NMR-Based Metabolic Profiling of Field-Grown Leaves from Sugar Beet Plants Harbouring Different Levels of Resistance to Cercospora Leaf Spot Disease
Abstract
:1. Introduction
2. Results
2.1. Annotation of Aqueous Metabolites in Sugar Beet Leaves
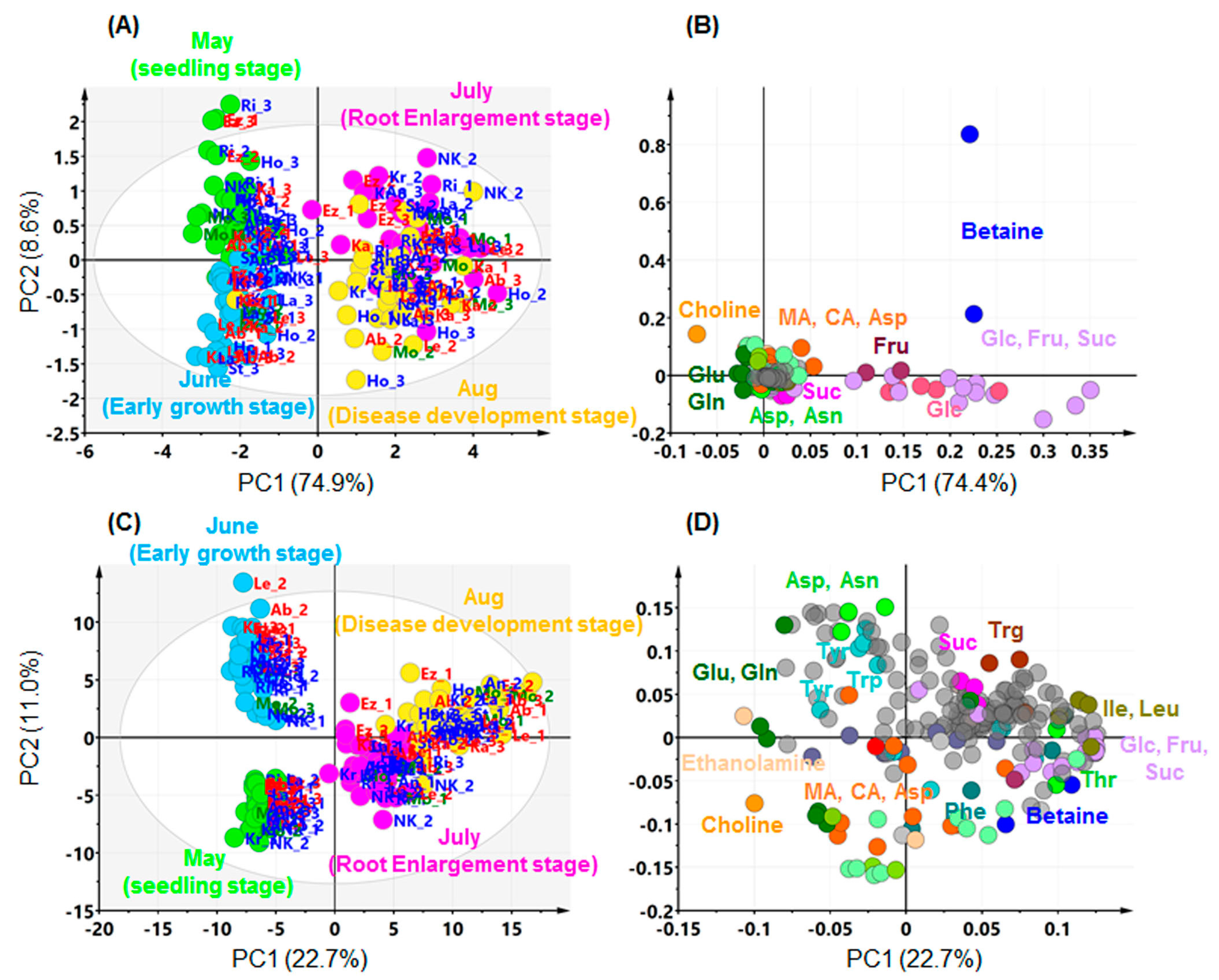
2.2. Effects of Growth Stage on Metabolite Profile in Sugar Beet Leaves
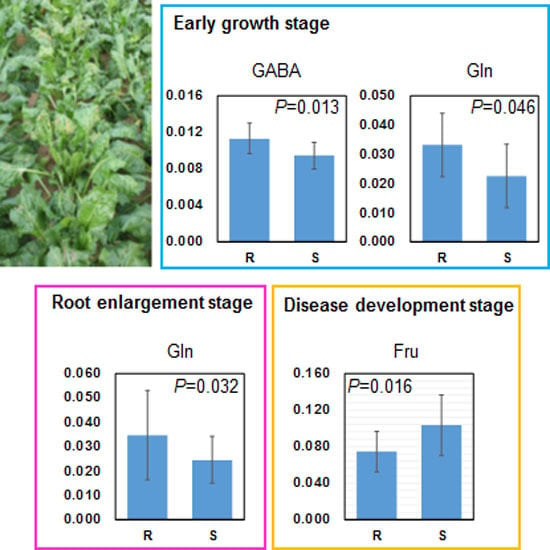
2.3. Relation between Leaf Metabolites and Disease Severity
2.4. Comparing Annotated Candidate Metabolites
3. Discussion
4. Materials and Methods
4.1. Preparation of NMR Samples
4.1.1. Plant Materials and Sampling
4.1.2. Metabolite Extraction
4.2. NMR Analysis
4.2.1. NMR Spectral Measurements
4.2.2. Metabolite Annotation
4.3. 1H NMR-Based Metabolic Profiling
4.3.1. Dataset Preparation
4.3.2. Multivariate Analysis
4.3.3. Significance Test
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khan, M.F.R. Sugar Beet Diseases: Cercospora Leaf Spot. In Fungicide Resistance in Plant Pathogens; Springer: Berlin/Heidelberg, Germany, 2015; pp. 379–387. [Google Scholar]
- Weiland, J.; Koch, G. Sugarbeet Leaf Spot Disease (Cercospora beticola Sacc.). Mol. Plant Pathol. 2004, 5, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.A.; Gaskill, J.O. Inheritance of Resistance to Cercospora Leaf Spot in Sugarbeet. J. Am. Soc. Sugar Beet Technol. 1970, 16, 172–180. [Google Scholar] [CrossRef]
- Rossi, V.; Rossi, V.; Battilani, P.; Chiusa, G.; Giosuè, S.; Languasco, L.; Racca, P. Components of rate-reducinse resistance to Cercospora leaf spot in sugar beet: Incubation length, infection efficiency, lesion size. J. Plant Pathol. 1999, 81, 25–35. [Google Scholar]
- Weltmeier, F.; Maser, A.; Menze, A.; Hennig, S.; Schad, M.; Breuer, F.; Schulz, B.; Holtschulte, B.; Nehls, R.; Stahl, D.J. Transcript Profiles in Sugar Beet Genotypes Uncover Timing and Strength of Defense Reactions to Cercospora beticola Infection. Mol. Plant-Microbe Interact. 2011, 24, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Kubo, T.; Takahashi, H.; Abe, H. Identification and Precise Mapping of Resistant QTLs of Cercospora Leaf Spot Resistance in Sugar Beet (Beta vulgaris L.). G3-Genes Genomes Genet. 2011, 1, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, A.; Koch, G.; Barnes, S.R.; Jung, C. Mapping quantitative trait loci (QTLs) for resistance to Cercospora leaf spot disease (Cercospora beticola Sacc.) in sugar beet (Beta vulgaris L.). Theor. Appl. Genet. 2000, 100, 1176–1182. [Google Scholar] [CrossRef]
