Role of Polyunsaturated Fatty Acids (PUFAs) and Eicosanoids on Dry Eye Symptoms and Signs
Abstract
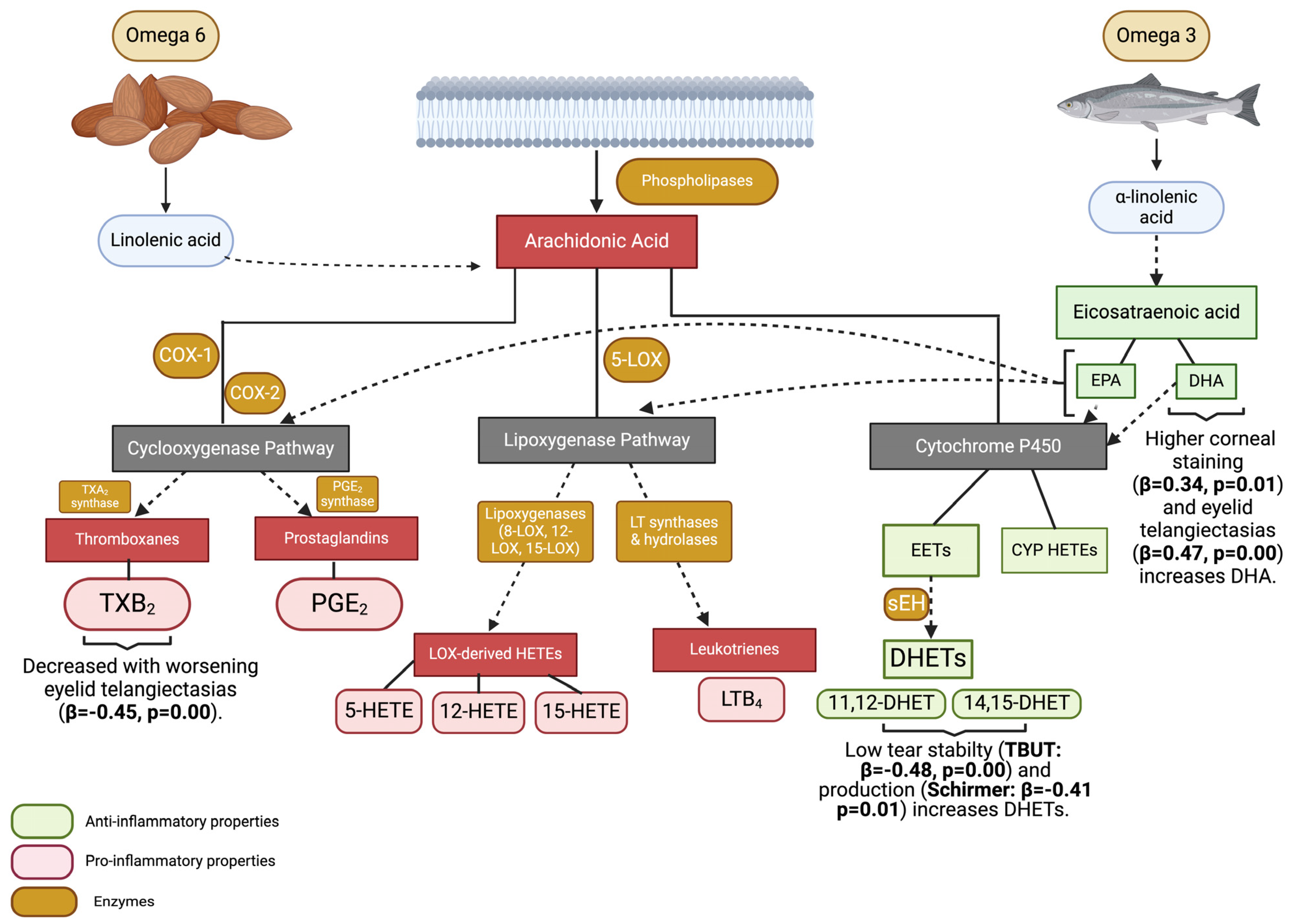
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Ocular Symptoms
2.4. Ocular Surface Assessment
- (1)
- Measurement of tear film osmolarity (TearLAB, San Diego, CA, USA).
