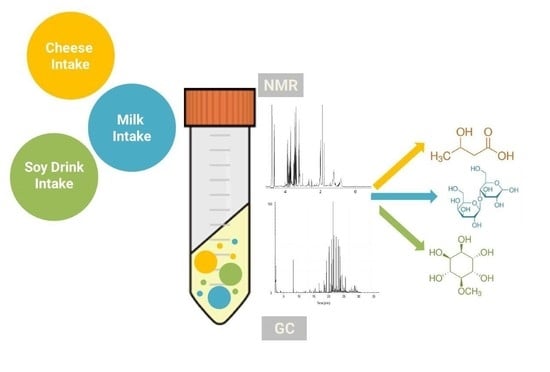
GC-MS Based Metabolomics and NMR Spectroscopy Investigation of Food Intake Biomarkers for Milk and Cheese in Serum of Healthy Humans
Abstract
:1. Introduction
2. Results
2.1. GC-MS
2.1.1. Candidate Fibs for Milk
2.1.2. Candidate Fibs for Cheese
2.1.3. Candidate FIBs for Soy Drink
2.1.4. Targeted Evaluation of C15:0 and C17:0 in Serum Samples
2.1.5. Fatty Acid Amounts in Test Foods
2.2. NMR
2.2.1. Candidate FIBs for Milk
2.2.2. Candidate FIBs for Cheese
2.2.3. Candidate FIBs for Soy Drink
2.3. Comparison of the Serum and Urinary Postprandial Profiles of Unmetabolized Candidate FIBs for Milk, Cheese, and Soy Drink
3. Discussion
3.1. Stengtening the Evidence by Measuring Postprandial Kinetics of Candidate FIBs both in Serum and Urine by GC-MS and NMR
3.2. Lactose-Derived Metabolites as Candidate FIBs for Milk Intake
3.3. Amino Acid-Derived Metabolites as Candidate FIBs for Cheese
- A survey of the free amino acids released during the ripening of a range of Swiss cheese showed that proline was among the most released amino acids in Gruyère [35]. Postprandial proline appeared in the plasma of healthy adults after ingestion of whey proteins [36] and in infants fed a milk-based formula [37].
- Methionine was among the most released amino acids during the ripening of Appenzeller cheese [35]. Postprandial methionine levels were increased in infants fed a milk-based formula [37] as well as in the plasma of obese subjects fed a whey isolate [38] but not as much after the intake by obese subjects of a whey hydrolysate [39], probably due to oxidation into its sulfoxide in the latter product. Of note, as whey is removed during the production of Gruyère cheese, the high content of methionine in casein could still explain our finding. In that context, it is interesting to note that Stanstrup et al. [38] reported similar postprandial kinetics than ours, although casein and whey proteins are digested with different kinetics. The cheese ripening process is, however, likely to eliminate these differences as a consequence of the pre-digestive properties associated with the fermentation process.
- Leucine was among the most released amino acids during the ripening of Gruyère [35]. The iAUC of this amino acid could be increased in infants fed a milk-based formula [37], in a dose-dependent manner in healthy adults fed whey protein [36], and in healthy older people by supplementing a whey protein extract with leucine [40].
- Tyrosine is also one predominant amino acid in casein [42]. The concentration of tyrosine was shown to increase during cheese ripening in a model cheese [43] as well as in Gruyère [35]. This amino acid was also found in higher concentration in urine when cheese was consumed, both in comparison with a control meal and with milk [23]. Postprandial tyrosine also appeared in a dose-dependent manner in the plasma of healthy adults after ingestion of whey proteins [36] and in infants fed a milk-based formula [37].
- Valine and isoleucine are two of the main amino acids in casein [42] and their concentrations increase during ripening [35]. The iAUC of these two amino acids could be increased in infants fed a milk-based formula [37], in a dose-dependent manner in healthy adults fed whey protein [36], and in healthy older people by supplementing a whey protein extract with leucine [40]. Both amino acids were also increased in the plasma of obese subjects after the intake of a whey isolate [39] as well as after intake of a caseinoglycomacropeptide [38], a whey protein derivate rich in these two amino acids. These insulinotropic amino acids are rapidly taken up by the organism peaking postprandially at 1 to 2 h [38,44,45], as also reported in our results. Interestingly, 3-hydroxyisobutyrate was found by NMR in higher concentrations in the serum of our subjects after cheese intake. The postprandial concentration of this organic acid, which is an intermediate in the catabolism of valine, was also increased in infants fed a milk-based formula [37].
