements on Combating Obesity by Targeting Human Brown/Beige Adipose Tissues. Front. Endocrinol. 2022, 13, 884944. [Google Scholar] [CrossRef] [PubMed]


| Male Mice | Female Mice | |||||
|---|---|---|---|---|---|---|
| CD | HSD | HFD | CD | HSD | HFD | |
| Body weight (g) | 44.3 ± 4.1 | 53.5 ± 8.3 * | 59.8 ± 9.7 * | 33.1 ± 4.9 | 38.2 ± 3.7 * | 41.4 ± 10.3 * |
| Blood glucose (mmol/L) | 11.2 ± 4.6 | 14.8 ± 8.4 Ns | 15.2 ± 8.2 Ns | 11.3 ± 5.1 | 10.5 ± 3.5 Ns | 10.6 ± 5.4 Ns |
| Total ketone bodies (mmol/L) | 0.42 ± 0.19 | 0.73 ± 0.40 Ns | 1.02 ± 0.13 *** | 0.27 ± 0.18 | 0.28 ± 0.13 Ns | 1.36 ± 0.43 *** |
| Control | Isoproterenol (1.0 μM) | |
|---|---|---|
| Total ketone bodies (μmol/L) | 7.8 ± 10.2 | 43.6 ± 20.1 *** |
| Control | Nonsense siRNA | AACS siRNA | |
|---|---|---|---|
| Total ketone bodies (μmol/L) | 7.8 ± 10.2 ns | 6.8 ± 12.3 ns | 18.6 ± 9.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamasaki, M.; Hasegawa, S.; Ozaki, S.; Imai, M.; Saito, D.; Takahashi, N. High-Fat-Diet Suppressed Ketone Body Utilization for Lipogenic Pathway in Brown Adipose Tissues. Metabolites 2023, 13, 519. https://doi.org/10.3390/metabo13040519
Yamasaki M, Hasegawa S, Ozaki S, Imai M, Saito D, Takahashi N. High-Fat-Diet Suppressed Ketone Body Utilization for Lipogenic Pathway in Brown Adipose Tissues. Metabolites. 2023; 13(4):519. https://doi.org/10.3390/metabo13040519
Chicago/Turabian StyleYamasaki, Masahiro, Shinya Hasegawa, Shotaro Ozaki, Masahiko Imai, Daisuke Saito, and Noriko Takahashi. 2023. "High-Fat-Diet Suppressed Ketone Body Utilization for Lipogenic Pathway in Brown Adipose Tissues" Metabolites 13, no. 4: 519. https://doi.org/10.3390/metabo13040519