- (2)
- Assessment of ocular surface inflammation via InflammaDry (Quidel, San Diego, CA, USA), identifying matrix metallopeptidase 9 (MMP9) graded as 1 = present or 0 = absent based on the appearance of pink stripe.
- (3)
- Upper or lower eyelid laxity determined by rotation (0 = 0–25%, 1 = 25–50%, and 2 = 50–100%) and the snap back test (0 = prompt snapback, 1 = slowed return, and 2 = does not return fully until blinking), respectively.
- (4)
- Anterior blepharitis graded as 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
- (5)
- Telangiectasias seen on the lower eyelids as 0 = none, 1 = mild vessel engorgement, 2 = moderate vessel engorgement, and 3 = severe vessel engorgement.
- (6)
- Inferior meibomian gland plugging graded as 0 = none, 1 = less than 1/3, 2 = between 1/3 and 2/3, and 3 = greater than 2/3 lid involvement.
- (7)
- Tear stability measured by placing 5 µL fluorescein in the superior conjunctivae and assessing the tear break-up time (TBUT).
- (8)
- Fluorescein corneal staining graded to the National Eye Institute (NEI) scale with five areas assessed the inferior, nasal, superior, temporal, and central, and each scored 0–3 (maximum score: 15).
- (9)
- Conjunctivochalasis in each area of the lower eyelid (nasally, medially, and temporally) graded as 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
- (10)
- Tear production graded as millimeter (mm) wetting of anesthetized Schirmer’s test placed in the inferior fornix at 5 min.
- (11)
- Inferior meibomian gland drop out graded to the Meiboscale (range: 0–4) [21].
- (12)
- Meibum quality graded as 0 = clear, 1 = cloudy, 2 = granular, 3 = toothpaste, and 4 = no meibum extracted.
2.5. Tear Collection, PUFA, and Eicosanoid Extraction and Analysis
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Tear PUFAs and Eicosanoids
3.3. Relationships between Tear Eicosanoids and Clinical Metrics
3.4. Relationships between Tear PUFAs, Eicosanoids, and DE/MGD Metrics
3.5. Linear Regression Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Invest Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef]
- Pouyeh, B.; Viteri, E.; Feuer, W.; Lee, D.J.; Florez, H.; Fabian, J.A.; Perez, V.L.; Galor, A. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am. J. Ophthalmol. 2012, 153, 1061–1066.e3. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gadaria-Rathod, N.; Epstein, S.; Asbell, P. Tear cytokine profile as a noninvasive biomarker of inflammation for ocular surface diseases: Standard operating procedures. Invest. Ophthalmol. Vis. Sci. 2013, 54, 8327–8336. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, Y.A.; Chao, C.; Ji, S.; Raida, M.; Torta, F.; Wenk, M.R.; Tong, L. Tear eicosanoids in healthy people and ocular surface disease. Sci. Rep. 2018, 8, 11296. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Claria, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- English, J.T.; Norris, P.C.; Hodges, R.R.; Dartt, D.A.; Serhan, C.N. Identification and Profiling of Specialized Pro-Resolving Mediators in Human Tears by Lipid Mediator Metabolomics. Prostaglandins Leukot. Essent. Fat. Acids 2017, 117, 17–27. [Google Scholar] [CrossRef]
- Walter, S.D.; Gronert, K.; McClellan, A.L.; Levitt, R.C.; Sarantopoulos, K.D.; Galor, A. omega-3 Tear Film Lipids Correlate With Clinical Measures of Dry Eye. Invest Ophthalmol. Vis. Sci. 2016, 57, 2472–2478. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Gancharova, O.S.; Baksheeva, V.E.; Tiulina, V.V.; Goriainov, S.V.; Azbukina, N.V.; Tsarkova, M.S.; Zamyatnin, A.A., Jr.; Philippov, P.P.; Sergeeva, M.G.; et al. Inflammation in Dry Eye Syndrome: Identification and Targeting of Oxylipin-Mediated Mechanisms. Biomedicines 2020, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, M.L.; Abraham, N.G.; Masferrer, J.; Dunn, M.W.; McGiff, J.C. Cytochrome P450 dependent metabolism of arachidonic acid in bovine corneal epithelium. Biochem. Biophys. Res. Commun. 1985, 132, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Peng, R.; Guo, Y.; Shen, L.; Zhao, S.; Xu, D. The role of 14,15-dihydroxyeicosatrienoic acid levels in inflammation and its relationship to lipoproteins. Lipids Health Dis. 2013, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.L.; Begley, C.G.; Caffery, B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact Lens Anterior Eye 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Kalangara, J.P.; Galor, A.; Levitt, R.C.; Covington, D.B.; McManus, K.T.; Sarantopoulos, C.D.; Felix, E.R. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens 2017, 43, 192–198. [Google Scholar] [CrossRef]
- Farhangi, M.; Feuer, W.; Galor, A.; Bouhassira, D.; Levitt, R.C.; Sarantopoulos, C.D.; Felix, E.R. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain 2019, 160, 1541–1550. [Google Scholar] [CrossRef]
- Heiko, D.P. Improved Meiboscale for Meibography. v. 2023. Available online: https://www.heiko-pult.de/media/files/MEIBOSCALE-2016--Einseiter.pdf (accessed on 18 October 2023).