3.4. Heterogeneous Molecular Pattern of Candidate FIBs for Soy Drink
3.5. Targeted Lipid Analysis in Food and Serum
3.6. Limitations of Study
4. Materials and Methods
4.1. Study Design
4.2. Untargeted GC-MS Analysis of Serum Samples
4.3. Assessment of Serum Pentadecanoic and Heptadecanoic Acid by GC-MS
4.4. Fatty Acid Profiles of Total Lipids of Test Foods Assessed by High Resolution GC FID
4.5. NMR Sample Preparation and Analysis
4.6. Analysis of NMR Spectra
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kristensen, M.; Engelsen, S.B.; Dragsted, L.O. LC-MS metabolomics top-down approach reveals new exposure and effect biomarkers of apple and apple-pectin intake. Metabolomics 2012, 8, 64–73. [Google Scholar] [CrossRef]
- Jenab, M.; Slimani, N.; Bictash, M.; Ferrari, P.; Bingham, S.A. Biomarkers in nutritional epidemiology: Applications, needs and new horizons. Hum. Genet. 2009, 125, 507–525. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Day, N.E.; Welch, A.A.; Runswick, S.A.; Luben, R.N.; Mulligan, A.A.; McTaggart, A.; Bingham, S.A. Use of biological markers to validate self-reported dietary intake in a random sample of the european prospective investigation into cancer united kingdom norfolk cohort. Am. J. Clin. Nutr. 2001, 74, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, A.; Capozzi, F. Foodomics for healthy nutrition. Curr. Opin. Clin. Nutr. 2014, 17, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis chemical analysis working group (cawg) metabolomics standards initiative (msi). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cuparencu, C.S.; Andersen, M.-B.S.; Gürdeniz, G.; Schou, S.S.; Mortensen, M.W.; Raben, A.; Astrup, A.; Dragsted, L.O. Identification of urinary biomarkers after consumption of sea buckthorn and strawberry, by untargeted lc–ms metabolomics: A meal study in adult men. Metabolomics 2016, 12, 31. [Google Scholar] [CrossRef]
- Pujos-Guillot, E.; Hubert, J.; Martin, J.F.; Lyan, B.; Quintana, M.; Claude, S.; Chabanas, B.; Rothwell, J.A.; Bennetau-Pelissero, C.; Scalbert, A.; et al. Mass spectrometry-based metabolomics for the discovery of biomarkers of fruit and vegetable intake: Citrus fruit as a case study. J. Proteome Res. 2013, 12, 1645–1659. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Brennan, L. The role of metabolomics in determination of new dietary biomarkers. Proc. Nutr. Soc. 2017, 76, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, H.; Brennan, L. Metabolomics as a tool in the identification of dietary biomarkers. Proc. Nutr. Soc. 2017, 76, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Brennan, L.; Drevon, C.A.; van Kranen, H.; Manach, C.; Dragsted, L.O.; Roche, H.M.; Andres-Lacueva, C.; Bakker, S.J.L.; Bouwman, J.; et al. Combining traditional dietary assessment methods with novel metabolomics techniques: Present efforts by the food biomarker alliance. Proc. Nutr. Soc. 2017, 76, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Pratico, G.; Scalbert, A.; Vergeres, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Andres-Lacueva, C.; et al. A scheme for a flexible classification of dietary and health biomarkers. Genes Nutr. 2017, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Pratico, G.; Gao, Q.; Scalbert, A.; Vergeres, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Pedapati, S.H.; Afman, L.A.; Wishart, D.S.; et al. Guidelines for biomarker of food intake reviews (bfirev): How to conduct an extensive literature search for biomarker of food intake discovery. Genes Nutr. 2018, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.C.; Hoppe, C.; Petersen, B.O.; Duus, J.O.; Molgaard, C.; Michaelsen, K.F. An nmr-based metabonomic investigation on effects of milk and meat protein diets given to 8-year-old boys. Br. J. Nutr. 2007, 97, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Albani, V.; Celis-Morales, C.; Marsaux, C.F.; Forster, H.; O’Donovan, C.B.; Woolhead, C.; Macready, A.L.; Fallaize, R.; Navas-Carretero, S.; San-Cristobal, R.; et al. Exploring the association of diary product intake with the fatty acids c15:0 and c17:0 measured from dried blood spots in a multi-population cohort: Findings from the food4me study. Mol. Nutr. Food Res. 2016, 60, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Brevik, A.; Veierod, M.B.; Drevon, C.A.; Andersen, L.F. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur. J. Clin. Nutr. 2005, 59, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Smedman, A.E.M.; Gustafsson, I.B.; Berglund, L.G.T.; Vessby, B.O.H. Pentadecanoic acid in serum as a marker for intake of milk fat: Relations between intake of milk fat and metabolic risk factors. Am. J. Clin. Nutr. 1999, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wolk, A.; Furuheim, M.; Vessby, B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J. Nutr. 2001, 131, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.M.; Cyr, A.; Lepine, M.C.; Labonte, M.E.; Couture, P.; Jones, P.J.; Lamarche, B. Recommended dairy product intake modulates circulating fatty acid profile in healthy adults: A multi-centre cross-over study. Br. J. Nutr. 2015, 113, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Golley, R.K.; Hendrie, G.A. Evaluation of the relative concentration of serum fatty acids c14:0, c15:0 and c17:0 as markers of children’s dairy fat intake. Ann. Nutr. Metab. 2014, 65, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.M. Concerns about the use of 15:0, 17:0, and trans-16:1n-7 as biomarkers of dairy fat intake in recent observational studies that suggest beneficial effects of dairy food on incidence of diabetes and stroke. Am. J. Clin. Nutr. 2015, 101, 1102–1103. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.J.; Seyssel, K.; Chiu, S.; Pan, P.H.; Lin, S.Y.; Stanley, E.; Ament, Z.; West, J.A.; Summerhill, K.; Griffin, J.L.; et al. Odd chain fatty acids; new insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose intolerance. Sci. Rep. 2017, 7, 44845. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.M.M.; Nebel, C.; Nielsen, N.C.; Andersen, H.J.; Olsson, J.; Simren, M.; Ohman, L.; Svensson, U.; Bertram, H.C.; Malmendal, A. A gc-ms-based metabonomic investigation of blood serum from irritable bowel syndrome patients undergoing intervention with acidified milk products. Eur. Food Res. Technol. 2011, 233, 1013–1021. [Google Scholar] [CrossRef]
- Zheng, H.; Yde, C.C.; Clausen, M.R.; Kristensen, M.; Lorenzen, J.; Astrup, A.; Bertram, H.C. Metabolomics investigation to shed light on cheese as a possible piece in the french paradox puzzle. J. Agric. Food Chem. 2015, 63, 2830–2839. [Google Scholar] [CrossRef] [PubMed]
- Hjerpsted, J.B.; Ritz, C.; Schou, S.S.; Tholstrup, T.; Dragsted, L.O. Effect of cheese and butter intake on metabolites in urine using an untargeted metabolomics approach. Metabolomics 2014, 10, 1176–1185. [Google Scholar] [CrossRef]
- Münger, L.H.; Trimigno, A.; Picone, G.; Freiburghaus, C.; Pimentel, G.; Burton, K.J.; Pralong, F.P.; Vionnet, N.; Capozzi, F.; Badertscher, R.; et al. Identification of urinary food intake biomarkers for milk, cheese, and soy-based drink by untargeted gc-ms and nmr in healthy humans. J. Proteome Res. 2017, 16, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.S.; Savorani, F.; Rasmussen, M.A.; Jespersen, B.M.; Kristensen, M.; Engelsen, S.B. New insights from a β-glucan human intervention study using nmr metabolomics. Food Res. Int. 2014, 63, 210–217. [Google Scholar] [CrossRef]
- Pimentel, G.; Burton, K.J.; Rosikiewicz, M.; Freiburghaus, C.; von Ah, U.; Munger, L.H.; Pralong, F.P.; Vionnet, N.; Greub, G.; Badertscher, R.; et al. Blood lactose after dairy product intake in healthy men. Br. J. Nutr. 2017, 118, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lee, M.J.; Sheng, S.; Meng, X.; Prabhu, S.; Winnik, B.; Huang, B.; Chung, J.Y.; Yan, S.; Ho, C.T.; et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem. Res. Toxicol. 2000, 13, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Pellis, L.; van Erk, M.J.; van Ommen, B.; Bakker, G.C.; Hendriks, H.F.; Cnubben, N.H.; Kleemann, R.; van Someren, E.P.; Bobeldijk, I.; Rubingh, C.M.; et al. Plasma metabolomics and proteomics profiling after a postprandial challenge reveal subtle diet effects on human metabolic status. Metabolomics 2012, 8, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, G.; Burton, K.J.; von Ah, U.; Bütikofer, U.; Pralong, F.P.; Vionnet, N.; Portmann, R.; Vergères, G. Metabolic footprinting of yogurt intake in serum of healthy men. J. Nutr. 2018, in press. [Google Scholar]
