Radiolabelled FGF-2 for Imaging Activated Fibroblasts in the Tumor Micro-Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Conjugation
2.2. Radiolabeling with 99mTc
2.3. Quality Controls
2.4. Stability to Challenge with Cysteine
2.5. In Vitro Cell Binding Assay
2.6. In Vivo and Ex Vivo Experiments in Mice
2.7. In Vivo Imaging in Mice
3. Results
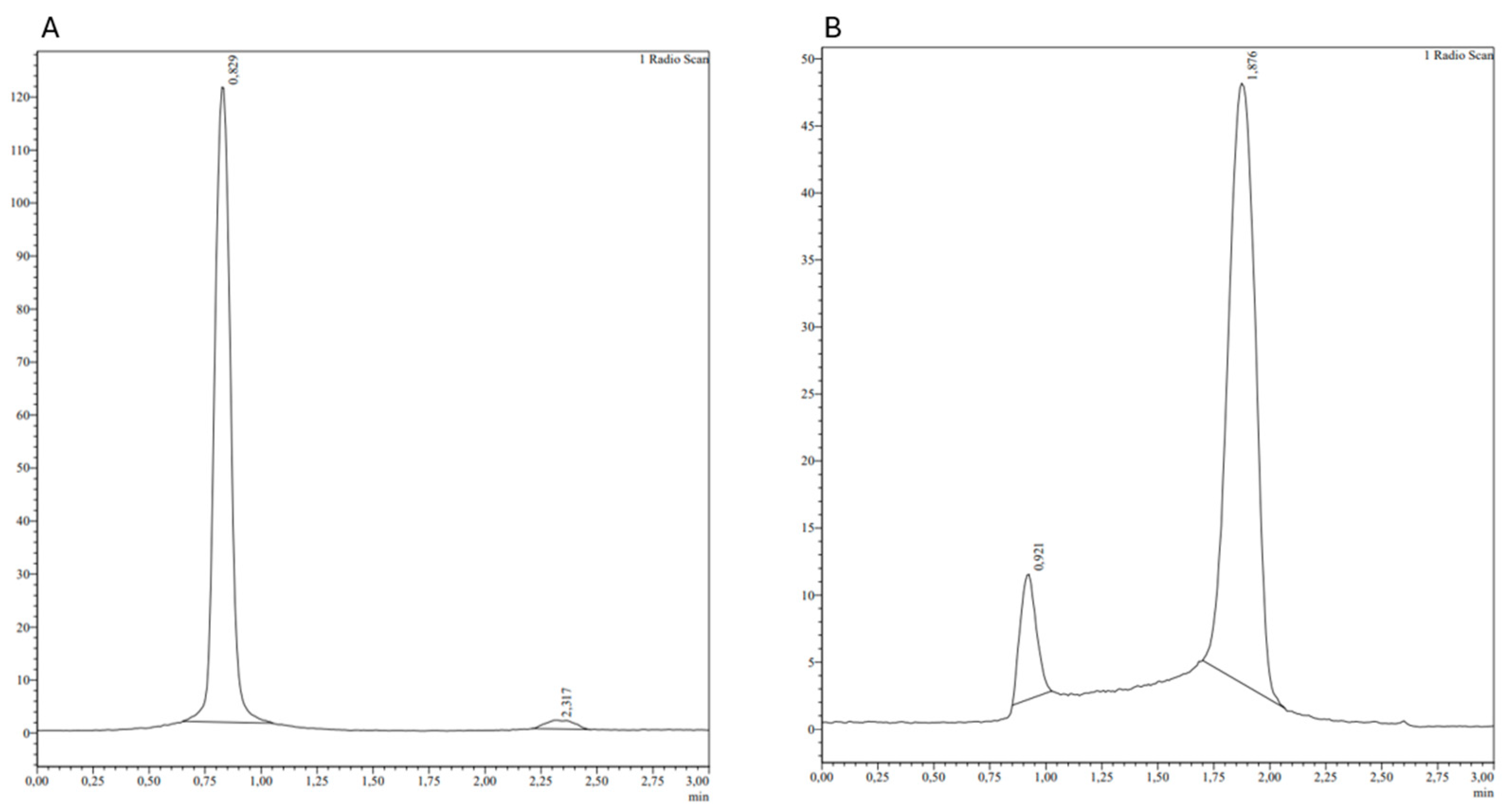
3.1. Radiolabeling with 99mTc and Quality Controls
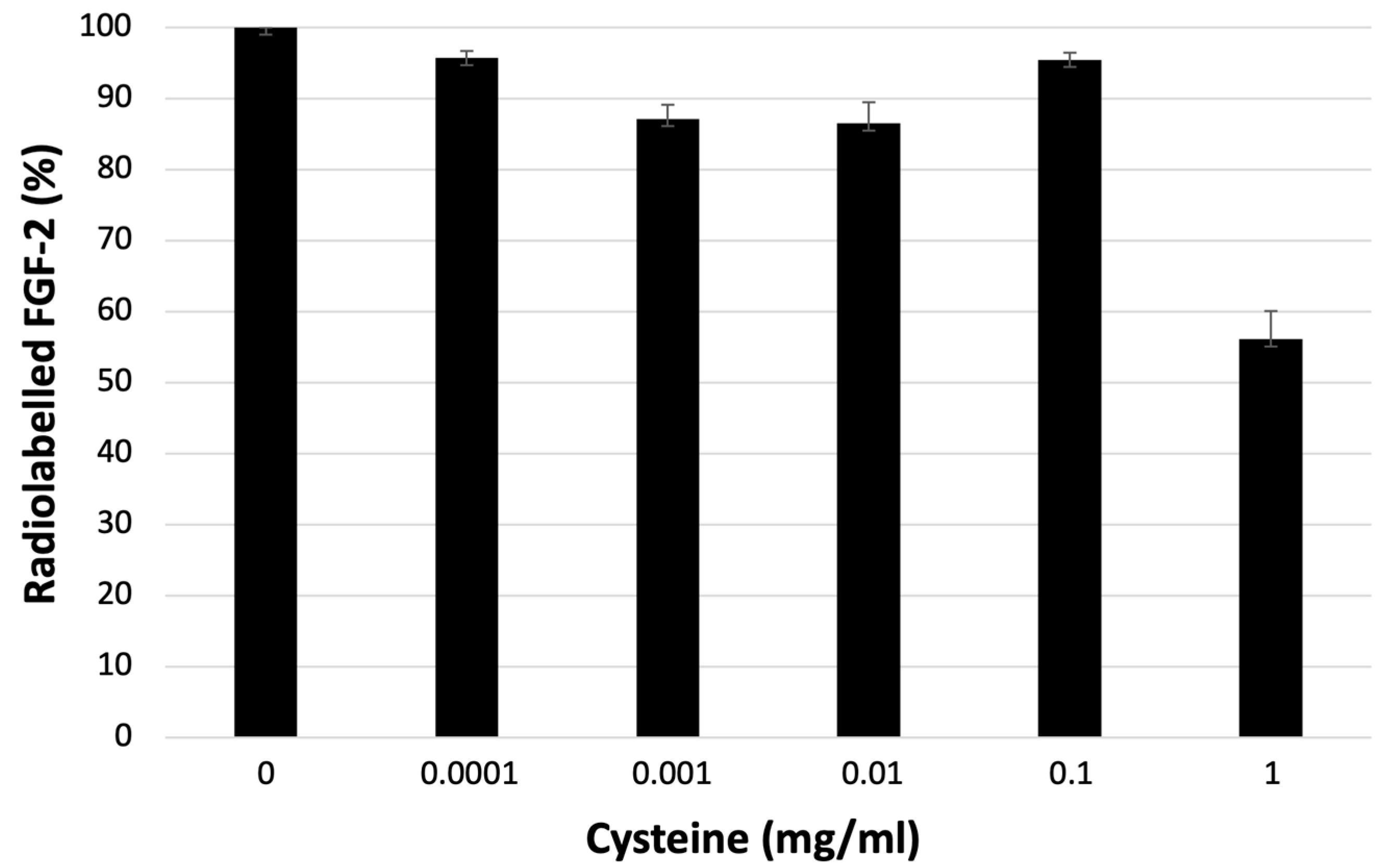
