Ammoniagenic Action of Valproate without Signs of Hepatic Dysfunction in Rats: Possible Causes and Supporting Evidence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Section
2.2.1. Experimental Design
2.2.2. Animals
2.2.3. Preparative and Analytical Methods
2.3. Statistical Analysis
3. Results
3.1. Ammonia, Urea Concentration, and Alanine Aminotransferase and Aspartate Aminotransferase Activities in the Plasma of Rats
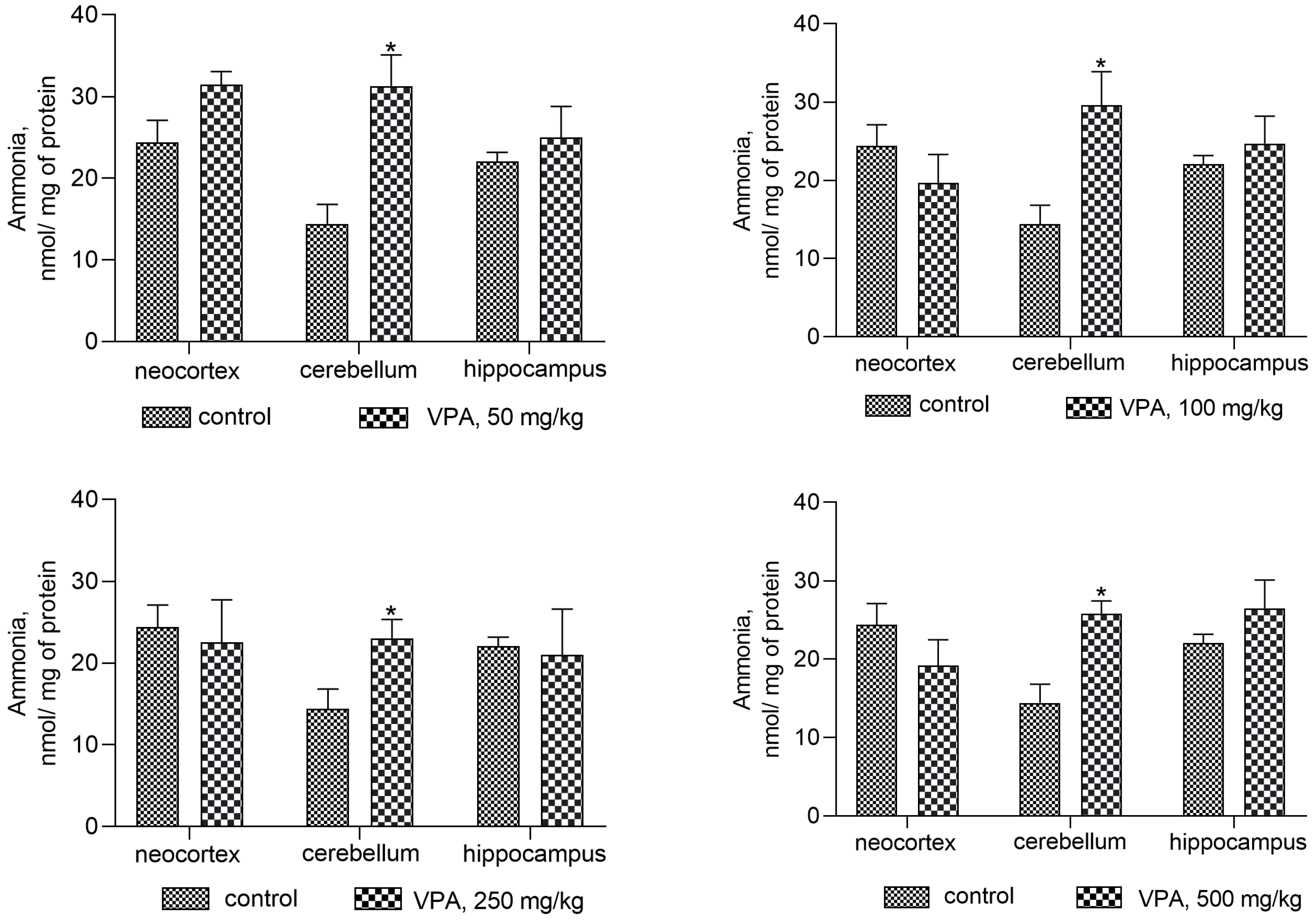
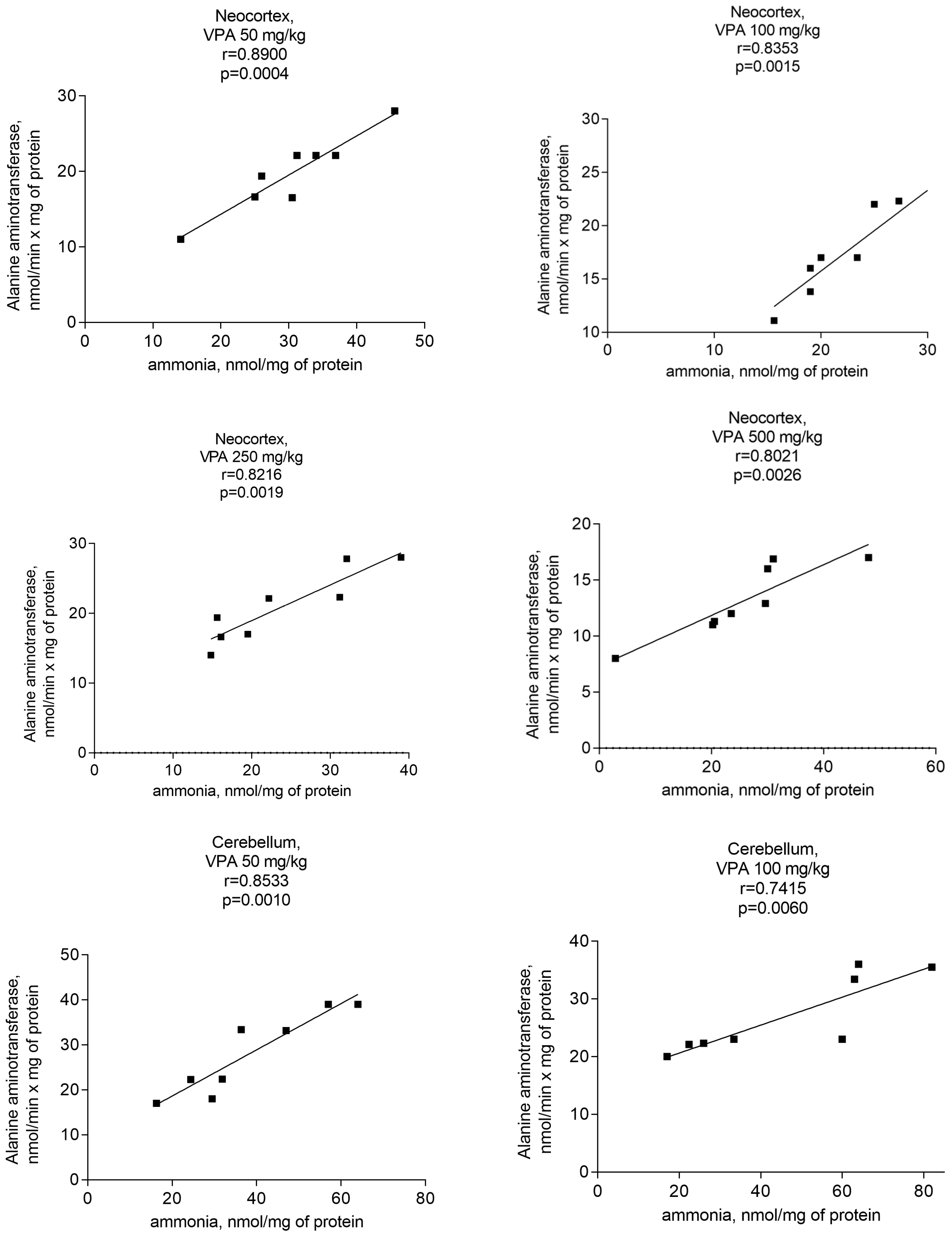
3.2. The Effect of Different Doses of VPA on the Concentration of Ammonia in the Mitochondria and Cytosol in Different Rat Brain Regions
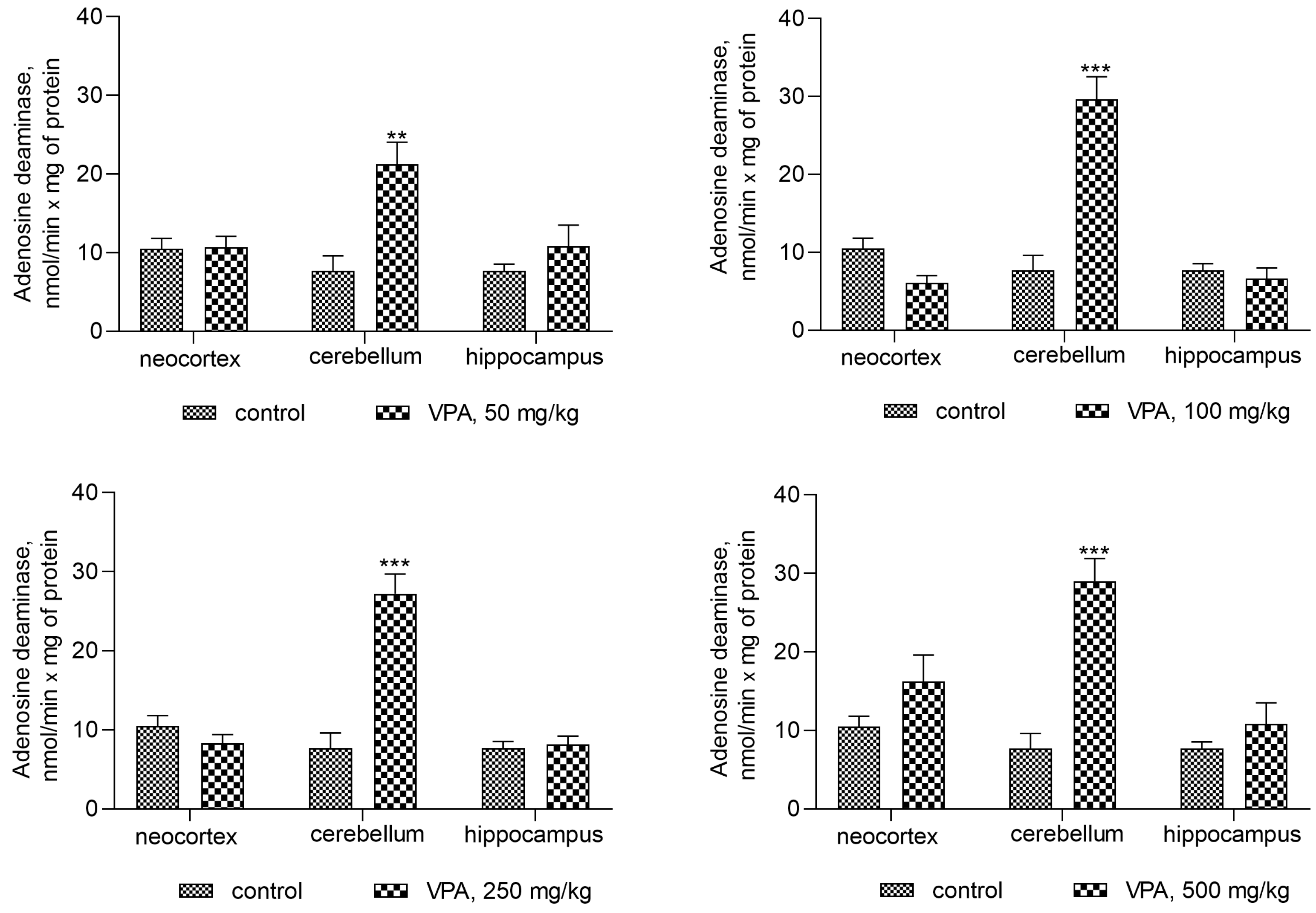
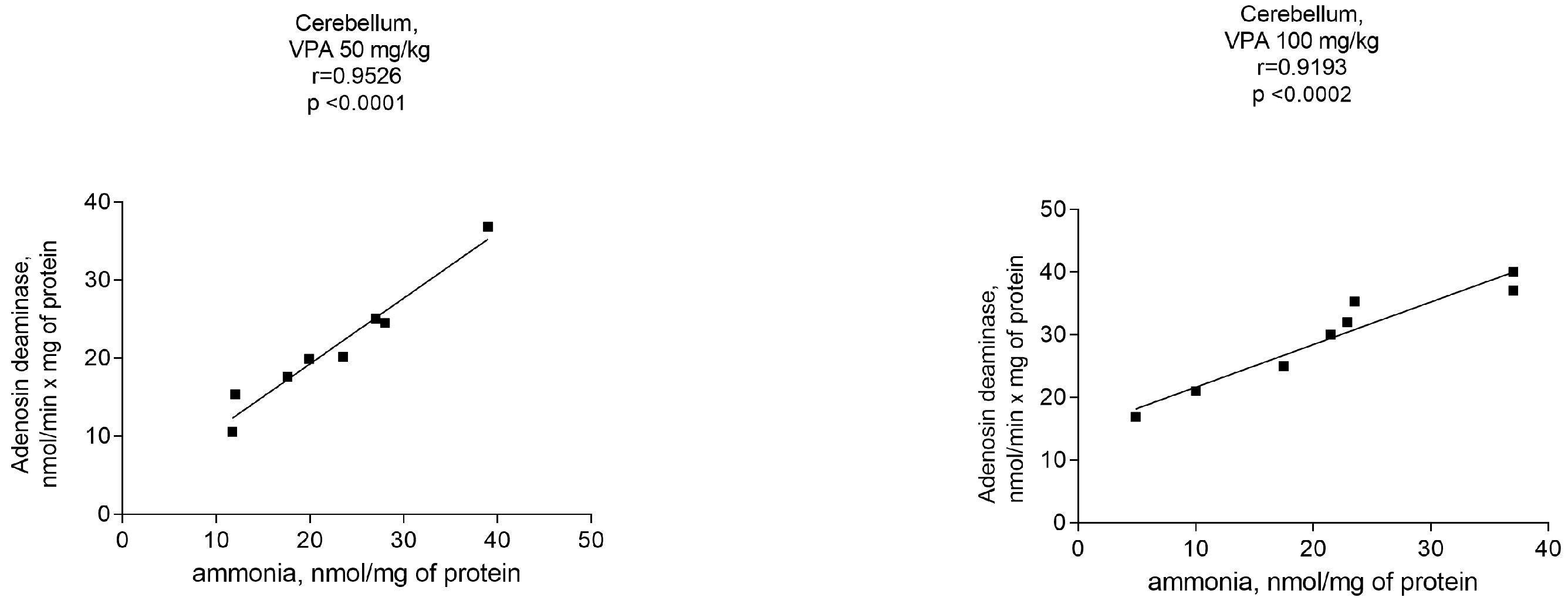
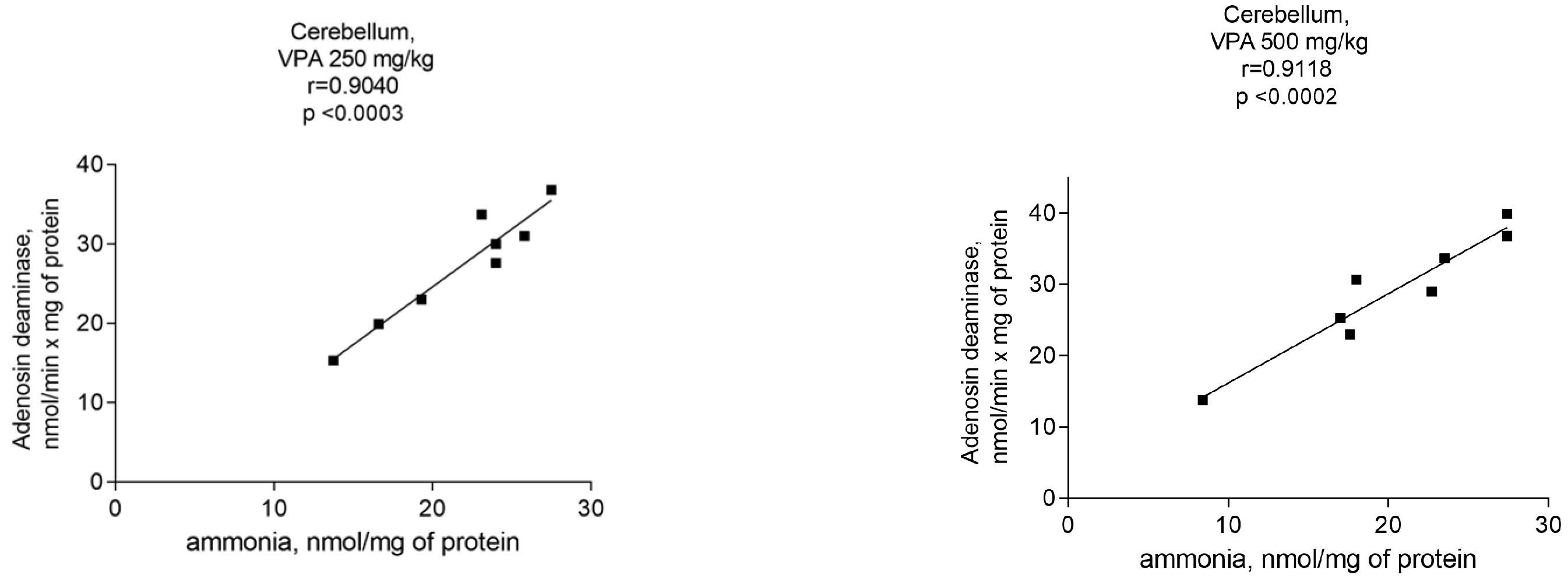
3.3. The Effect of Valproate on AMP-Deaminase and Adenosine Deaminase Activities in the Cytosol of Different Rat Brain Structures
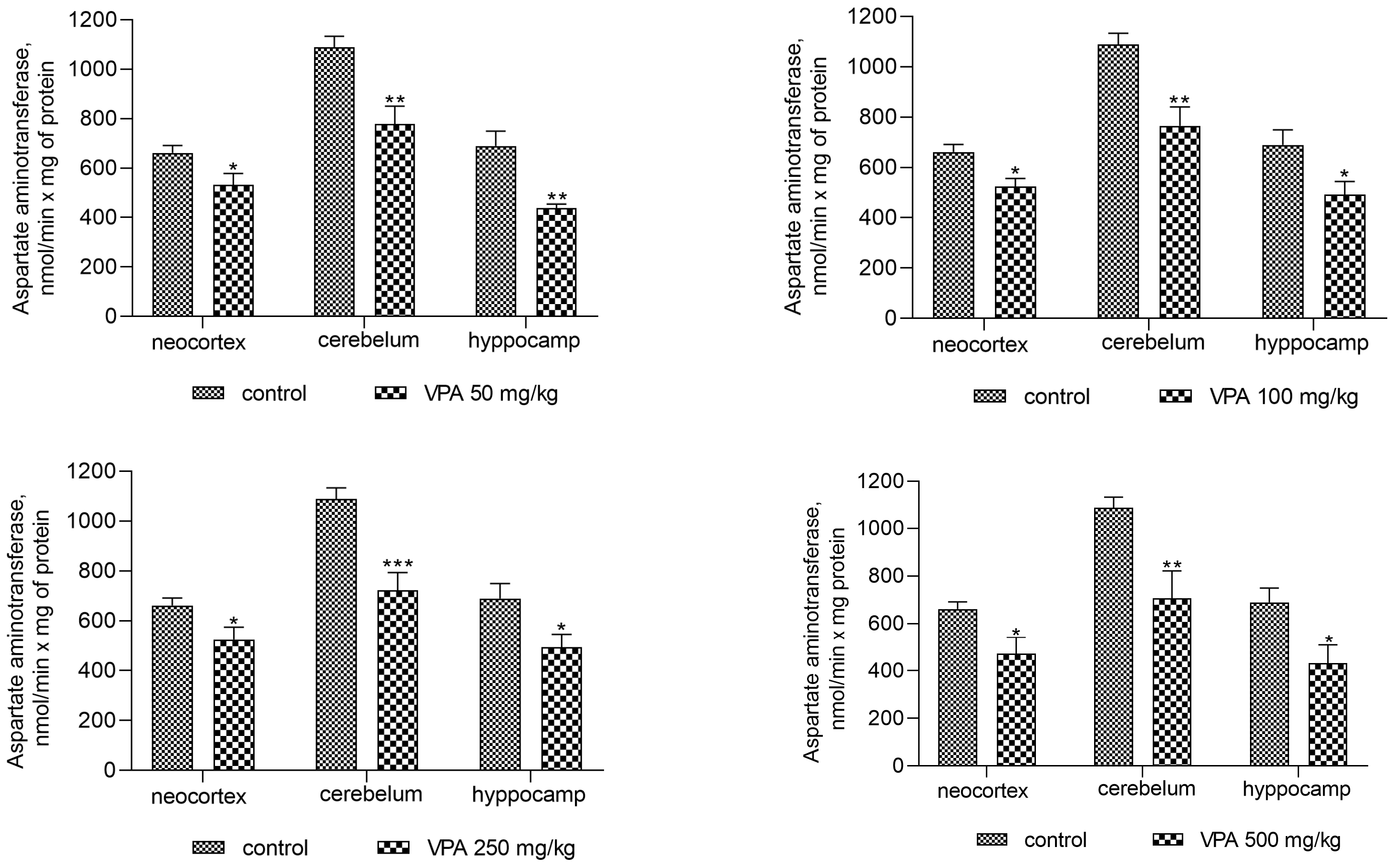
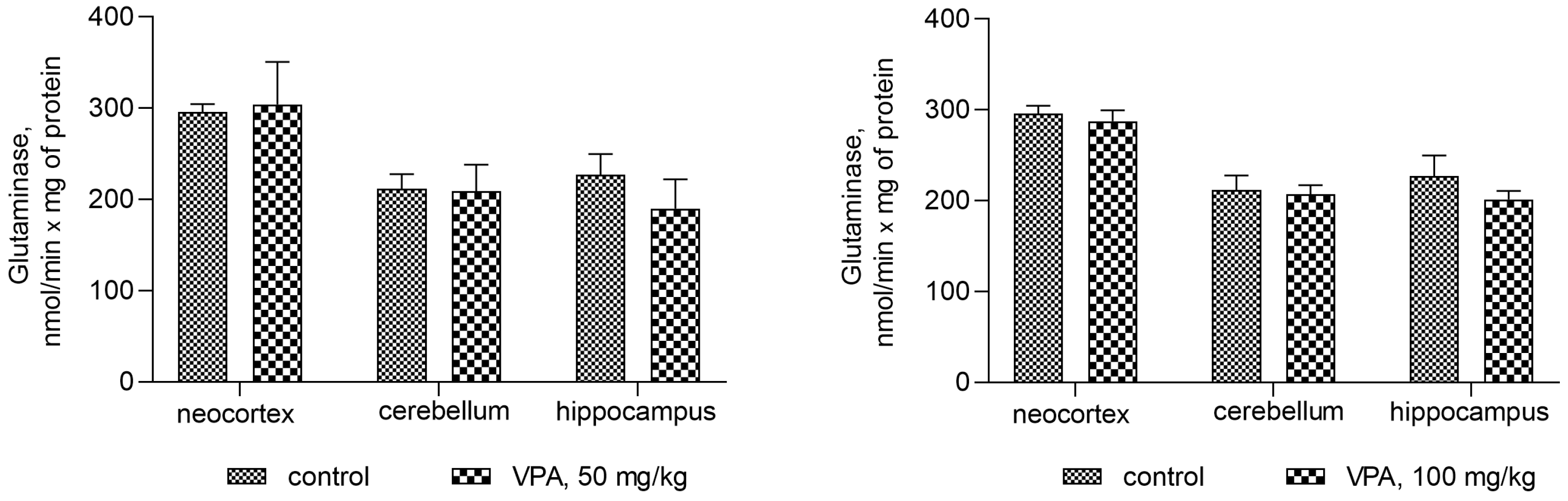
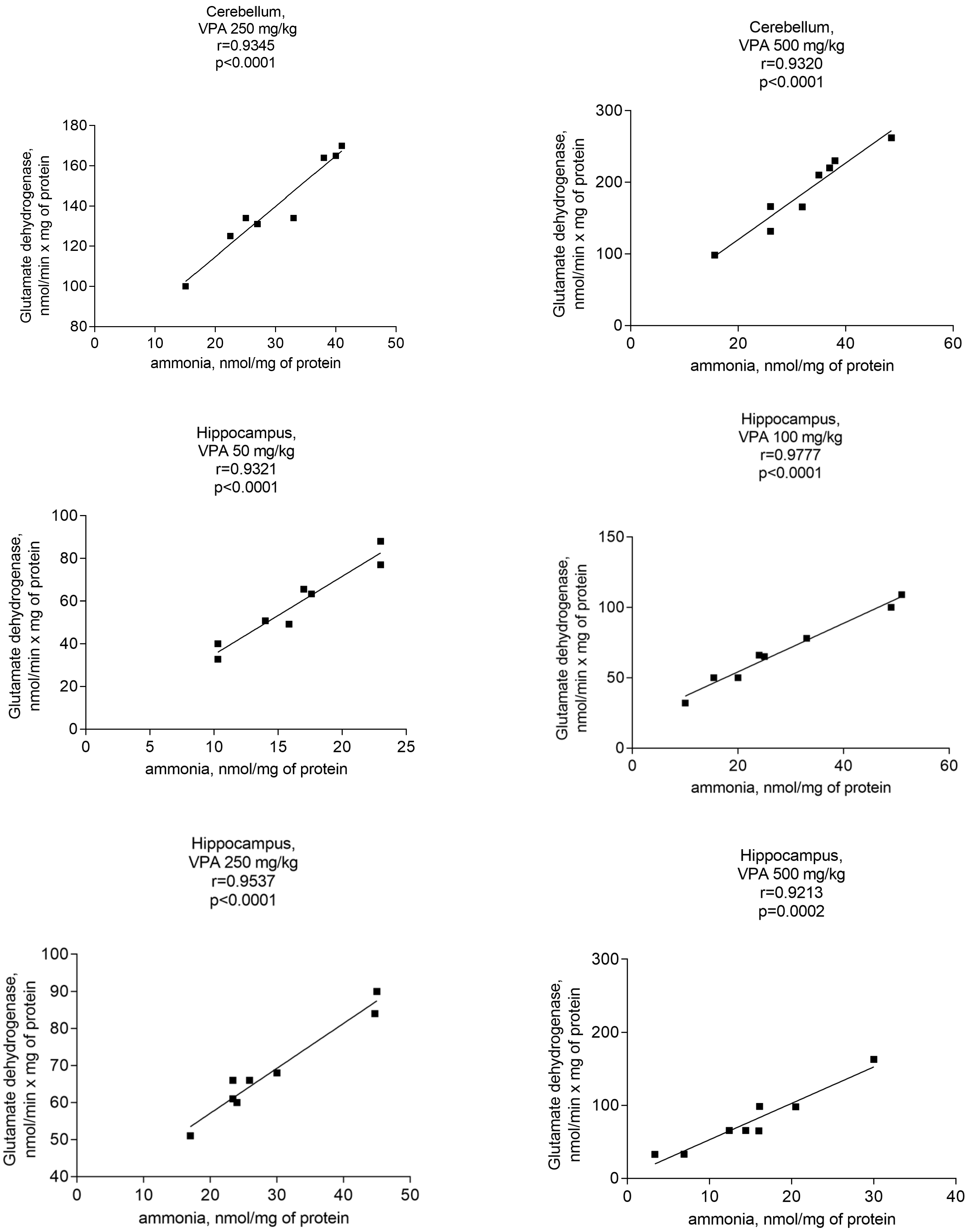
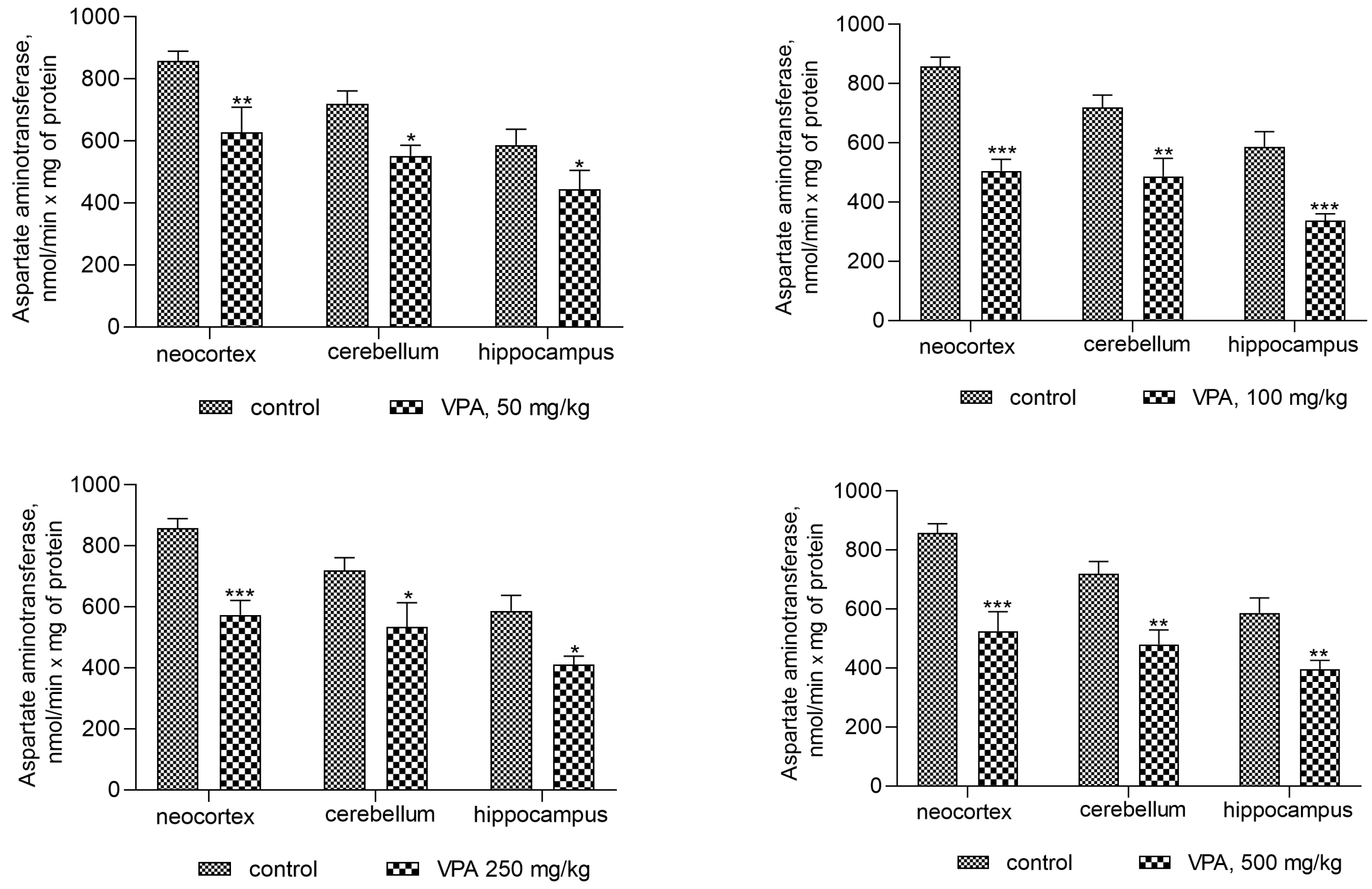
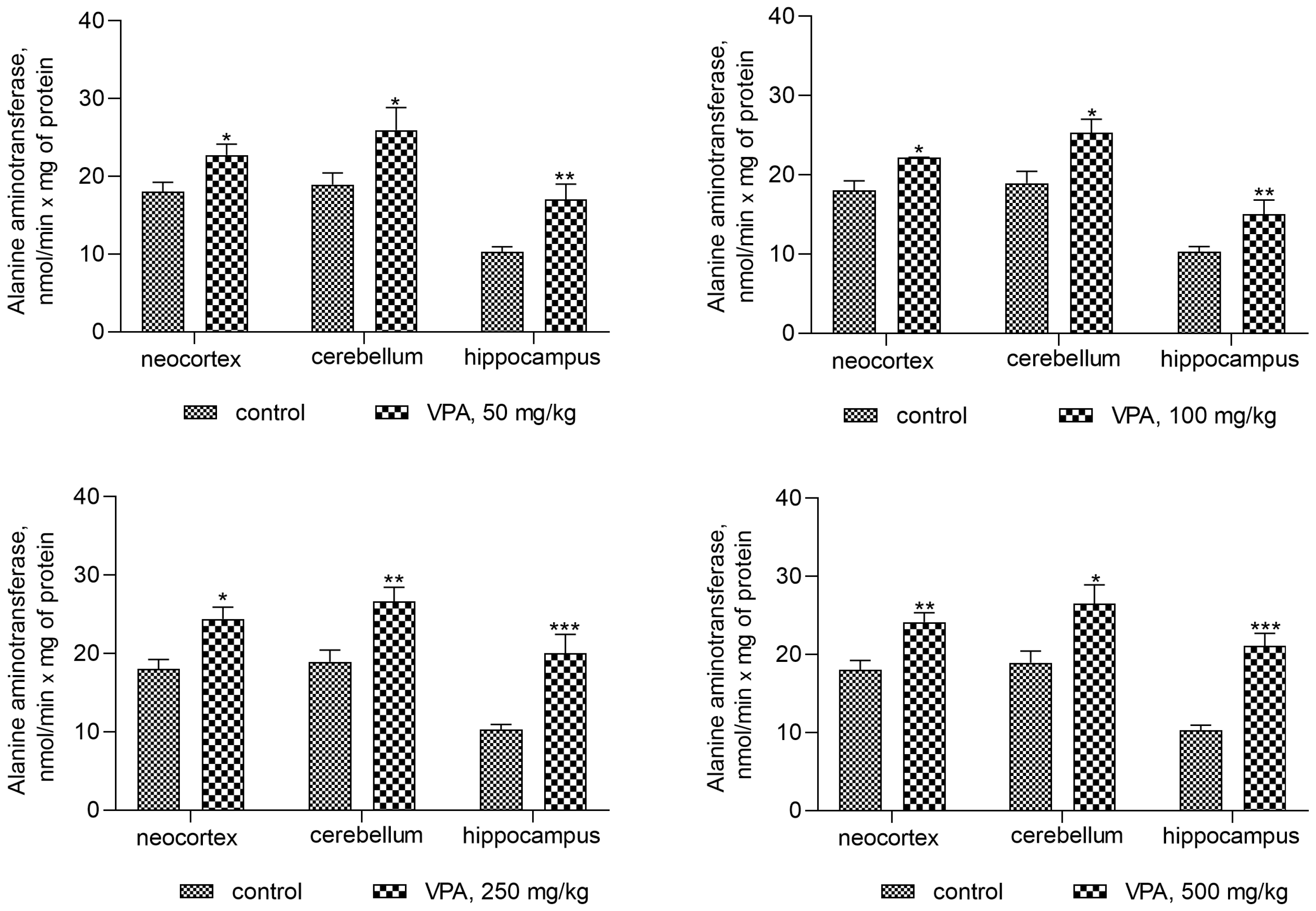
3.4. The Effect of VPA on Glutaminase, Glutamate Dehydrogenase, Alanine Aminotransferase, and Aspartate Aminotransferase Activities in the Mitochondria of Different Regions of the Rat Brain
3.5. The Effect of VPA Treatment on Brain GS Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanner, A.M.; Balabanov, A. Valproate: A Practical Review of Its Uses in Neurological and Psychiatric Disorders. Expert Rev. Neurother. 2002, 2, 151–165. [Google Scholar] [CrossRef]
- Sztajnkrycer, M.D. Valproic Acid Toxicity: Overview and Management. J. Toxicol. Clin. Toxicol. 2002, 40, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Nanau, R.M.; Neuman, M.G. Adverse Drug Reactions Induced by Valproic Acid. Clin. Biochem. 2013, 46, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Kulick, S.K.; Kramer, D.A. Hyperammonemia Secondary to Valproic Acid as a Cause of Lethargy in a Postictal Patient. Ann. Emerg. Med. 1993, 22, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Walczak, T. Do Antiepileptic Drugs Play a Role in Sudden Unexpected Death in Epilepsy? Drug Saf. 2003, 26, 673–683. [Google Scholar] [CrossRef]
- Zaccara, G.; Paganini, M.; Campostrini, R.; Arnetoli, G.; Zappoli, R.; Moroni, F. Hyperammonemia and Valproate-Induced Alterations of the State of Consciousness. A Report of 8 Cases. Eur. Neurol. 1984, 23, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.O.; Ritland, S. Life Threatening Intoxication with Sodium Valproate. J. Toxicol. Clin. Toxicol. 1995, 33, 279–284. [Google Scholar] [CrossRef]
- Löscher, W.; Nau, H. Pharmacological Evaluation of Various Metabolites and Analogues of Valproic Acid. Anticonvulsant and Toxic Potencies in Mice. Neuropharmacology 1985, 24, 427–435. [Google Scholar] [CrossRef]