- Coelho, A.I.; Berry, G.T.; Rubio-Gozalbo, M.E. Galactose metabolism and health. Curr. Opin.Clin. Nutr. 2015, 18, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Bierenstiel, M.; Schlaf, M. Δ-galactonolactone: Synthesis, isolation, and comparative structure and stability analysis of an elusive sugar derivative. Eur. J. Org. Chem. 2004, 2004, 1474–1481. [Google Scholar] [CrossRef]
- Cuatrecasas, P.; Segal, S. Galactose conversion to d-xylulose: An alternate route of galactose metabolism. Science 1966, 153, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Bütikofer, U.; Fuchs, D. Development of free amino acids in appenzeller, emmentaler, gruyère, raclette, sbrinz and tilsiter cheese. Le Lait 1997, 77, 91–100. [Google Scholar] [CrossRef]
- Gunnerud, U.J.; Ostman, E.M.; Bjorck, I.M. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose-response study. Eur. J. Clin. Nutr. 2013, 67, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Slupsky, C.M.; He, X.; Hernell, O.; Andersson, Y.; Rudolph, C.; Lonnerdal, B.; West, C.E. Postprandial metabolic response of breast-fed infants and infants fed lactose-free vs. regular infant formula: A randomized controlled trial. Sci. Rep. 2017, 7, 3640. [Google Scholar] [CrossRef] [PubMed]
- Stanstrup, J.; Rasmussen, J.E.; Ritz, C.; Holmer-Jensen, J.; Hermansen, K.; Dragsted, L.O. Intakes of whey protein hydrolysate and whole whey proteins are discriminated by lc-ms metabolomics. Metabolomics 2014, 10, 719–736. [Google Scholar] [CrossRef]
- Stanstrup, J.; Schou, S.S.; Holmer-Jensen, J.; Hermansen, K.; Dragsted, L.O. Whey protein delays gastric emptying and suppresses plasma fatty acids and their metabolites compared to casein, gluten, and fish protein. J. Proteome Res. 2014, 13, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Deutz, N.E.; Memelink, R.G.; Verlaan, S.; Wolfe, R.R. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: A randomized controlled trial. Nutr. J. 2014, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Le Boucher, C.; Gagnaire, V.; Briard-Bion, V.; Jardin, J.; Maillard, M.B.; Dervilly-Pinel, G.; Le Bizec, B.; Lortal, S.; Jeanson, S.; Thierry, A. Spatial distribution of lactococcus lactis colonies modulates the production of major metabolites during the ripening of a model cheese. Appl. Environ. Microb. 2015, 82, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Von Post-Skagegård, M.; Vessby, B.; Karlström, B. Glucose and insulin responses in healthy women after intake of composite meals containing cod-, milk-, and soy protein. Eur. J. Clin. Nutr. 2006, 60, 949. [Google Scholar] [CrossRef] [PubMed]
- Le Boucher, C.; Courant, F.; Royer, A.-L.; Jeanson, S.; Lortal, S.; Dervilly-Pinel, G.; Thierry, A.; Le Bizec, B. Lc–hrms fingerprinting as an efficient approach to highlight fine differences in cheese metabolome during ripening. Metabolomics 2015, 11, 1117–1130. [Google Scholar] [CrossRef]
- Nilsson, M.; Stenberg, M.; Frid, A.H.; Holst, J.J.; Björck, I.M. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: The role of plasma amino acids and incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Holmer-Jensen, J.; Mortensen, L.S.; Astrup, A.; de Vrese, M.; Holst, J.J.; Thomsen, C.; Hermansen, K. Acute differential effects of dietary protein quality on postprandial lipemia in obese non-diabetic subjects. Nutr. Res. 2013, 33, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Barillaro, C.; Liperoti, R.; Martone, A.M.; Onder, G.; Landi, F. The new metabolic treatments for sarcopenia. Aging Clin. Exp. Res. 2013, 25, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Woo, J. Nutritional interventions in sarcopenia: Where do we stand? Curr. Opin. Clin. Nutr. 2018, 21, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Negishi, O.; Mun’im, A.; Negishi, Y. Content of methylated inositols in familiar edible plants. J. Agric. Food Chem. 2015, 63, 2683–2688. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Kristal, A.R.; Shikany, J.M.; Wilson, A.C.; Chen, C.; Mares-Perlman, J.A.; Masaki, K.H.; Caan, B.J. Correlates of serum alpha- and gamma-tocopherol in the women’s health initiative. Ann. Epidemiol. 2001, 11, 136–144. [Google Scholar] [CrossRef]
