TSG-6 Inhibits the NF-κB Signaling Pathway and Promotes the Odontogenic Differentiation of Dental Pulp Stem Cells via CD44 in an Inflammatory Environment
Abstract
:1. Background
2. Methods
2.1. Cell Culture and Identification
2.2. Cell Induction
2.3. Real-Time Polymerase Chain Reaction
2.4. P65 Nuclear Transfer Assay
2.5. Cell Counting Kit-8
2.6. Determination of Alkaline Phosphatase Activity
2.7. 5-Ethynyl-2’-deoxyuridine Cell Activity Assay
2.8. Western Blotting Analysis
2.9. Subcutaneous Transplantation
2.10. Rat Mandibular Bone Culture
2.11. Statistical Analysis
3. Results
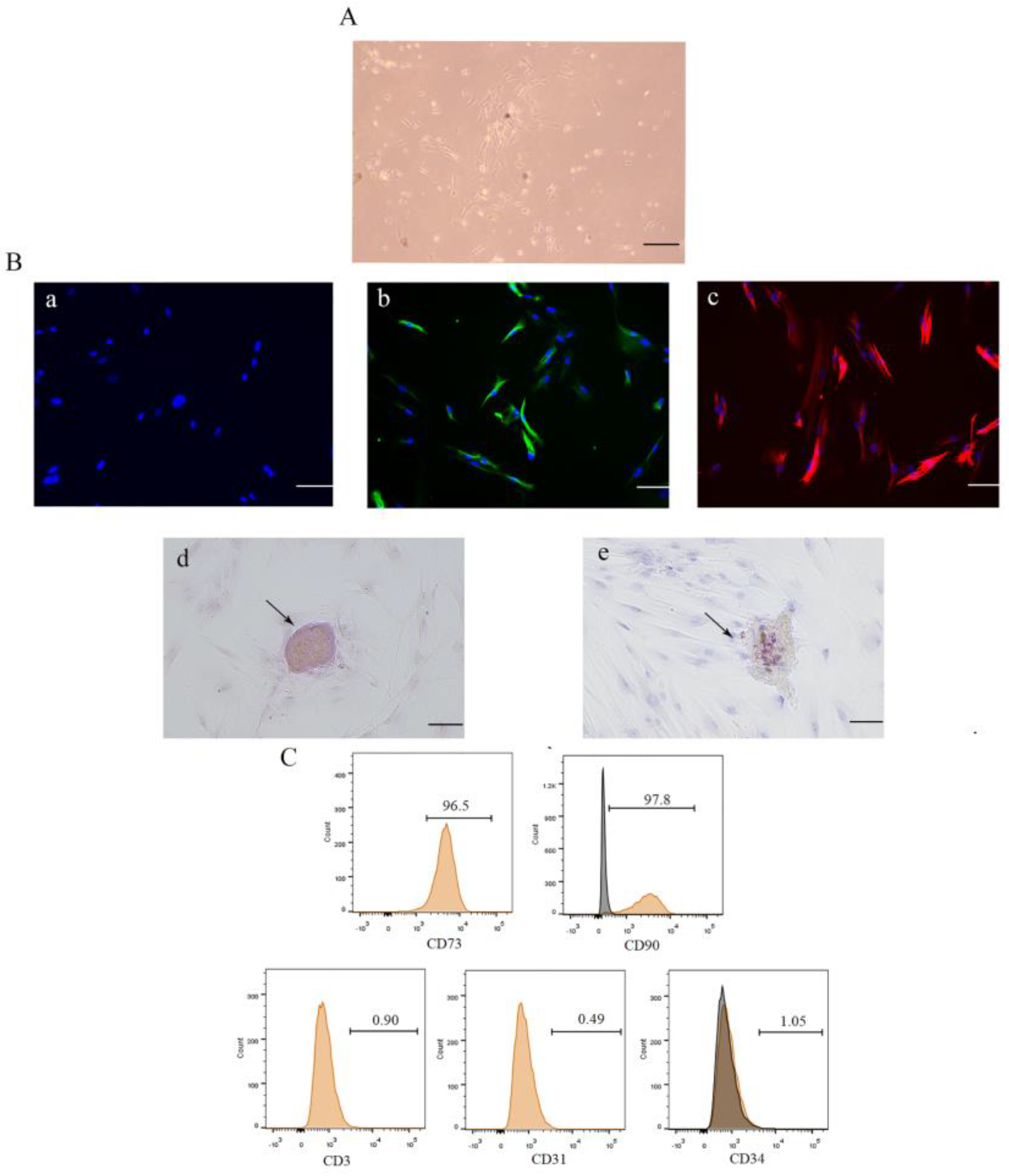
3.1. Isolation, Culture, and Identification of DPSCs
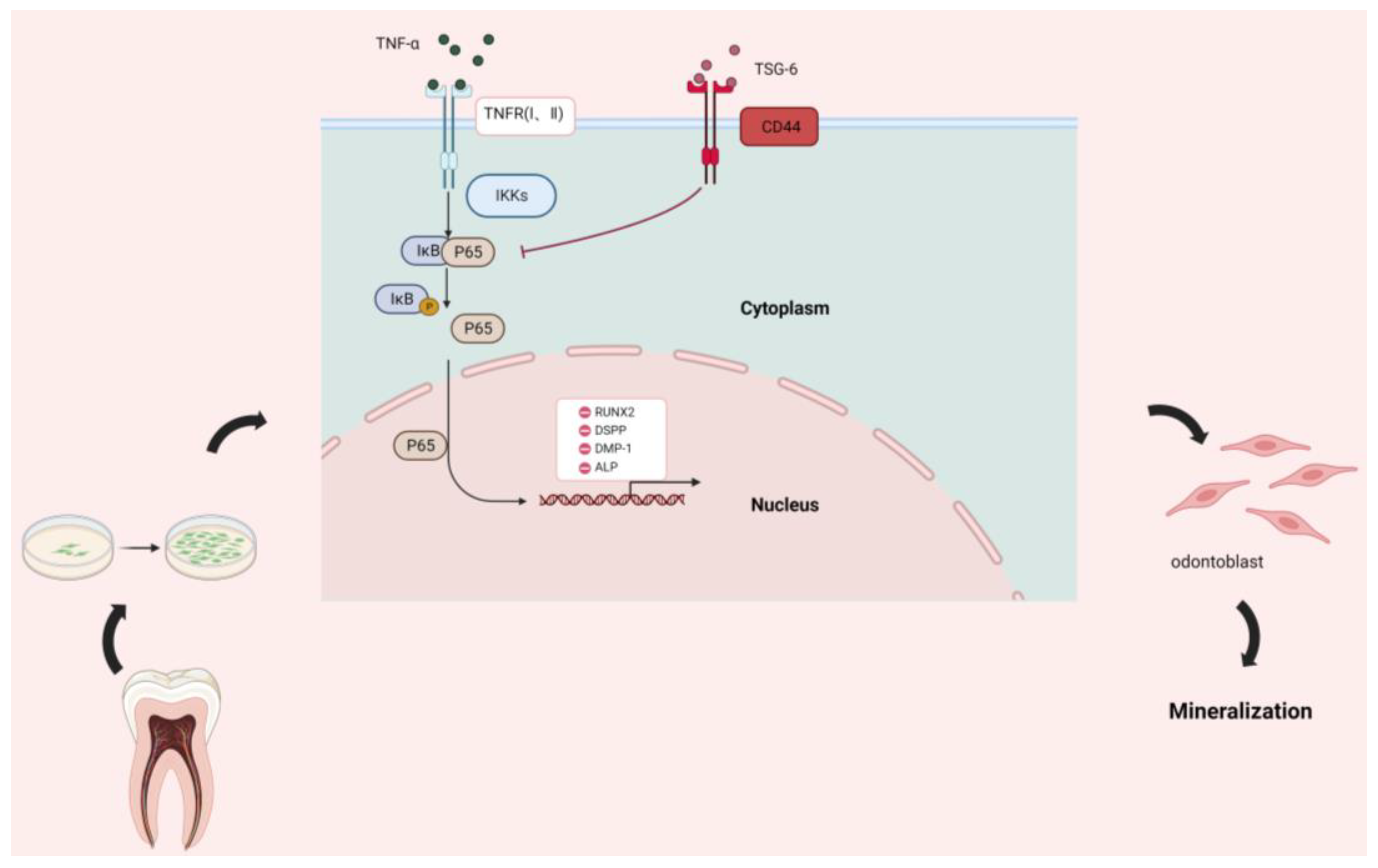
3.1.1. Exogenous TSG-6 Inhibited the NF-κB Signaling Pathway and Partially Rescued the Suppressed Mineralization of DPSCs in an Inflammatory Environment
3.1.2. Exogenous TSG-6 Treatment Promoted the Mineralization of DPSCs by Inhibiting the NF-κB Signaling Pathway
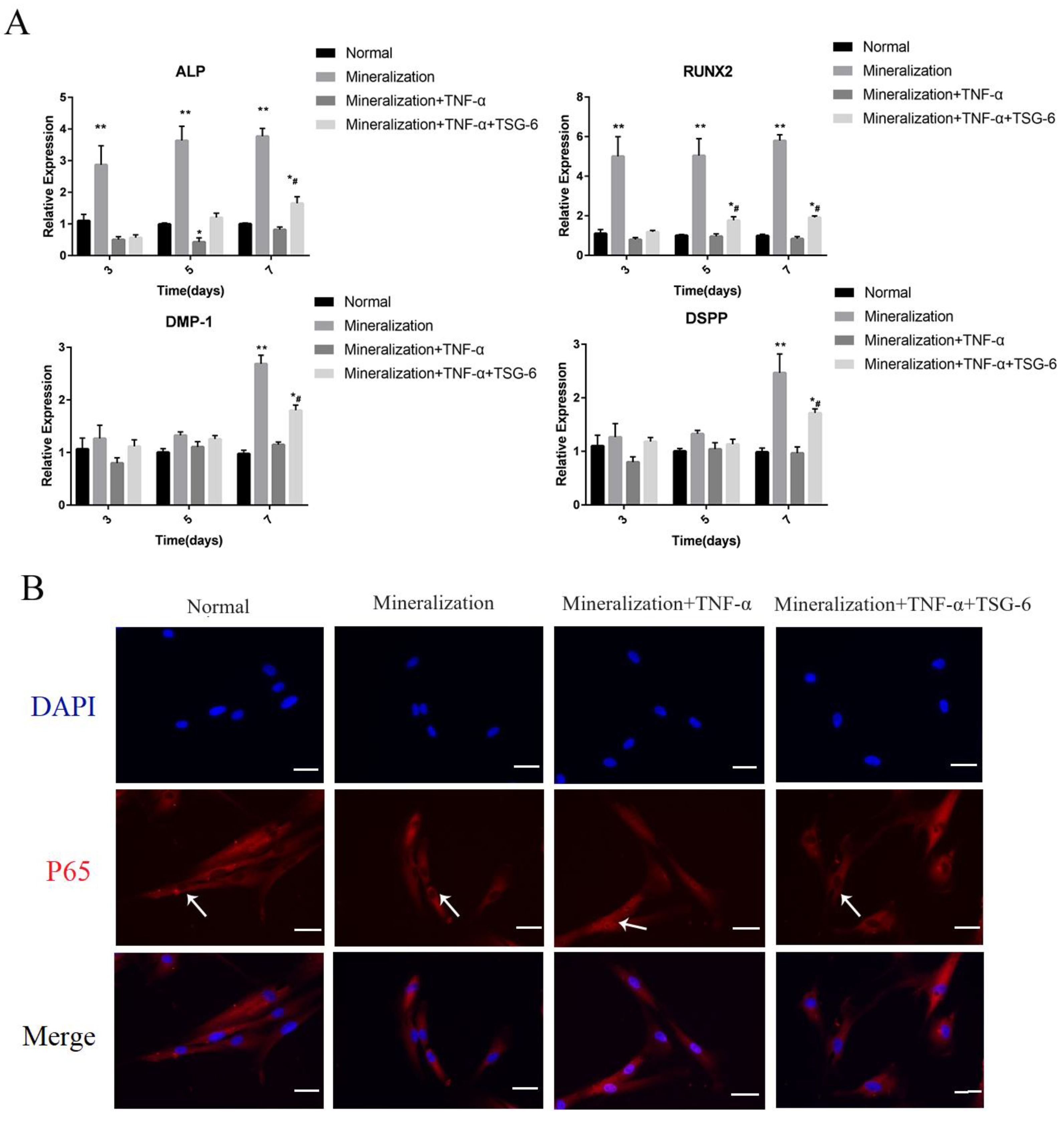
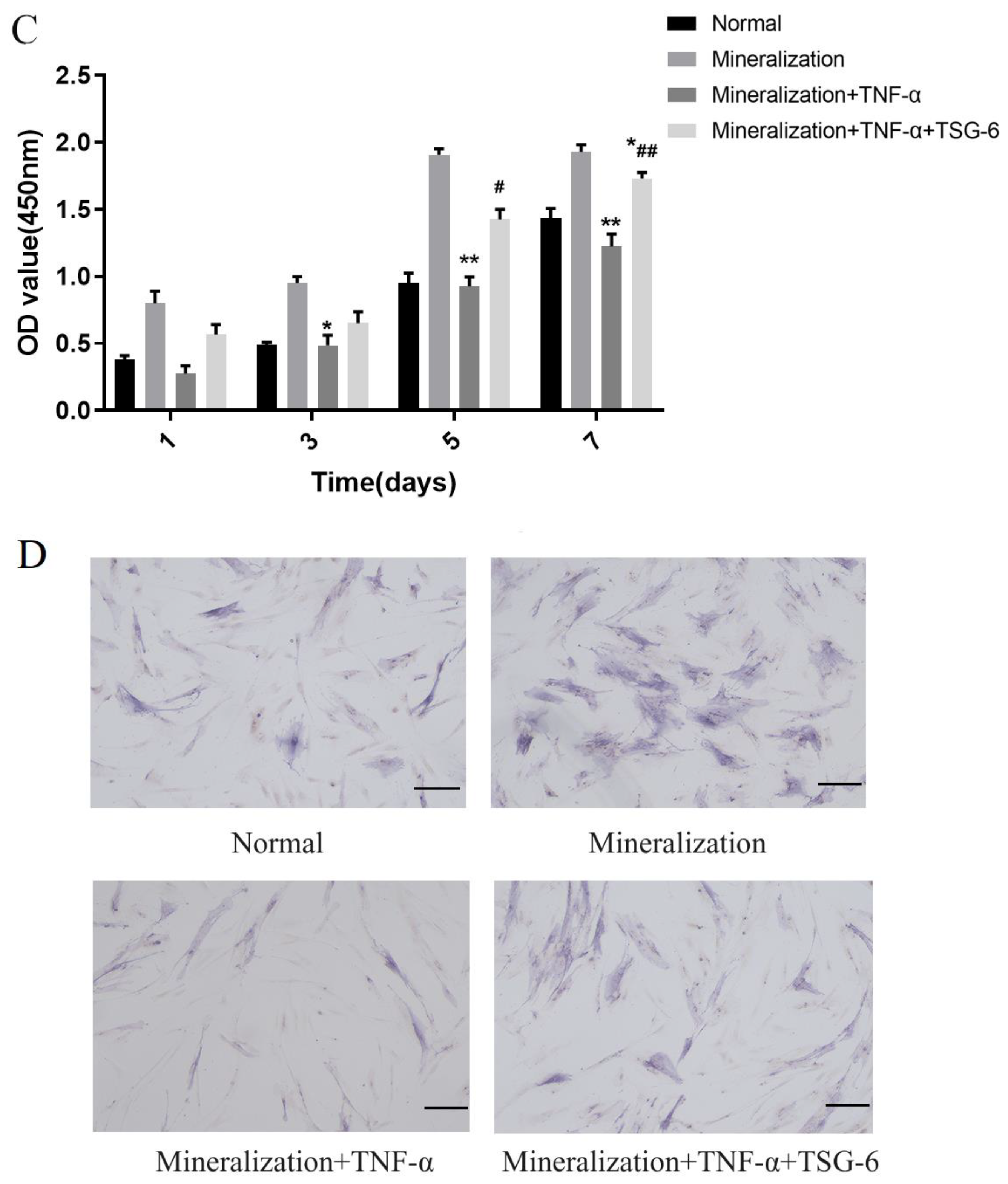

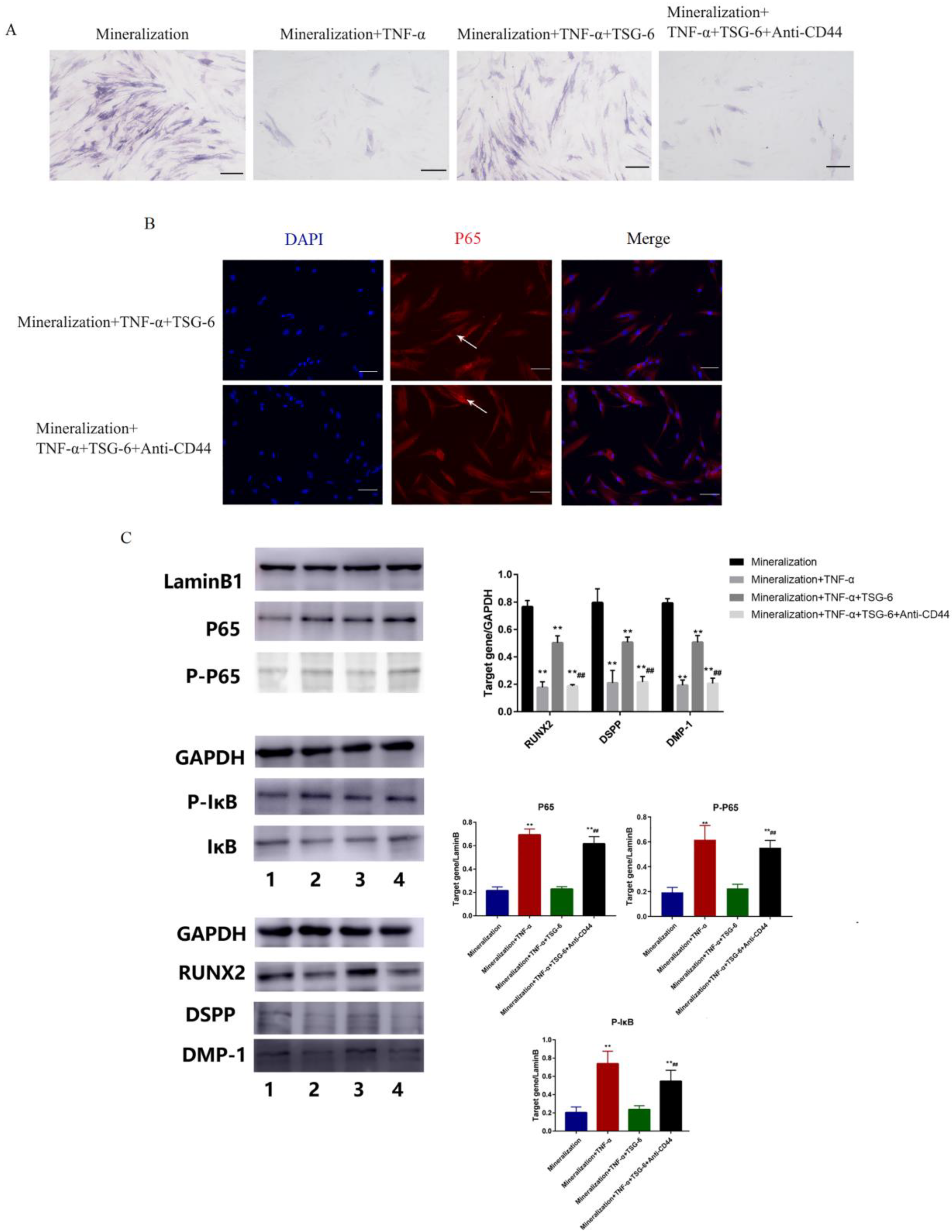
3.1.3. CD44 was the “Switch” Whereby TSG-6 Inhibited the NF-κB Signaling Pathway and Enhanced the Mineralization of DPSCs
3.1.4. TSG-6 Was Beneficial for Subcutaneous Osteogenesis and Mandibular Bone Culture in an Inflammatory Environment
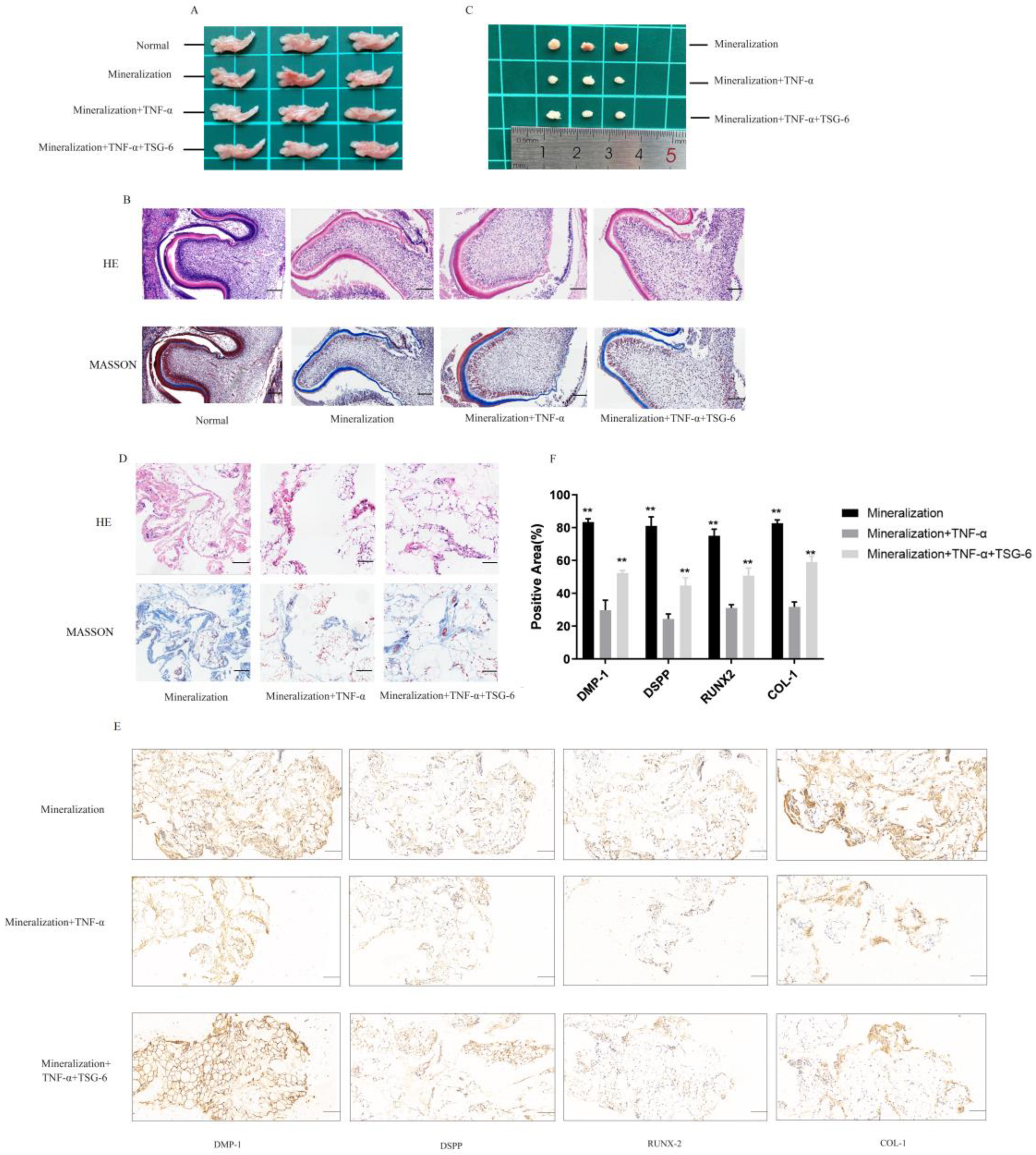