- Windels, C.E.; Lamey, H.A.; Hilde, D.; Widner, J.; Knudsen, T. A Cercospora leaf spot model for sugar beet: In practice by an industry. Plant Dis. 1998, 82, 716–726. [Google Scholar] [CrossRef]
- Feindt, F.; Mendgen, K.; Heitefuss, R. The Influence of Stomatal Opening and Leaf Age on the Infection by Cercospora-Beticola in Resistant and Susceptible Leaves of Beta-Vulgaris. Phytopathol. Z. 1981, 101, 281–297. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Nan, J.D.; Yu, B. OMICS Technologies and Applications in Sugar Beet. Front. Plant Sci. 2016, 7, 900. [Google Scholar] [CrossRef] [PubMed]
- Rellan-Alvarez, R.; Andaluz, S.; Rodriguez-Celma, J.; Wohlgemuth, G.; Zocchi, G.; Alvarez-Fernandez, A.; Fiehn, O.; Lopez-Millan, A.F.; Abadia, J. Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafta, A.M.; Fugate, K.K. Metabolic profile of wound-induced changes in primary carbon metabolism in sugarbeet root. Phytochemistry 2011, 72, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.M.; Freeman, C.; Broeckling, C.D. Metabolome profiling to understand the defense response of sugar beet (Beta vulgaris) to Rhizoctonia solani AG 2–2 IIIB. Physiol. Mol. Plant Pathol. 2016, 94, 108–117. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Hallmann, E.; Lipowski, J.; Drela, N.; Kowalik, A.; Pussa, T.; Matt, D.; Luik, A.; Gozdowski, D.; Rembialkowska, E. Beetroot (Beta vulgaris L.) and naturally fermented beetroot juices from organic and conventional production: Metabolomics, antioxidant levels and anticancer activity. J. Sci. Food Agric. 2014, 94, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Fester, T. Plant metabolite profiles and the buffering capacities of ecosystems. Phytochemistry 2015, 110, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Ikeda, S.; Tsuda, S.; Someya, N.; Asano, K.; Kikuchi, J.; Chikayama, E.; Ono, H.; Sekiyama, Y. A survey of metabolic changes in potato leaves by NMR-based metabolic profiling in relation to resistance to late blight disease under field conditions. Magn. Reson. Chem. 2017, 55, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lowman, D.W.; Maciel, G.E. Determination of Sucrose in Sugar-Beet Juices by Nuclear Magnetic-Resonance Spectrometry. Anal. Chem. 1979, 51, 85–90. [Google Scholar] [CrossRef]
- Omarzad, O.; Pichon, R.; Kervarec, N.; Hagege, D. NMR natural abundance estimation of 13C-metabolic solutes in normal and habituated sugarbeet cell lines. Protoplasma 1998, 202, 145–152. [Google Scholar] [CrossRef]