- MacKnight, H.P.; Stephenson, D.J.; Hoeferlin, L.A.; Benusa, S.D.; DeLigio, J.T.; Maus, K.D.; Ali, A.N.; Wayne, J.S.; Park, M.A.; Hinchcliffe, E.H.; et al. The interaction of ceramide 1-phosphate with group IVA cytosolic phospholipase A(2) coordinates acute wound healing and repair. Sci. Signal 2019, 12, eaav5918. [Google Scholar] [CrossRef]
- Stephenson, D.J.; MacKnight, H.P.; Hoeferlin, L.A.; Washington, S.L.; Sawyers, C.; Archer, K.J.; Strauss, J.F., III.; Walsh, S.W.; Chalfant, C.E. Bioactive lipid mediators in plasma are predictors of preeclampsia irrespective of aspirin therapy. J. Lipid Res. 2023, 64, 100377. [Google Scholar] [CrossRef]
- Maus, K.D.; Stephenson, D.J.; Ali, A.N.; MacKnight, H.P.; Huang, H.-J.; Serrats, J.; Kim, M.; Diegelmann, R.F.; Chalfant, C.E. Ceramide kinase regulates acute wound healing by suppressing 5-oxo-ETE biosynthesis and signaling via its receptor OXER1. J. Lipid Res. 2022, 63, 100187. [Google Scholar] [CrossRef]
- Maus, K.D.; Stephenson, D.J.; Macknight, H.P.; Vu, N.T.; Hoeferlin, L.A.; Kim, M.; Diegelmann, R.F.; Xie, X.; Chalfant, C.E. Skewing cPLA(2)alpha activity toward oxoeicosanoid production promotes neutrophil N2 polarization, wound healing, and the response to sepsis. Sci. Signal 2023, 16, eadd6527. [Google Scholar] [CrossRef]
- Wijesinghe, D.S.; Allegood, J.C.; Gentile, L.B.; Fox, T.E.; Kester, M.; Chalfant, C.E. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J. Lipid Res. 2010, 51, 641–651. [Google Scholar] [CrossRef]
- Vu, N.T.; Kim, M.; Stephenson, D.J.; MacKnight, H.P.; Chalfant, C.E. Ceramide Kinase Inhibition Drives Ferroptosis and Sensitivity to Cisplatin in Mutant KRAS Lung Cancer by Dysregulating VDAC-Mediated Mitochondria Function. Mol. Cancer Res. 2022, 20, 1429–1442. [Google Scholar] [CrossRef]
- Wijesinghe, D.S.; Brentnall, M.; Mietla, J.A.; Hoeferlin, L.A.; Diegelmann, R.F.; Boise, L.H.; Chalfant, C.E. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J. Lipid Res. 2014, 55, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.K.; Kamlekar, R.K.; Wijesinghe, D.S.; Zou, X.; Zhai, X.; Mishra, S.K.; Molotkovsky, J.G.; Malinina, L.; Hinchcliffe, E.H.; Chalfant, C.E.; et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 2013, 500, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, Y.A.; Fuchs, D.; Raida, M.; Mazengia, N.T.; Torta, F.; Wheelock, C.E.; Wenk, M.R.; Tong, L. Changes of tear lipid mediators after eyelid warming or thermopulsation treatment for meibomian gland dysfunction. Prostaglandins Other Lipid Mediat. 2020, 151, 106474. [Google Scholar] [CrossRef]