- Cooper, A.J.; Plum, F. Biochemistry and Physiology of Brain Ammonia. Physiol. Rev. 1987, 67, 440–519. [Google Scholar] [CrossRef]
- Ong, J.P.; Aggarwal, A.; Krieger, D.; Easley, K.A.; Karafa, M.T.; Van Lente, F.; Arroliga, A.C.; Mullen, K.D. Correlation between Ammonia Levels and the Severity of Hepatic Encephalopathy. Am. J. Med. 2003, 114, 188–193. [Google Scholar] [CrossRef]
- Butterworth, R.F.; Giguère, J.F.; Michaud, J.; Lavoie, J.; Layrargues, G.P. Ammonia: Key Factor in the Pathogenesis of Hepatic Encephalopathy. Neurochem. Pathol. 1987, 6, 1–12. [Google Scholar] [CrossRef]
- Munoz, S.J. Hepatic Encephalopathy. Med. Clin. N. Am. 2008, 92, 795–812. [Google Scholar] [CrossRef]
- Aires, C.C.P.; van Cruchten, A.; Ijlst, L.; de Almeida, I.T.; Duran, M.; Wanders, R.J.A.; Silva, M.F.B. New Insights on the Mechanisms of Valproate-Induced Hyperammonemia: Inhibition of Hepatic N-Acetylglutamate Synthase Activity by Valproyl-CoA. J. Hepatol. 2011, 55, 426–434. [Google Scholar] [CrossRef]
- Rumbach, L.; Mutet, C.; Cremel, G.; Marescaux, C.A.; Micheletti, G.; Warter, J.M.; Waksman, A. Effects of Sodium Valproate on Mitochondrial Membranes: Electron Paramagnetic Resonance and Transmembrane Protein Movement Studies. Mol. Pharmacol. 1986, 30, 270–273. [Google Scholar]
- Rumbach, L.; Warter, J.M.; Rendon, A.; Marescaux, C.; Micheletti, G.; Waksman, A. Inhibition of Oxidative Phosphorylation in Hepatic and Cerebral Mitochondria of Sodium Valproate-Treated Rats. J. Neurol. Sci. 1983, 61, 417–423. [Google Scholar] [CrossRef]
- Haas, R.; Stumpf, D.A.; Parks, J.K.; Eguren, L. Inhibitory Effects of Sodium Valproate on Oxidative Phosphorylation. Neurology 1981, 31, 1473–1476. [Google Scholar] [CrossRef]
- Benavides, J.; Martin, A.; Ugarte, M.; Valdivieso, F. Inhibition by Valproic Acid of Pyruvate Uptake by Brain Mitochondria. Biochem. Pharmacol. 1982, 31, 1633–1636. [Google Scholar] [CrossRef]
- Surendran, I.; Sahoo, S.; Gupta, G.; Chauhan, N.; Grover, S. Valproate-Induced Hyperammonemic Encephalopathy in an Elderly Patient with Bipolar Disorder. J. Geriatr. Ment. Health 2016, 3, 172–175. [Google Scholar] [CrossRef]
- Bae, K.-Y.; Jang, J.-E.; Kim, Y.-H.; Kim, J.-M.; Yoon, J.-S. Valproate-Induced Hyperammonemic Encephalopathy Caused by Free Carnitine Deficiency in a Patient with Bipolar Disorder: A Case Report. Clin. Psychopharmacol. Neurosci. 2009, 7, 57–62. [Google Scholar]
- Weng, T.-I.; Shih, F.F.-Y.; Chen, W.-J. Unusual Causes of Hyperammonemia in the ED. Am. J. Emerg. Med. 2004, 22, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-F.; Yang, R.-C.; Chen, C.Y.; Jong, Y.-J. Valproate-Induced Hyperammonemic Encephalopathy. Pediatr. Neonatol. 2008, 49, 201–204. [Google Scholar] [CrossRef]
- Rath, A.; Naryanan, T.J.; Chowdhary, G.V.S.; Murthy, J.M.K. Valproate-Induced Hyperammonemic Encephalopathy with Normal Liver Function. Neurol. India 2005, 53, 226–228. [Google Scholar] [CrossRef]