- De Juan, C. Carbon metabolism in sugar beet leaves experiencing heat stress. In Proceedings of the 12th Annual Conference of the Metabolomics Society, Dublin, Ireland, 27–30 June 2016.
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, A.M.; Wheelock, C.E. Trials and tribulations of ’omics data analysis: Assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst. 2013, 9, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Riedl, J.; Esslinger, S.; Fauhl-Hassek, C. Review of validation and reporting of non-targeted fingerprinting approaches for food authentication. Anal. Chim. Acta 2015, 885, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.Y.; Pasikanti, K.K.; Nicholson, J.K. Global urinary metabolic profiling procedures using gas chromatography-mass spectrometry. Nat. Protoc. 2011, 6, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.L. The significance of the difference between two means when the population variances are unequal. Biometrika 1938, 29, 350–362. [Google Scholar] [CrossRef]
- Hoffmann, C.M. Sucrose Accumulation in Sugar Beet Under Drought Stress. J. Agron. Crop Sci. 2010, 196, 243–252. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mack, G.; Hoffmann, C.M. Organ-specific adaptation to low precipitation in solute concentration of sugar beet (Beta vulgaris L.). Eur. J. Agron. 2006, 25, 270–279. [Google Scholar] [CrossRef]
- Tomita, S.; Nemoto, T.; Matsuo, Y.; Shoji, T.; Tanaka, F.; Nakagawa, H.; Ono, H.; Kikuchi, J.; Ohnishi-Kameyama, M.; Sekiyama, Y. A NMR-based, non-targeted multistep metabolic profiling revealed L-rhamnitol as a metabolite that characterised apples from different geographic origins. Food Chem. 2015, 174, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, Y.; Chikayama, E.; Kikuchi, J. Evaluation of a Semipolar Solvent System as a Step toward Heteronuclear Multidimensional NMR-Based Metabolomics for 13C-Labelled Bacteria, Plants, and Animals. Anal. Chem. 2011, 83, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, Y.; Chikayama, E.; Kikuchi, J. Profiling Polar and Semipolar Plant Metabolites throughout Extraction Processes Using a Combined Solution-State and High-Resolution Magic Angle Spinning NMR Approach. Anal. Chem. 2010, 82, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- SOP for Preparation of NMR Extracts. Available online: http://www.metabolomics.bbsrc.ac.uk/techniques.htm (accessed on25 January 2017).