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neuhaus, K. Teeth: Malignant neoplasms in the dental pulp? Lancet Oncol. 2007, 8, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, H.; Wu, X.; Duan, Y.; Zhang, S.; Hu, H.; Liao, Y.; Wang, T.; Yang, Y.; Chen, G.; et al. Regeneration of pulpo-dentinal-like complex by a group of unique multipotent CD24a stem cells. Sci. Adv. 2020, 6, eaay1514. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Huang, H.; Wang, M.; Rong, W.; Wang, J. Applications of Antioxidants in Dental Procedures. Antioxidants 2022, 11, 2492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, W.; Yan, Z.; Zhao, L.; Bian, X.; Liu, C.; Qi, Z.; Zhang, S.; Tang, Z. Root canal treatment planning by automatic tooth and root canal segmentation in dental CBCT with deep multi-task feature learning. Med. Image Anal. 2023, 85, 102750. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, Q.; Jiang, Y.; Wu, X.; Yue, X.; Geng, Y.; Shen, J.; Ding, D. Semiconducting Polymer Nanoparticles with Intramolecular Motion-Induced Photothermy for Tumor Phototheranostics and Tooth Root Canal Therapy. Adv. Mater. 2022, 34, e2200179. [Google Scholar] [CrossRef]
- Kaukua, N.; Shahidi, M.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.; Wang, L.; Hultman, I.; Ahrlund-Richter, L.; et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Achilleos, A.; Trainor, P. Neural crest stem cells: Discovery, properties and potential for therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef]
- D’Esposito, V.; Lecce, M.; Marenzi, G.; Cabaro, S.; Ambrosio, M.; Sammartino, G.; Misso, S.; Migliaccio, T.; Liguoro, P.; Oriente, F.; et al. Platelet-rich plasma counteracts detrimental effect of high-glucose concentrations on mesenchymal stem cells from Bichat fat pad. J. Tissue Eng. Regen. Med. 2020, 14, 701–713. [Google Scholar] [CrossRef]