- Dixit, S.; Namdeo, M.; Azad, S. Valproate Induced Delirium Due to Hyperammonemia in a Case of Acute Mania: A Diagnostic Dilemma. J. Clin. Diagn. Res. JCDR 2015, 9, VD01–VD02. [Google Scholar] [CrossRef]
- Duarte, J.; Macias, S.; Coria, F.; Fernandez, E.; Clavería, L.E. Valproate-Induced Coma: Case Report and Literature Review. Ann. Pharmacother. 1993, 27, 582–583. [Google Scholar] [CrossRef]
- Rawat, S.; Borkowski, W.J.; Swick, H.M. Valproic Acid and Secondary Hyperammonemia. Neurology 1981, 31, 1173–1174. [Google Scholar] [CrossRef]
- Zaret, B.S.; Beckner, R.R.; Marini, A.M.; Wagle, W.; Passarelli, C. Sodium Valproate-Induced Hyperammonemia without Clinical Hepatic Dysfunction. Neurology 1982, 32, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Farooq, O.; Zunga, P.M.; Dar, M.I.; Rather, A.Q.; Rashid, S.; Basu, J.; Dar, I.H.; Ashraf, M. Non-Hyperammonemic Valproate Encephalopathy. Ann. Neurosci. 2014, 21, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Caruana Galizia, E.; Isaacs, J.D.; Cock, H.R. Non-Hyperammonaemic Valproate Encephalopathy after 20 Years of Treatment. Epilepsy Behav. Case Rep. 2017, 8, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Guin, D.S.; Biswas, S.; Saha, K.; Mishra, A.; Das, S.K. A Case of Non-Hyperammonemic Valproate-Induced Encephalopathy. Neurol. Asia 2006, 11, 135–137. [Google Scholar]
- Agrawal, A.; Agrawal, R. Valproate-Induced Non-Hepatic Hyperammonaemic Encephalopathy: A Rare Complication of Chronic Valproate Therapy. Int. J. Clin. Pediatr. 2012, 1, 85–87. [Google Scholar] [CrossRef]
- Kaminsky, Y.G.; Beloushko, E.E.; Kosenko, E.A. Antioxidant Defense in the Rat Brain Cortex, Cerebellum, Hippocampus, and Striatum and Its Alterations during Portacaval Shunting. Neurochem. J. 2014, 8, 289–294. [Google Scholar] [CrossRef]
- Hoyer, S.; Oesterreich, K.; Wagner, O. Glucose Metabolism as the Site of the Primary Abnormality in Early-Onset Dementia of Alzheimer Type? J. Neurol. 1988, 235, 143–148. [Google Scholar] [CrossRef]
- Hoyer, S.; Nitsch, R.; Oesterreich, K. Ammonia Is Endogenously Generated in the Brain in the Presence of Presumed and Verified Dementia of Alzheimer Type. Neurosci. Lett. 1990, 117, 358–362. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Jeitner, T.M. Central Role of Glutamate Metabolism in the Maintenance of Nitrogen Homeostasis in Normal and Hyperammonemic Brain. Biomolecules 2016, 6, 16. [Google Scholar] [CrossRef]
- Jafarian, I.; Eskandari, M.R.; Mashayekhi, V.; Ahadpour, M.; Hosseini, M.-J. Toxicity of Valproic Acid in Isolated Rat Liver Mitochondria. Toxicol. Mech. Methods 2013, 23, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.A.; Tikhonova, L.A.; Alilova, G.A.; Montoliu, C.; Barreto, G.E.; Aliev, G.; Kaminsky, Y.G. Portacaval Shunting Causes Differential Mitochondrial Superoxide Production in Brain Regions. Free Radic. Biol. Med. 2017, 113, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Nau, H.; Löscher, W. Valproic Acid: Brain and Plasma Levels of the Drug and Its Metabolites, Anticonvulsant Effects and Gamma-Aminobutyric Acid (GABA) Metabolism in the Mouse. J. Pharmacol. Exp. Ther. 1982, 220, 654–659. [Google Scholar] [PubMed]