- Akiyama, K.; Chikayama, E.; Yuasa, H.; Shimada, Y.; Tohge, T.; Shinozaki, K.; Hirai, M.Y.; Sakurai, T.; Kikuchi, J.; Saito, K. PRIMe: A Web site that assembles tools for metabolomics and transcriptomics. In Silico Biol. 2008, 8, 339–345. [Google Scholar] [PubMed]
- Chikayama, E.; Sekiyama, Y.; Okamoto, M.; Nakanishi, Y.; Tsuboi, Y.; Akiyama, K.; Saito, K.; Shinozaki, K.; Kikuchi, J. Statistical Indices for Simultaneous Large-Scale Metabolite Detections for a Single NMR Spectrum. Anal. Chem. 2010, 82, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Platform for RIKEN Metabolomics. Available online: http://prime.psc.riken.jp/ (accessed on 25 January 2017).
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. Nmrpipe—A Multidimensional Spectral Processing System Based on Unix Pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]
- The Human Metabolome Database. Available online: http://www.hmdb.ca/ (accessed on 25 January 2017).
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res. 2008, 36, D402–D408. [Google Scholar] [CrossRef] [PubMed]
- The Biological Magnetic Resonance Data Bank. Available online: http://www.bmrb.wisc.edu/ (accessed on 25 January 2017).
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [PubMed]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 24 January 2017).
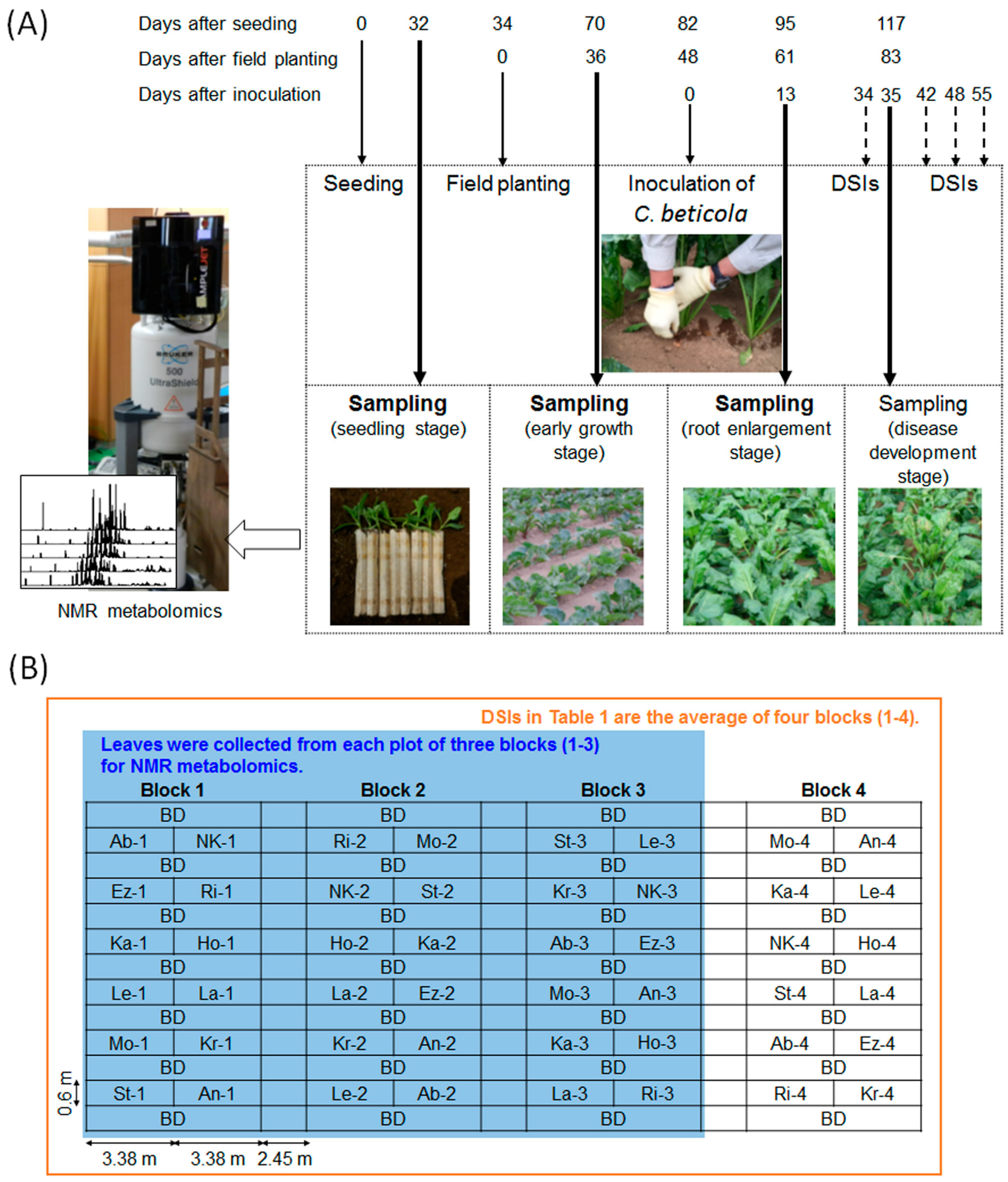

| Cultivars/Line (Abbreviations) 1 | Supplier 1 | Known Resistance Level to CLS | Disease Severity Indices in 2015 2,3 | |||
|---|---|---|---|---|---|---|
| 3 August | 11 August | 17 August | 24 August | |||
| Ezomaru (Ez) | KS | Weak | 3.0 a | 3.8 a | 4.6 a | 5.0 a |
| Abend (Ab) | SH | Weak | 2.8 a | 3.7 a,b | 4.5 a | 4.9 a |
| Kachimaru (Ka) | KS | Weak | 2.8 a,b | 3.6 a,b | 4.4 a | 4.9 a |