- Kim, J.; Irfan, M.; Hossain, M.; George, A.; Chung, S. BDNF/TrkB Is a Crucial Regulator in the Inflammation-Mediated Odontoblastic Differentiation of Dental Pulp Stem Cells. Cells 2023, 12, 1851. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, J.; Chen, M.; Lin, C.; Kim, S.; Zhou, Y.; Xiang, L.; Xie, M.; Bai, H.; Yao, H.; et al. Parenchymal and stromal tissue regeneration of tooth organ by pivotal signals reinstated in decellularized matrix. Nat. Mater. 2019, 18, 627–637. [Google Scholar] [CrossRef]
- Chan, Y.; Ho, K.; Lee, Y.; Chou, M.; Lew, W.; Huang, H.; Lai, P.; Feng, S. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res. Ther. 2022, 13, 73. [Google Scholar] [CrossRef]
- Kanno, K.; Shimizu, K.; Shinoda, M.; Hayashi, M.; Takeichi, O.; Iwata, K. Role of macrophage-mediated Toll-like receptor 4-interleukin-1R signaling in ectopic tongue pain associated with tooth pulp inflammation. J. Neuroinflamm. 2020, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Z.; Luo, Z.; Yu, Q.; Jiang, Y.; Zhang, Y.; Zhou, Z.; Smith, A.; Cooper, P. LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway. J. Cell. Physiol. 2015, 230, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.; Oh, J. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Pulin, A.; Seo, M.; Kota, D.; Ylostalo, J.; Larson, B.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Kui, L.; Chan, G.; Lee, P. TSG-6 Downregulates IFN-Alpha and TNF-Alpha Expression by Suppressing IRF7 Phosphorylation in Human Plasmacytoid Dendritic Cells. Mediat. Inflamm. 2017, 2017, 7462945. [Google Scholar] [CrossRef]