- Cattaneo, C.I.; Ressico, F.; Valsesia, R.; D’Innella, P.; Ballabio, M.; Fornaro, M. Sudden Valproate-Induced Hyperammonemia Managed with L-Carnitine in a Medically Healthy Bipolar Patient: Essential Review of the Literature and Case Report. Medicine 2017, 96, e8117. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, A.L.; Aguirre, B.; Bourgeois, J.A. Severe Acute Hyperammonemia after Brief Exposure to Valproate. Psychosomatics 2008, 49, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Connacher, A.A.; Macnab, M.S.; Moody, J.P.; Jung, R.T. Fatality Due to Massive Overdose of Sodium Valproate. Scott. Med. J. 1987, 32, 85–86. [Google Scholar] [CrossRef]
- Anderson, G.D.; Acheampong, A.A.; Wilensky, A.J.; Levy, R.H. Effect of Valproate Dose on Formation of Hepatotoxic Metabolites. Epilepsia 1992, 33, 736–742. [Google Scholar] [CrossRef]
- Tomasiewicz, H.C.; Mague, S.D.; Cohen, B.M.; Carlezon, W.A. Behavioral Effects of Short-Term Administration of Lithium and Valproic Acid in Rats. Brain Res. 2006, 1093, 83–94. [Google Scholar] [CrossRef]
- Kerscher, L.; Ziegenhorn, J. Urea. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publishers Ltd.: Cambridge, UK, 1990; Volume VIII, pp. 444–453. [Google Scholar]
- Kvamme, E.; Torgner, I.A.; Svenneby, G. Glutaminase from Mammalian Tissues. In Methods in Enzymology; Meister, A., Ed.; Glutamate, Glutamine, Glutathione, and Related Compounds; Academic Press: New York, NY, USA, 1985; Volume 113, pp. 241–256. [Google Scholar]
- Schmidt, E.; Schmidt, F.W. Glutamate Dehydrogenase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1984; Volume III, pp. 216–227. [Google Scholar]
- Meister, A. Glutamine Synthetase from Mammalian Tissues. Methods Enzymol. 1985, 113, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, Y.; Kosenko, E. AMP Deaminase and Adenosine Deaminase Activities in Liver and Brain Regions in Acute Ammonia Intoxication and Subacute Toxic Hepatitis. Brain Res. 2010, 1311, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Hörstermann, D. Differential Effects of Vigabatrin, Gamma-Acetylenic GABA, Aminooxyacetic Acid, and Valproate on Levels of Various Amino Acids in Rat Brain Regions and Plasma. Naunyn. Schmiedebergs Arch. Pharmacol. 1994, 349, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.; Keane, P.E.; Meldrum, B.S.; Simiand, J.; Vernieres, J.C. Mechanism of Anticonvulsant Action of Valproate. Prog. Neurobiol. 1982, 19, 315–359. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Norenberg, M.D. Glutamine: A Trojan Horse in Ammonia Neurotoxicity. Hepatology 2006, 44, 788–794. [Google Scholar] [CrossRef]