- Matthaus, B.; Ozcan, M.M. Fatty acid and tocopherol contents of several soybean oils. Nat. Prod. Res. 2014, 28, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.S.; Seguin, P. Factors affecting tocopherol concentrations in soybean seeds. J. Agric. Food Chem. 2016, 64, 9465–9474. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Lee, S.; Leonard, S.W.; Atkinson, J.K.; Kamal-Eldin, A.; Traber, M.G. Sex differences in the inhibition of gamma-tocopherol metabolism by a single dose of dietary sesame oil in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Schwab, U. Biomarkers of dairy fat. Am. J. Clin. Nutr. 2015, 101, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Trimigno, A. Using metabolomics to describe food in detail. In Metabolomics as a Tool in Nutrition Research; Sébédio, J.L., Brennan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 203–230. [Google Scholar]
- Roxborough, H.E.; Burton, G.W.; Kelly, F.J. Inter- and intra-individual variation in plasma and red blood cell vitamin e after supplementation. Free Radic. Res. 2000, 33, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomas-Barberan, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Almenara, X.; Brezmes, J.; Vinaixa, M.; Samino, S.; Ramirez, N.; Ramon-Krauel, M.; Lerin, C.; Diaz, M.; Ibanez, L.; Correig, X.; et al. Erah: A computational tool integrating spectral deconvolution and alignment with quantification and identification of metabolites in gc/ms-based metabolomics. Anal. Chem. 2016, 88, 9821–9829. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Gel, Y.; Brunner, E.; Konietschke, F. Nparld: An R software package for the nonparametric analysis of longitudinal data in factorial experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef]
- Collomb, M.; Bühler, T. Analyse de la composition en acides gras de la graisse de lait, I. Optimisation et validation d’une méthode générale à haute résolution. Mitt. Lebensm. Hyg. 2000, 91, 306–332. (In French) [Google Scholar]
- Saude, E.J.; Sykes, B.D. Urine stability for metabolomic studies: Effects of preparation and storage. Metabolomic 2007, 3, 19–27. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.S.; Koenig, B.G.; Metcalfe, C.D. Analysis of acidic drugs in the effluents of sewage treatment plants using liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2002, 952, 139–147. [Google Scholar] [CrossRef]
- Wu, N.; Peck, T.L.; Webb, A.G.; Magin, R.L.; Sweedler, J.V. 1H-NMR spectroscopy on the nanoliter scale for static and on-line measurements. Anal. Chem. 1994, 66, 3849–3857. [Google Scholar] [CrossRef]












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimigno, A.; Münger, L.; Picone, G.; Freiburghaus, C.; Pimentel, G.; Vionnet, N.; Pralong, F.; Capozzi, F.; Badertscher, R.; Vergères, G. GC-MS Based Metabolomics and NMR Spectroscopy Investigation of Food Intake Biomarkers for Milk and Cheese in Serum of Healthy Humans. Metabolites 2018, 8, 26. https://doi.org/10.3390/metabo8020026
Trimigno A, Münger L, Picone G, Freiburghaus C, Pimentel G, Vionnet N, Pralong F, Capozzi F, Badertscher R, Vergères G. GC-MS Based Metabolomics and NMR Spectroscopy Investigation of Food Intake Biomarkers for Milk and Cheese in Serum of Healthy Humans. Metabolites. 2018; 8(2):26. https://doi.org/10.3390/metabo8020026
Chicago/Turabian StyleTrimigno, Alessia, Linda Münger, Gianfranco Picone, Carola Freiburghaus, Grégory Pimentel, Nathalie Vionnet, François Pralong, Francesco Capozzi, René Badertscher, and Guy Vergères. 2018. "GC-MS Based Metabolomics and NMR Spectroscopy Investigation of Food Intake Biomarkers for Milk and Cheese in Serum of Healthy Humans" Metabolites 8, no. 2: 26. https://doi.org/10.3390/metabo8020026