- Choi, H.; Lee, R.; Bazhanov, N.; Oh, J.; Prockop, D. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.; Li, F.; Han, Y.; Rabson, A.; Wang, Y.; Shi, Y.; et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Szántó, S.; Bárdos, T.; Gál, I.; Glant, T.; Mikecz, K. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 2004, 50, 3012–3022. [Google Scholar] [CrossRef]
- Zhu, L.; Donhou, S.; Burleigh, A.; Miotla Zarebska, J.; Curtinha, M.; Parisi, I.; Khan, S.; Dell’Accio, F.; Chanalaris, A.; Vincent, T. TSG-6 Is Weakly Chondroprotective in Murine OA but Does not Account for FGF2-Mediated Joint Protection. ACR Open Rheumatol. 2020, 2, 605–615. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78, 60–83. [Google Scholar] [CrossRef]
- Um, S.; Kim, H.; Lee, J.; Song, I.; Seo, B. TSG-6 secreted by mesenchymal stem cells suppresses immune reactions influenced by BMP-2 through p38 and MEK mitogen-activated protein kinase pathway. Cell Tissue Res. 2017, 368, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D.; Mikecz, K.; Ali, T.; Mabilleau, G.; Benayahu, D.; Plaas, A.; Milner, C.; Day, A.; Sabokbar, A. TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J. Biol. Chem. 2008, 283, 25952–25962. [Google Scholar] [CrossRef]
- Yang, H.; Aprecio, R.; Zhou, X.; Wang, Q.; Zhang, W.; Ding, Y.; Li, Y. Therapeutic effect of TSG-6 engineered iPSC-derived MSCs on experimental periodontitis in rats: A pilot study. PLOS ONE 2014, 9, e100285. [Google Scholar] [CrossRef]