- Jeavons, P.M. Non-Dose-Related Side Effects of Valproate. Epilepsia 1984, 25 (Suppl. S1), S50–S55. [Google Scholar] [CrossRef]
- Adedapo, A.D.A.; Demaki, W.E.; Lagunju, I. Non-Dose-Dependent Changes in Liver Enzyme Levels of Children With Epilepsy on Treatment With Sodium Valproate. Dose Response Publ. Int. Hormesis Soc. 2020, 18, 1559325820918445. [Google Scholar] [CrossRef]
- Siemes, H.; Nau, H.; Schultze, K.; Wittfoht, W.; Drews, E.; Penzien, J.; Seidel, U. Valproate (VPA) Metabolites in Various Clinical Conditions of Probable VPA-Associated Hepatotoxicity. Epilepsia 1993, 34, 332–346. [Google Scholar] [CrossRef]
- Ahl, B.; Weissenborn, K.; van den Hoff, J.; Fischer-Wasels, D.; Köstler, H.; Hecker, H.; Burchert, W. Regional Differences in Cerebral Blood Flow and Cerebral Ammonia Metabolism in Patients with Cirrhosis. Hepatology 2004, 40, 73–79. [Google Scholar] [CrossRef]
- Rodrigo, R.; Cauli, O.; Gomez-Pinedo, U.; Agusti, A.; Hernandez-Rabaza, V.; Garcia-Verdugo, J.-M.; Felipo, V. Hyperammonemia Induces Neuroinflammation That Contributes to Cognitive Impairment in Rats with Hepatic Encephalopathy. Gastroenterology 2010, 139, 675–684. [Google Scholar] [CrossRef]
- Chapman, A.G.; Riley, K.; Evans, M.C.; Meldrum, B.S. Acute Effects of Sodium Valproate and Gamma-Vinyl GABA on Regional Amino Acid Metabolism in the Rat Brain: Incorporation of 2-[14C]Glucose into Amino Acids. Neurochem. Res. 1982, 7, 1089–1105. [Google Scholar] [CrossRef]
- Plaitakis, A.; Kalef-Ezra, E.; Kotzamani, D.; Zaganas, I.; Spanaki, C. The Glutamate Dehydrogenase Pathway and Its Roles in Cell and Tissue Biology in Health and Disease. Biology 2017, 6, 11. [Google Scholar] [CrossRef]
- Kanamori, K.; Ross, B.D.; Chung, J.C.; Kuo, E.L. Severity of Hyperammonemic Encephalopathy Correlates with Brain Ammonia Level and Saturation of Glutamine Synthetase in Vivo. J. Neurochem. 1996, 67, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Manoguerra, A.S.; Erdman, A.R.; Woolf, A.D.; Chyka, P.A.; Caravati, E.M.; Scharman, E.J.; Booze, L.L.; Christianson, G.; Nelson, L.S.; Cobaugh, D.J.; et al. Valproic Acid Poisoning: An Evidence-Based Consensus Guideline for out-of-Hospital Management. Clin. Toxicol. 2008, 46, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.A.; Tikhonova, L.A.; Kaminsky, Y.G. Ammonia and Enzymes of Ammonia Metabolism in Different Brain Regions in Hyperammonemia. Neurochem. J. 2015, 9, 133–140. [Google Scholar] [CrossRef]
- Scorziello, A.; Pellegrini, C.; Forte, L.; Tortiglione, A.; Gioielli, A.; Iossa, S.; Amoroso, S.; Tufano, R.; Di Renzo, G.; Annunziato, L. Differential Vulnerability of Cortical and Cerebellar Neurons in Primary Culture to Oxygen Glucose Deprivation Followed by Reoxygenation. J. Neurosci. Res. 2001, 63, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Renovell, A.; Giner, J.; Portoles, M. Loss of Granule Neurons in the Aging Human Cerebellar Cortex. Int. J. Dev. Biol. 1996, 40 (Suppl. S1), 193S–194S. [Google Scholar]