- Anderson, J.S.; Nettleton, J.A.; Herrington, D.M.; Johnson, W.C.; Tsai, M.Y.; Siscovick, D. Relation of omega-3 fatty acid and dietary fish intake with brachial artery flow-mediated vasodilation in the Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2010, 92, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Miettinen, H.; Stern, M.P.; Agil, A.; Jialal, I. Plasma oxidizability in Mexican-Americans and non-Hispanic whites. Metabolism 1996, 45, 876–881. [Google Scholar] [CrossRef]
- Steffen, B.T.; Steffen, L.M.; Tracy, R.; Siscovick, D.; Jacobs, D.; Liu, K.; He, K.; Hanson, N.Q.; Nettleton, J.A.; Tsai, M.Y. Ethnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation biomarkers in the Multi-Ethnic Study of Atherosclerosis (MESA). Eur. J. Clin. Nutr. 2012, 66, 600–605. [Google Scholar] [CrossRef]
- Issan, Y.; Hochhauser, E.; Guo, A.; Gotlinger, K.H.; Kornowski, R.; Leshem-Lev, D.; Lev, E.; Porat, E.; Snir, E.; Thompson, C.I.; et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. 2013, 100–101, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pascale, J.V.; Lucchesi, P.A.; Garcia, V. Unraveling the Role of 12- and 20- HETE in Cardiac Pathophysiology: G-Protein-Coupled Receptors, Pharmacological Inhibitors, and Transgenic Approaches. J. Cardiovasc. Pharmacol. 2021, 77, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Titz, B.; Luettich, K.; Leroy, P.; Boue, S.; Vuillaume, G.; Vihervaara, T.; Ekroos, K.; Martin, F.; Peitsch, M.C.; Hoeng, J. Alterations in Serum Polyunsaturated Fatty Acids and Eicosanoids in Patients with Mild to Moderate Chronic Obstructive Pulmonary Disease (COPD). Int. J. Mol. Sci. 2016, 17, 1583. [Google Scholar] [CrossRef] [PubMed]
- Dry Eye Assessment and Management Study Research Group; Asbell, P.A.; Maguire, M.G.; Pistilli, M.; Ying, G.-S.; Szczotka-Flynn, L.B.; Hardten, D.R.; Lin, M.C.; Shtein, R.M. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N. Engl. J. Med. 2018, 378, 1681–1690. [Google Scholar]
- Downie, L.E.; Ng, S.M.; Lindsley, K.B.; Akpek, E.K. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst. Rev. 2019, 12, CD011016. [Google Scholar] [CrossRef] [PubMed]
- Oydanich, M.; Maguire, M.G.; Pistilli, M.; Hamrah, P.; Greiner, J.V.; Lin, M.C.; Asbell, P.A.; Dry Eye Assessment and Management Study Research Group. Effects of Omega-3 Supplementation on Exploratory Outcomes in the Dry Eye Assessment and Management Study. Ophthalmology 2020, 127, 136–138. [Google Scholar] [CrossRef]
- Ng, A.; Woods, J.; Jahn, T.; Jones, L.W.; Ritter, J.S. Effect of a Novel Omega-3 and Omega-6 Fatty Acid Supplement on Dry Eye Disease: A 3-month Randomized Controlled Trial. Optom. Vis. Sci. 2022, 99, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of Omega-3 Fatty Acid Supplementation for Treatment of Dry Eye Disease: A Meta-Analysis of Randomized Clinical Trials. Cornea 2019, 38, 565–573. [Google Scholar] [CrossRef]