- Peng, R.; Yao, X.; Cao, B.; Tang, J.; Ding, J. The effect of culture conditions on the adipogenic and osteogenic inductions of mesenchymal stem cells on micropatterned surfaces. Biomaterials 2012, 33, 6008–6019. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W.; et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix—Based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, D.; Li, Q.; Tian, W.; Guo, W. The establishment of a chemically defined serum-free culture system for human dental pulp stem cells. Stem Cell Res. Ther. 2018, 9, 191. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Hu, Y.; Sun, J.; Guo, W.; Li, H.; Chen, J.; Huo, F.; Tian, W.; Li, S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent. Mater. 2019, 35, 1238–1253. [Google Scholar] [CrossRef]
- Jung, J.; Park, S.; Seo, E.; Jang, I.; Park, Y.; Lee, D.; Kim, D.; Kim, J. Functional expression of oxytocin receptors in pulp-dentin complex. Biomaterials 2023, 293, 121977. [Google Scholar] [CrossRef]
- Yin, W.; Liu, G.; Li, J.; Bian, Z. Landscape of Cell Communication in Human Dental Pulp. Small Methods 2021, 5, e2100747. [Google Scholar] [CrossRef]
- Browne, R.; Tobias, R.; Plant, C. A method for testing the toxicity of temporary crown and bridge materials. Biomaterials 1984, 5, 149–152. [Google Scholar] [CrossRef]
- Ai, T.; Zhang, J.; Wang, X.; Zheng, X.; Qin, X.; Zhang, Q.; Li, W.; Hu, W.; Lin, J.; Chen, F.; et al. DNA methylation profile is associated with the osteogenic potential of three distinct human odontogenic stem cells. Signal Transduct. Target. Ther. 2018, 3, 1. [Google Scholar] [CrossRef]