| Lemiel (Le) | SH | Weak | 2.8 a,b | 3.7 a,b | 4.2 a | 5.0 a |
| Monohikari (Mo) | HS | Moderate | 2.0 b | 3.1 b | 3.5 b | 4.1 b |
| Angy (An) | SS | Strong | 1.4 c,d | 2.4 c | 3.0 b,c | 3.8 b,c |
| La terre (La) | SH | Strong | 1.6 c,d | 2.4 c | 2.9 b,c,d | 3.6 b,c |
| Hokkaimitsuboshi (Ho) | HC | Strong | 1.4 c,d | 2.2 c | 2.8 c,d | 3.7 c |
| Krister (Kr) | SS | Strong | 1.3 c,d | 2.2 c,d | 2.7 c,d | 3.8 c |
| Stout (St) | SH | Strong | 1.4 c,d,e | 2.1 c,d,e | 2.5 c,d,e | 3.1 c |
| Rivolta (Ri) | SS | Strong | 0.8 d,e | 1.6 d,e | 2.3 d,e | 2.8 c,d |
| NK-310mm-O (NK) [6] | HS | Strong | 0.7 e | 1.5 e | 2.0 e | 2.6 d |
| Bucket (ppm) | Presumed Compounds | PLS-VIP | Pearson’s Correlation for DSIs on August | Welch’s t-Test p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y Variable = DSIs on August | |||||||||||
| 4 | 11 | 17 | 24 | 4 | 11 | 17 | 24 | ||||
| Seedling stage | 3.96–3.92 | Unidentified | 2.46 | 2.40 | 2.32 | 2.51 | 0.55 | 0.52 | 0.52 | 0.52 | 0.00404 |
| Early growth stage | 3.00–2.96 | GABA, Asn | 2.52 | 2.55 | 2.44 | 2.60 | −0.70 | −0.69 | −0.66 | −0.69 | 0.00011 |
| 2.36–2.32 | Glu, MA | 2.33 | 2.33 | 2.22 | 2.24 | −0.65 | −0.63 | −0.60 | −0.60 | 0.00068 | |
| 2.44–2.40 | Gln | 2.26 | 2.36 | 2.27 | 2.45 | −0.63 | −0.63 | −0.61 | −0.65 | 0.00266 | |
| 8.00–7.96 | Unidentified | 2.13 | 2.04 | 2.09 | * | 0.59 | 0.55 | 0.56 | 0.52 | 0.00048 | |
| 2.32–2.28 | GABA, Glu, Pro | 2.12 | 2.13 | 2.17 | 2.15 | −0.59 | −0.57 | −0.59 | −0.57 | 0.00048 | |
| 2.28–2.24 | GABA, Val | 2.11 | 2.07 | 2.15 | 2.06 | −0.59 | −0.55 | −0.58 | −0.55 | 0.00016 | |
| 5.04–5.00 | Unidentified | 2.10 | 2.06 | 2.13 | 2.01 | 0.58 | 0.55 | 0.57 | 0.54 | 0.00046 | |
| 1.92–1.88 | GABA, Acetate | 2.07 | 2.07 | 2.12 | 2.02 | −0.58 | −0.57 | −0.55 | −0.54 | 0.00356 | |