- Dykens, J.A. Isolated Cerebral and Cerebellar Mitochondria Produce Free Radicals When Exposed to Elevated Ca2+ and Na+: Implications for Neurodegeneration. J. Neurochem. 1994, 63, 584–591. [Google Scholar] [CrossRef]
- Rapoport, M.; van Reekum, R.; Mayberg, H. The Role of the Cerebellum in Cognition and Behavior: A Selective Review. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 193–198. [Google Scholar] [CrossRef]
- Fiez, J.A. Cerebellar Contributions to Cognition. Neuron 1996, 16, 13–15. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum 2012, 11, 777–807. [Google Scholar] [CrossRef]
- Luder, A.S.; Parks, J.K.; Frerman, F.; Parker, W.D. Inactivation of Beef Brain Alpha-Ketoglutarate Dehydrogenase Complex by Valproic Acid and Valproic Acid Metabolites. Possible Mechanism of Anticonvulsant and Toxic Actions. J. Clin. Investig. 1990, 86, 1574–1581. [Google Scholar] [CrossRef]
- Ponchaut, S.; van Hoof, F.; Veitch, K. In Vitro Effects of Valproate and Valproate Metabolites on Mitochondrial Oxidations. Relevance of CoA Sequestration to the Observed Inhibitions. Biochem. Pharmacol. 1992, 43, 2435–2442. [Google Scholar] [CrossRef]
- Leiderman, D.B.; Balish, M.; Bromfield, E.B.; Theodore, W.H. Effect of Valproate on Human Cerebral Glucose Metabolism. Epilepsia 1991, 32, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Komulainen, T.; Lodge, T.; Hinttala, R.; Bolszak, M.; Pietilä, M.; Koivunen, P.; Hakkola, J.; Poulton, J.; Morten, K.J.; Uusimaa, J. Sodium Valproate Induces Mitochondrial Respiration Dysfunction in HepG2 in Vitro Cell Model. Toxicology 2015, 331, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wardas, J. Neuroprotective Role of Adenosine in the CNS. Pol. J. Pharmacol. 2002, 54, 313–326. [Google Scholar] [PubMed]
- Hall, B.; George, J.G.; Allen, S.P. Adenosine Deaminase, Not Immune to a Mechanistic Rethink in Central Nervous System Disorders? Histol. Histopathol. 2022, 37, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Cerebral Ammonia Homeostasis. Neurochem. Res. 2012, 37, 2439–2455. [Google Scholar] [CrossRef]
- Badawy, A.A.; Elghaba, R.; Soliman, M.; Hussein, A.M.; AlSadrah, S.A.; Awadalla, A.; Abulseoud, O.A. Chronic Valproic Acid Administration Increases Plasma, Liver, and Brain Ammonia Concentration and Suppresses Glutamine Synthetase Activity. Brain Sci. 2020, 10, 759. [Google Scholar] [CrossRef]
- Collins, R.M.; Zielke, H.R.; Woody, R.C. Valproate Increases Glutaminase and Decreases Glutamine Synthetase Activities in Primary Cultures of Rat Brain Astrocytes. J. Neurochem. 1994, 62, 1137–1143. [Google Scholar] [CrossRef]