- Han, B.; Cao, C.; Wang, A.; Zhao, Y.; Jin, M.; Wang, Y.; Chen, S.; Yu, M.; Yang, Z.; Qu, X.; et al. Injectable Double-Network Hydrogel-Based Three-Dimensional Cell Culture Systems for Regenerating Dental Pulp. ACS Appl. Mater. Interfaces 2023, 15, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, M.; Liu, S.; Liu, X.; Huan, Y.; Ye, Q.; Yang, X.; Guo, H.; Liu, A.; Huang, X.; et al. Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration. Mol. Ther. 2022, 30, 3193–3208. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Sun, H.; Xu, X.; Zhong, H.; Wu, Y.; Wang, J. YAP1 inhibits the induction of TNF-α-stimulated bone-resorbing mediators by suppressing the NF-κB signaling pathway in MC3T3-E1 cells. J. Cell. Physiol. 2020, 235, 4698–4708. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Ryu, J.; Kim, H.; Kouvatsos, N.; Dodd, R.; Choi, S.; Kim, Y.; Milner, C.; Day, A. The Link module of human TSG-6 (Link_TSG6) promotes wound healing, suppresses inflammation and improves glandular function in mouse models of Dry Eye Disease. Ocul. Surf. 2022, 24, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kim, J.; Lee, C.; Oh, D.; Han, J.; Kim, T.; Kim, S.; Seo, Y.; Oh, S.; Jung, Y. Tumor necrosis factor-inducible gene 6 reprograms hepatic stellate cells into stem-like cells, which ameliorates liver damage in mouse. Biomaterials 2019, 219, 119375. [Google Scholar] [CrossRef]
- Vallés, G.; Bensiamar, F.; Crespo, L.; Arruebo, M.; Vilaboa, N.; Saldaña, L. Topographical cues regulate the crosstalk between MSCs and macrophages. Biomaterials 2015, 37, 124–133. [Google Scholar] [CrossRef]