- Christen, W.G.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Lee, I.-M.; Bubes, V.; Friedenberg, G.; Dushkes, R.; Smith, D.; Schaumberg, D.A.; et al. Efficacy of Marine omega-3 Fatty Acid Supplementation vs Placebo in Reducing Incidence of Dry Eye Disease in Healthy US Adults: A Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Martin, E.; Enriquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Frequencies |
|---|---|
| Demographics, % (n) | |
| Sex, male | 95% (38) |
| Race, White | 30% (12) |
| Ethnicity, non-Hispanic | 85% (34) |
| Comorbidities, % (n) | |
| Smoking, current | 35% (14) |
| Hypertension | 68% (27) |
| Hypercholesterolemia | 63% (25) |
| Diabetes | 40% (16) |
| PTSD | 30% (12) |
| Depression | 65% (26) |
| Osteoarthritis | 55% (22) |
| Sleep apnea | 35% (14) |
| BPH | 18% (7) |
| Medications, % (n) | |
| Betablockers | 18% (7) |
| Statins | 53% (21) |
| Antidepressants | 65% (26) |
| Anxiolytics | 63% (25) |
| Antihistamines | 20% (8) |
| NSAIDs | 33% (13) |
| ASA | 43% (17) |
| Fish oil supplements | 10% (4) |
| Devices, % (n) | |
| CPAP | 15% (6) |
| Dry eye symptoms and ocular pain, median (IQR) | |
| DEQ-5 | 12.5 (7.0) |
| OSDI | 34.4 (37.6) |
| NRS of average pain 1 week (0–10) | 3.0 (4.0) |
| NPSI-Eye total (0–100) | 21.5 (29.8) |
| * Dry eye signs, % (n) | |
| Tear osmolarity, mOsm/L, median (IQR) | 299.5 (15.8) |
| MMP-9, =1, (0–1) | 60% (24) |
| Upper lid laxity, ≥2, (0–2) | 25% (10) |
| Lower lid laxity, ≥2, (0–2) | 18% (7) |
| Anterior blepharitis, ≥2, (0–3) | 5% (2) |
| Telangiectasias, ≥2 (0–3) | 10% (4) |
| Inferior meibomian gland plugging, ≥2, (0–3) | 45% (18) |
| TBUT, seconds, median (IQR), | 9.5 (6.7) |
| Corneal staining, ≥2 (0–15) | 45% (18) |
| Conjunctivochalasis, ≥2, (0–3) | 58% (23) |
| Schirmer’s, mm, median (IQR) | 11.5 (13) |
| Meibomian gland dropout, ≥2, (0–4) | 58% (23) |
| Meibum quality, ≥2, (0–4) | 50% (20) |
| Group | Eicosanoid | Median (IQR), (pmol Select Lipid/mg Protein]) * | n | Mann–Whitney U | p-Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Gender | |||||
| Males | 12-HETE | 3.02 (3.10) | 38 | 6.00 | 0.05 |
| Females | 0.72 (0) | 2 | |||
| Ethnicity | |||||
| Non-Hispanics | 15-HETE | 1.06 (1.44) | 34 | 43.00 | 0.03 |
| Hispanics | 2.80 (3.49) | 6 | |||
| Comorbidities | |||||
| Smoking | |||||
| Yes ** | 14,15 DHET | 0.10 (0.12) | 33 | 50.00 | 0.02 |
| No | 0.05 (0.08) | 7 | |||
| Hypercholesterolemia | |||||
| Yes | TXB2 | 0.00 (26.66) | 25 | 119.00 | 0.05 |
| No | 20.73 (52.11) | 15 | |||
| Diabetes | |||||
| Yes | 12 HETE | 4.10 (3.47) | 16 | 117.00 | 0.04 |
| No | 2.18 (2.51) | 24 | |||
| Sleep apnea | |||||
| Yes | AA: DHA | 6.24 (2.49) | 14 | 112.00 | 0.05 |
| No | 7.09 (3.94) | 26 | |||
| Yes | ω6: ω3 | 5.67 (2.44) | 15 | 107.00 | 0.03 |
| No | 6.81 (8.95) | 26 | |||
| Medications | |||||
| Betablockers | |||||
| Yes | 15 HETE | 4.65 (4.60) | 7 | 46.00 | 0.01 |