- Rivett, A.J. Preferential Degradation of the Oxidatively Modified Form of Glutamine Synthetase by Intracellular Mammalian Proteases. J. Biol. Chem. 1985, 260, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Milman, G.; Portnoff, L.S.; Tiemeier, D.C. Immunochemical Evidence for Glutamine-Mediated Degradation of Glutamine Synthetase in Cultured Chinese Hamster Cells. J. Biol. Chem. 1975, 250, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Rosier, F.; Lambert, D.; Mertens-Strijthagen, M. Effect of Glucose Deprivation on Rat Glutamine Synthetase in Cultured Astrocytes. Biochem. J. 1996, 315 Pt 2, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.; Kaminsky, Y.; Grau, E.; Miñana, M.D.; Grisolía, S.; Felipo, V. Nitroarginine, an Inhibitor of Nitric Oxide Synthetase, Attenuates Ammonia Toxicity and Ammonia-Induced Alterations in Brain Metabolism. Neurochem. Res. 1995, 20, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Miñana, M.D.; Kosenko, E.; Marcaida, G.; Hermenegildo, C.; Montoliu, C.; Grisolía, S.; Felipo, V. Modulation of Glutamine Synthesis in Cultured Astrocytes by Nitric Oxide. Cell. Mol. Neurobiol. 1997, 17, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.; Kaminsky, Y.; Grau, E.; Miñana, M.D.; Marcaida, G.; Grisolía, S.; Felipo, V. Brain ATP Depletion Induced by Acute Ammonia Intoxication in Rats Is Mediated by Activation of the NMDA Receptor and Na+,K(+)-ATPase. J. Neurochem. 1994, 63, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Rodríguez, N.; Coballase-Urrutia, E.; Rivera-Espinosa, L.; Romero-Toledo, A.; Sampieri, A.; Ortega-Cuellar, D.; Montesinos-Correa, H.; Floriano-Sánchez, E.; Carmona-Aparicio, L. Modulation of Antioxidant Enzymatic Activities by Certain Antiepileptic Drugs (Valproic Acid, Oxcarbazepine, and Topiramate): Evidence in Humans and Experimental Models. Oxid. Med. Cell. Longev. 2013, 2013, 598493. [Google Scholar] [CrossRef] [PubMed]
- Rundfeldt, C.; Koch, R.; Richter, A.; Mevissen, M.; Gerecke, U.; Löscher, W. Dose-Dependent Anticonvulsant and Proconvulsant Effects of Nitric Oxide Synthase Inhibitors on Seizure Threshold in a Cortical Stimulation Model in Rats. Eur. J. Pharmacol. 1995, 274, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-H.; Park, J.-H.; Joo Lee, E.; Jong Won, K.; Lee, S.-H.; Kim, Y.-H.; Hwang, S.; Ja Kwon, K.; Young Shin, C.; Song, K.-H.; et al. Valproic Acid Increases NO Production via the SH-PTP1-CDK5-eNOS-Ser(116) Signaling Cascade in Endothelial Cells and Mice. Free Radic. Biol. Med. 2014, 76, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Mairuae, N.; Cheepsunthorn, P. Valproic Acid Attenuates Nitric Oxide and Interleukin-1β Production in Lipopolysaccharide-Stimulated Iron-Rich Microglia. Biomed. Rep. 2018, 8, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Faradji, H.; Rousset, C.; Debilly, G.; Vergnes, M.; Cespuglio, R. Sleep and Epilepsy: A Key Role for Nitric Oxide? Epilepsia 2000, 41, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.; Felipo, V.; Montoliu, C.; Grisolía, S.; Kaminsky, Y. Effects of Acute Hyperammonemia in Vivo on Oxidative Metabolism in Nonsynaptic Rat Brain Mitochondria. Metab. Brain Dis. 1997, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Mastorodemos, V.; Kotzamani, D.; Zaganas, I.; Arianoglou, G.; Latsoudis, H.; Plaitakis, A. Human GLUD1 and GLUD2 Glutamate Dehydrogenase Localize to Mitochondria and Endoplasmic Reticulum. Biochem. Cell Biol. Biochim. Biol. Cell. 2009, 87, 505–516. [Google Scholar] [CrossRef]