- Xu, C.; Feng, C.; Huang, P.; Li, Y.; Liu, R.; Liu, C.; Han, Y.; Chen, L.; Ding, Y.; Shao, C.; et al. TNFα and IFNγ rapidly activate PI3K-AKT signaling to drive glycolysis that confers mesenchymal stem cells enhanced anti-inflammatory property. Stem Cell Res. Ther. 2022, 13, 491. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, P.; Zhang, F.; Chen, S.; Liu, Y.; Chen, F.; Wu, Y.; Li, S.; Wang, C.; Gong, Y.; et al. Abnormal expression of TSG-6 disturbs extracellular matrix homeostasis in chondrocytes from endemic osteoarthritis. Front. Genet. 2022, 13, 1064565. [Google Scholar] [CrossRef] [PubMed]
- Balog, A.; Varga, B.; Fülöp, F.; Lantos, I.; Toldi, G.; Vécsei, L.; Mándi, Y. Kynurenic Acid Analog Attenuates the Production of Tumor Necrosis Factor-α, Calgranulins (S100A 8/9 and S100A 12), and the Secretion of HNP1-3 and Stimulates the Production of Tumor Necrosis Factor-Stimulated Gene-6 in Whole Blood Cultures of Patients With Rheumatoid Arthritis. Front. Immunol. 2021, 12, 632513. [Google Scholar] [CrossRef]
- Kohda, D.; Morton, C.; Parkar, A.; Hatanaka, H.; Inagaki, F.; Campbell, I.; Day, A. Solution structure of the link module: A hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell 1996, 86, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Midgley, A.; Meran, S.; Woods, E.; Bowen, T.; Phillips, A.; Steadman, R. Tumor Necrosis Factor-stimulated Gene 6 (TSG-6)-mediated Interactions with the Inter-α-inhibitor Heavy Chain 5 Facilitate Tumor Growth Factor β1 (TGFβ1)-dependent Fibroblast to Myofibroblast Differentiation. J. Biol. Chem. 2016, 291, 13789–13801. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xing, M.; Zhou, Y.; Zhang, W.; Liu, Z.; Li, L.; Zheng, Z.; Ma, Y.; Li, P.; Liu, X.; et al. HSP90β promotes osteoclastogenesis by dual-activation of cholesterol synthesis and NF-κB signaling. Cell Death Differ. 2023, 30, 673–686. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Liu, H.; Cheng, Q.; Yang, S.; Yang, D. All-trans retinoic acid inhibits the osteogenesis of periodontal ligament stem cells by promoting IL-1β production via NF-κB signaling. Int. Immunopharmacol. 2022, 108, 108757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, Y.; Xue, N.; Xu, H.; Zhang, D.; Ji, N.; Chen, Q. TSG-6 Inhibits the NF-κB Signaling Pathway and Promotes the Odontogenic Differentiation of Dental Pulp Stem Cells via CD44 in an Inflammatory Environment. Biomolecules 2024, 14, 368. https://doi.org/10.3390/biom14030368
Wang Y, Xie Y, Xue N, Xu H, Zhang D, Ji N, Chen Q. TSG-6 Inhibits the NF-κB Signaling Pathway and Promotes the Odontogenic Differentiation of Dental Pulp Stem Cells via CD44 in an Inflammatory Environment. Biomolecules. 2024; 14(3):368. https://doi.org/10.3390/biom14030368
Chicago/Turabian StyleWang, Ying, Yulang Xie, Ningning Xue, Hao Xu, Dunfang Zhang, Ning Ji, and Qianming Chen. 2024. "TSG-6 Inhibits the NF-κB Signaling Pathway and Promotes the Odontogenic Differentiation of Dental Pulp Stem Cells via CD44 in an Inflammatory Environment" Biomolecules 14, no. 3: 368. https://doi.org/10.3390/biom14030368