| 5.72–5.68 | Unidentified | 2.07 | 2.07 | 2.18 | 2.37 | −0.57 | −0.58 | −0.56 | −0.63 | 0.00500 | |
| 2.12–2.08 | Glu/Gln/Pro | 1.83 | 1.92 | 1.94 | 1.92 | −0.51 | −0.52 | −0.52 | −0.51 | 0.00762 | |
| Root-enlargement stage | 5.04–5.00 | Unidentified | 2.79 | 2.75 | 2.79 | 2.63 | 0.66 | 0.63 | 0.65 | 0.59 | 0.00003 |
| 8.00–7.96 | Unidentified | 2.40 | 2.34 | 2.39 | 2.30 | 0.57 | 0.53 | 0.56 | 0.52 | 0.00075 | |
| 2.44–2.40 | Gln | 2.19 | 2.31 | 2.31 | 2.29 | −0.52 | −0.53 | −0.53 | −0.52 | 0.01508 | |
| 5.72–5.68 | Unidentified | 2.18 | 2.40 | 2.24 | 2.63 | −0.52 | −0.55 | −0.52 | −0.59 | 0.01293 | |
| Disease-development stage | 3.96–3.92 | Unidentified | 3.05 | 2.94 | 3.02 | 2.78 | 0.64 | 0.53 | 0.64 | 0.58 | 0.00014 |
| 8.00–7.96 | Unidentified | 2.79 | 2.59 | 2.75 | 2.58 | 0.59 | 0.51 | 0.58 | 0.54 | 0.00199 | |
| 3.60–3.56 | Suc, Fru | 2.35 | 2.46 | 2.47 | 2.41 | 0.54 | 0.53 | 0.56 | 0.56 | 0.01193 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekiyama, Y.; Okazaki, K.; Kikuchi, J.; Ikeda, S. NMR-Based Metabolic Profiling of Field-Grown Leaves from Sugar Beet Plants Harbouring Different Levels of Resistance to Cercospora Leaf Spot Disease. Metabolites 2017, 7, 4. https://doi.org/10.3390/metabo7010004
Sekiyama Y, Okazaki K, Kikuchi J, Ikeda S. NMR-Based Metabolic Profiling of Field-Grown Leaves from Sugar Beet Plants Harbouring Different Levels of Resistance to Cercospora Leaf Spot Disease. Metabolites. 2017; 7(1):4. https://doi.org/10.3390/metabo7010004
Chicago/Turabian StyleSekiyama, Yasuyo, Kazuyuki Okazaki, Jun Kikuchi, and Seishi Ikeda. 2017. "NMR-Based Metabolic Profiling of Field-Grown Leaves from Sugar Beet Plants Harbouring Different Levels of Resistance to Cercospora Leaf Spot Disease" Metabolites 7, no. 1: 4. https://doi.org/10.3390/metabo7010004