- Fisher, H.F. L-Glutamate Dehydrogenase from Bovine Liver. Methods Enzymol. 1985, 113, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Mans, A.M. Brain Metabolism in Encephalopathy Caused by Hyperammonemia. Adv. Exp. Med. Biol. 1994, 368, 11–21. [Google Scholar] [CrossRef]
- Alilova, G.A.; Tikhonova, L.A.; Montoliu, C.; Kosenko, E.A. Hepatoencephalopathy: The Role of Cerebral Ammonia-Forming Reactions. Medline 2020, 21, 1134–1156. [Google Scholar]
- McKenna, M.C.; Stridh, M.H.; McNair, L.F.; Sonnewald, U.; Waagepetersen, H.S.; Schousboe, A. Glutamate Oxidation in Astrocytes: Roles of Glutamate Dehydrogenase and Aminotransferases. J. Neurosci. Res. 2016, 94, 1561–1571. [Google Scholar] [CrossRef]
- Johannessen, C.U.; Petersen, D.; Fonnum, F.; Hassel, B. The Acute Effect of Valproate on Cerebral Energy Metabolism in Mice. Epilepsy Res. 2001, 47, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.A.; Solomadin, I.N.; Tikhonova, L.A.; Reddy, V.P.; Aliev, G.; Kaminsky, Y.G. Pathogenesis of Alzheimer Disease: Role of Oxidative Stress, Amyloid-β Peptides, Systemic Ammonia and Erythrocyte Energy Metabolism. CNS Neurol. Disord. Drug Targets 2014, 13, 112–119. [Google Scholar] [CrossRef] [PubMed]







| Control | VPA, 50 mg/kg | VPA, 100 mg/kg | VPA, 250 mg/kg | VPA, 500 mg/kg | |
|---|---|---|---|---|---|
| Ammonia, µM | 200.5 ± 35.22 | 196.2 ± 29.64 ns | 177.5 ± 17.59 ns | 171.4 ± 21.57 ns | 164.0 ± 13.74 ns |
| Urea, mM | 6.91 ± 0.7 | 7.18 ± 11 ns | 6.72 ± 0.59 ns | 6.4 ± 0.72 ns | 6.23 ± 0.96 ns |
| ALT, μmol/min × L | 24.1 ± 9.8 | 25.83 ± 9.8 ns | 26.34 ± 2.7 ns | 24.36 ± 4.5 ns | 26.17 ± 3.6 ns |
| AST, μmol/min × L | 26.3 ± 3.8 | 27.95 ± 9.6 ns | 30.03 ± 4.4 ns | 26.8 ± 4.9 ns | 25.7 ± 4.7 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alilova, G.; Tikhonova, L.; Montoliu, C.; Kosenko, E. Ammoniagenic Action of Valproate without Signs of Hepatic Dysfunction in Rats: Possible Causes and Supporting Evidence. Biomolecules 2024, 14, 370. https://doi.org/10.3390/biom14030370
Alilova G, Tikhonova L, Montoliu C, Kosenko E. Ammoniagenic Action of Valproate without Signs of Hepatic Dysfunction in Rats: Possible Causes and Supporting Evidence. Biomolecules. 2024; 14(3):370. https://doi.org/10.3390/biom14030370
Chicago/Turabian StyleAlilova, Gubidat, Lyudmila Tikhonova, Carmina Montoliu, and Elena Kosenko. 2024. "Ammoniagenic Action of Valproate without Signs of Hepatic Dysfunction in Rats: Possible Causes and Supporting Evidence" Biomolecules 14, no. 3: 370. https://doi.org/10.3390/biom14030370