| No | 1.07 (1.39) | 33 | |||
| Anxiolytics | |||||
| Yes | TXB2 | 16.89 (46.6) | 25 | 112.00 | 0.03 |
| No | 0.00 (16.60) | 15 | |||
| Fish oil supplements | |||||
| Yes | 5 HETE | 2.00 (1.16) | 4 | 28.00 | 0.05 |
| No | 0.76 (0.72) | 36 | |||
| Yes | EPA | 49.83 (66.85) | 4 | 23.00 | 0.03 |
| No | 7.61 (16.3) | 36 | |||
| Yes | AA: EPA | 35.43 (44.18) | 4 | 26.00 | 0.04 |
| No | 74.11 (75.56) | 36 | |||
| Yes | AA: DHA | 4.96 (2.33) | 4 | 25.00 | 0.03 |
| No | 7.15 (3.01) | 36 | |||
| Yes | ω6: ω3 | 4.30 (2.80) | 4 | 18.00 | 0.02 |
| No | 6.81 (2.31) | 36 | |||
| Multivitamin supplements | |||||
| Yes | 14,15 DHET | 0.10 (0.16) | 21 | 123.50 | 0.04 |
| No | 0.08 (0.11) | 19 | |||
| Yes | 11,12 DHET | 0.05 (0.07) | 21 | 103.00 | 0.00 |
| No | 0.00 (0.03) | 19 | |||
| Yes | 5 HETE | 1.21 (1.51) | 21 | 84.00 | 0.00 |
| No | 0.70 (0.79) | 19 | |||
| Yes | EPA | 15.47 (28.44) | 21 | 109.50 | 0.02 |
| No | 4.01 (10.85) | 19 | |||
| Yes | DHA | 138.60 (197.55) | 21 | 100.00 | 0.00 |
| No | 54.22 (68.68) | 19 | |||
| Yes | AA | 742.38 (1377.13) | 21 | 119.00 | 0.03 |
| No | 412.64 (735.95) | 19 | |||
| Yes | AA: EPA | 53.96 (34.25) | 21 | 125.50 | 0.05 |
| No | 102.87 (80.17) | 19 | |||
 pro-inflammatory;
pro-inflammatory;  anti-inflammatory;
anti-inflammatory;  pro/anti-inflammatory ratio. * Unit of each lipid is normalized with the amount of protein in the sample as a pico mole of lipid per milligram of protein (pmol/mg protein). ** Includes history of smoking and current smokers.
pro/anti-inflammatory ratio. * Unit of each lipid is normalized with the amount of protein in the sample as a pico mole of lipid per milligram of protein (pmol/mg protein). ** Includes history of smoking and current smokers.| Pro-Inflammatory | Anti-Inflammatory | Pro/Anti-Inflammatory Ratios | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | TXB2 | 5 HETE | 12 HETE | 15 HETE | DHA | EPA | 11,12 DHET | 14,15 DHET | AA: DHA | AA: EPA | ω6: ω3 | |
| ρ | ρ | ρ | ρ | ρ | ρ | ρ | ρ | ρ | ρ | ρ | ρ | |
| Symptoms | ||||||||||||
| DEQ-5 | −0.11 | 0.11 | 0.02 | 0.12 | 0.07 | −0.12 | −0.01 | 0.10 | 0.01 | 0.14 | 0.20 | 0.15 |
| OSDI | −0.04 | 0.10 | −0.00 | −0.03 | 0.04 | −0.11 | −0.02 | 0.01 | 0.04 | 0.24 | 0.14 | 0.22 |
| Avg. eye pain intensity over 1 week | 0.01 | 0.14 | 0.08 | 0.13 | 0.01 | −0.05 | 0.08 | 0.13 | 0.18 | 0.13 | −0.05 | 0.11 |
| NPSI-Eye total | −0.05 | 0.12 | 0.06 | 0.08 | 0.00 | −0.11 | 0.04 | 0.05 | 0.12 | 0.16 | −0.04 | 0.14 |
| § Signs | ||||||||||||
| Tear osmolarity | 0.18 | −0.02 | 0.14 | −0.01 | 0.02 | 0.30 | 0.24 | 0.10 | −0.01 | −0.25 | −0.34 | −0.31 |
| Upper lid laxity | −0.02 | −0.27 | 0.14 | 0.24 | 0.08 | 0.02 | 0.01 | 0.12 | −0.01 | −0.17 | 0.13 | −0.14 |
| Lower lid laxity | −0.05 | −0.25 | 0.02 | 0.13 | 0.02 | 0.01 | 0.06 | −0.05 | −0.06 | −0.20 | −0.09 | −0.22 |
| Anterior blepharitis | 0.14 | −0.01 | 0.03 | 0.08 | 0.24 | 0.11 | 0.03 | 0.11 | −0.04 | 0.09 | 0.29 | 0.09 |
| Eyelid telangiectasias | 0.24 | −0.18 | 0.17 | −0.06 | 0.32 * | 0.25 | 0.21 | −0.07 | 0.06 | −0.09 | −0.00 | −0.09 |
| Meibomian gland plugging | 0.11 | 0.16 | 0.03 | −0.01 | −0.12 | 0.12 | 0.07 | 0.03 | 0.08 | 0.09 | −0.03 | 0.05 |
| TBUT | −0.29 | −0.16 | −0.13 | 0.17 | −0.05 | −0.34 * | −0.29 | −0.34 * | −0.14 | 0.05 | 0.12 | 0.05 |
| Staining | 0.30 | 0.10 | 0.25 | 0.13 | 0.17 | 0.35 * | 0.23 | 0.26 | 0.26 | −0.05 | −0.05 | −0.06 |
| Conjunctivochalasis | 0.01 | 0.05 | 0.01 | 0.19 | −0.14 | 0.02 | 0.10 | 0.18 | 0.18 | 0.03 | −0.17 | −0.01 |
| Schirmer | −0.30 | −0.14 | −0.32 * | −0.20 | −0.24 | −0.31 | −0.26 | −0.22 | −0.40 ** | −0.09 | 0.01 | −0.07 |
| Meibomian glands drop out | 0.17 | −0.29 | 0.19 | −0.01 | 0.08 | 0.15 | 0.17 | −0.01 | 0.15 | 0.09 | 0.11 | 0.09 |
| Meibum quality | 0.19 | −0.01 | 0.13 | 0.02 | 0.06 | 0.22 | 0.28 | −0.18 | 0.08 | −0.07 | −0.04 | −0.12 |
| Unstandardized Coefficients | Standardized Coefficients | Sig. | 95% Confidence Intervals | Coefficient of Determination | ||||
|---|---|---|---|---|---|---|---|---|
| Models | B | SE | β | p Value | Lower | Upper | Adjusted R2 | |
| DE Signs | ||||||||
| Eyelid telangiectasias | DHA | 0.00 | 0.00 | 0.47 | 0.00 | 0.00 | 0.01 | 0.36 |
| TXB2 | −0.01 | 0.00 | −0.45 | 0.00 | −0.02 | −0.00 | ||
| Ethnicity | 0.54 | 0.25 | 0.29 | 0.04 | 0.03 | 1.05 | ||
| TBUT | 11,12-DHET | −65.0 | 19.6 | −0.48 | 0.00 | −104.64 | −25.30 | 0.54 |
| AA: DHA | 0.99 | 0.35 | 0.41 | 0.00 | 0.29 | 1.70 | ||
| Staining | DM | 1.93 | 0.63 | 0.43 | 0.00 | 0.66 | 3.19 | 0.30 |
| DHA | 0.01 | 0.00 | 0.34 | 0.02 | 0.00 | 0.01 | ||
| Schirmer | 14,15-DHET | −29.66 | 10.76 | −0.41 | 0.01 | −51.45 | −7.87 | 0.17 |
 pro-inflammatory;
pro-inflammatory;  anti-inflammatory;
anti-inflammatory;  pro/anti-inflammatory ratio.
pro/anti-inflammatory ratio.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangwani-Mordani, S.; Prislovsky, A.; Stephenson, D.; Chalfant, C.E.; Galor, A.; Mandal, N. Role of Polyunsaturated Fatty Acids (PUFAs) and Eicosanoids on Dry Eye Symptoms and Signs. Biomolecules 2024, 14, 376. https://doi.org/10.3390/biom14030376
Mangwani-Mordani S, Prislovsky A, Stephenson D, Chalfant CE, Galor A, Mandal N. Role of Polyunsaturated Fatty Acids (PUFAs) and Eicosanoids on Dry Eye Symptoms and Signs. Biomolecules. 2024; 14(3):376. https://doi.org/10.3390/biom14030376
Chicago/Turabian StyleMangwani-Mordani, Simran, Amanda Prislovsky, Daniel Stephenson, Charles E. Chalfant, Anat Galor, and Nawajes Mandal. 2024. "Role of Polyunsaturated Fatty Acids (PUFAs) and Eicosanoids on Dry Eye Symptoms and Signs" Biomolecules 14, no. 3: 376. https://doi.org/10.3390/biom14030376