3.2. Stability to Challenge with Cysteine
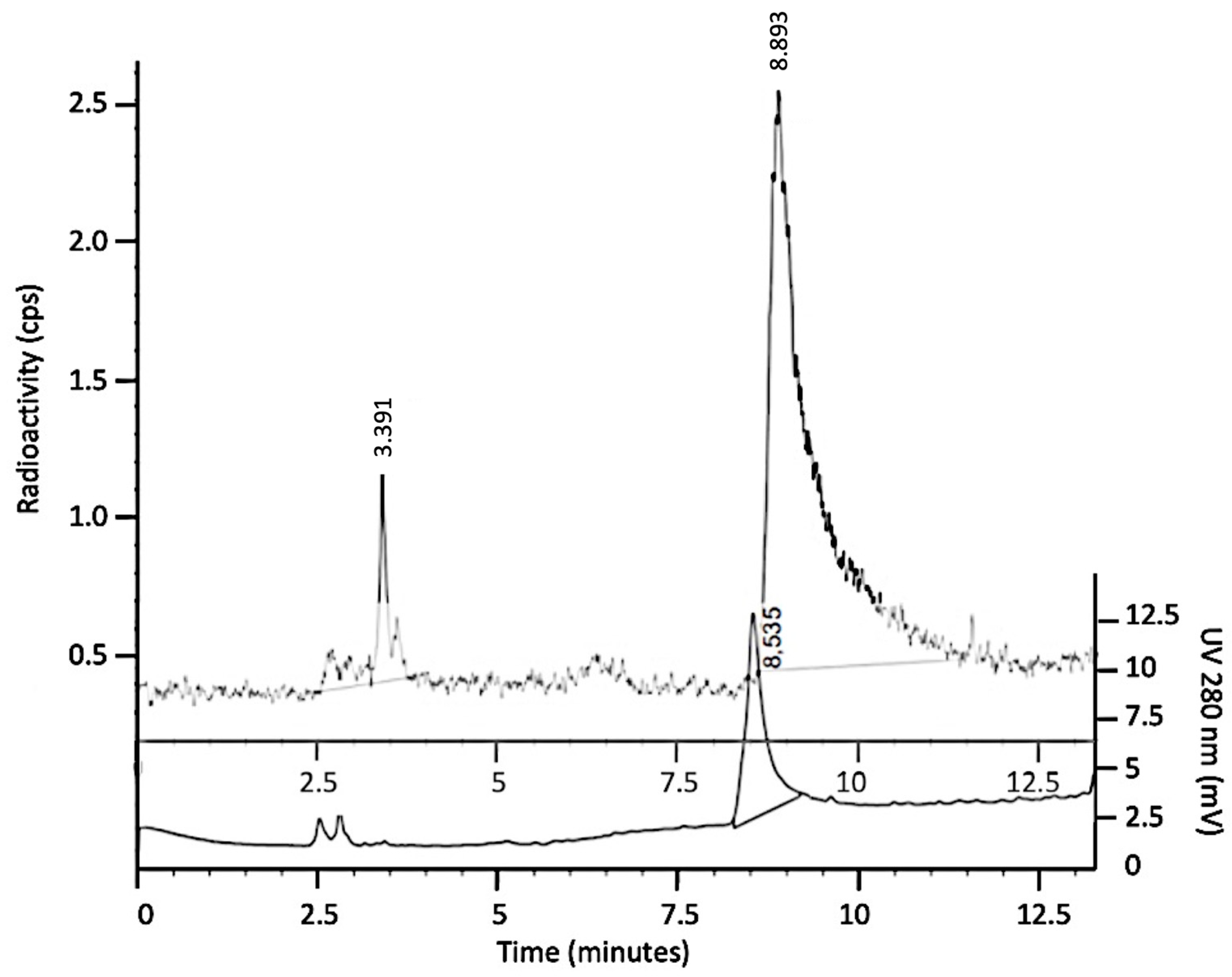
3.3. Binding Assay
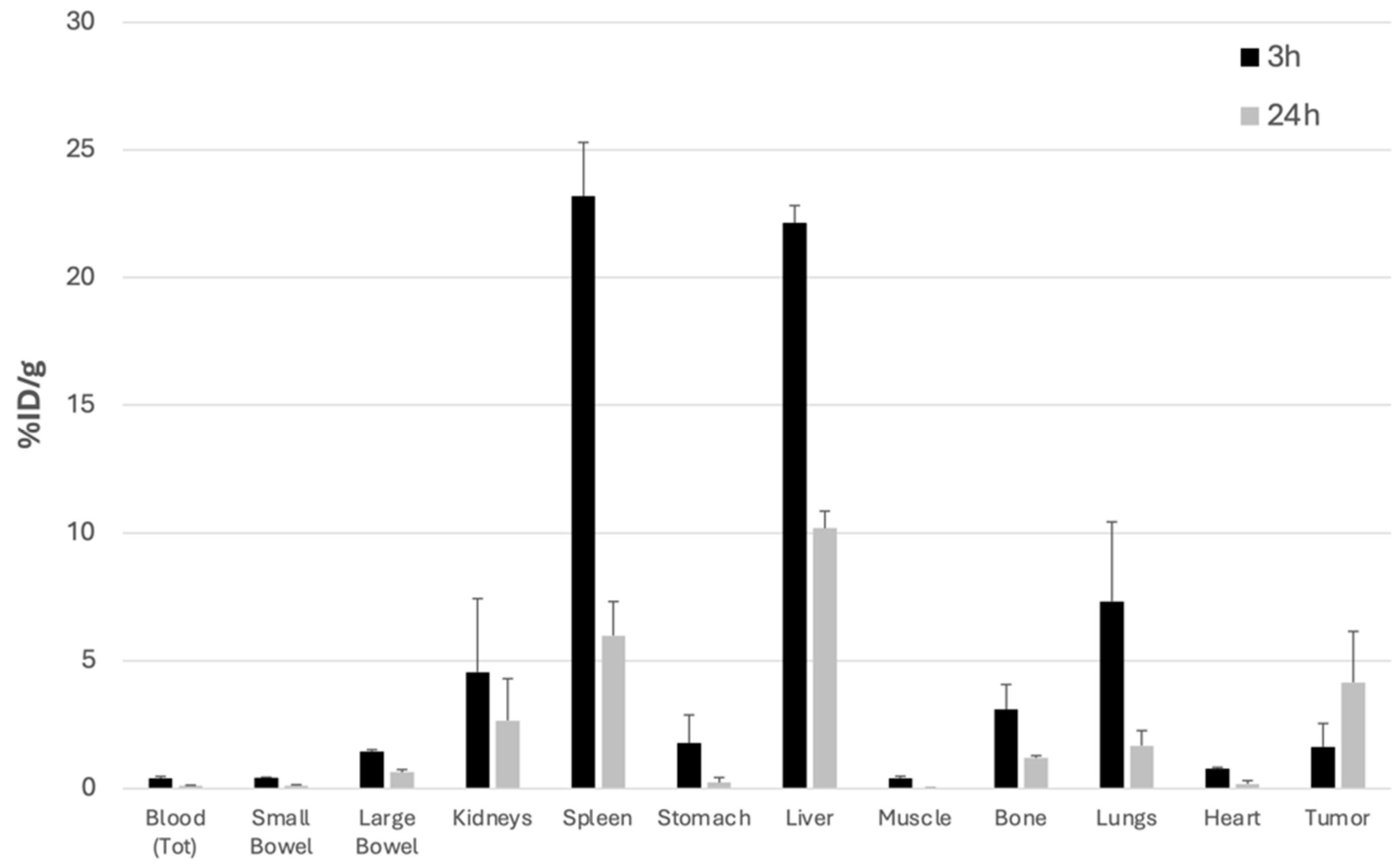
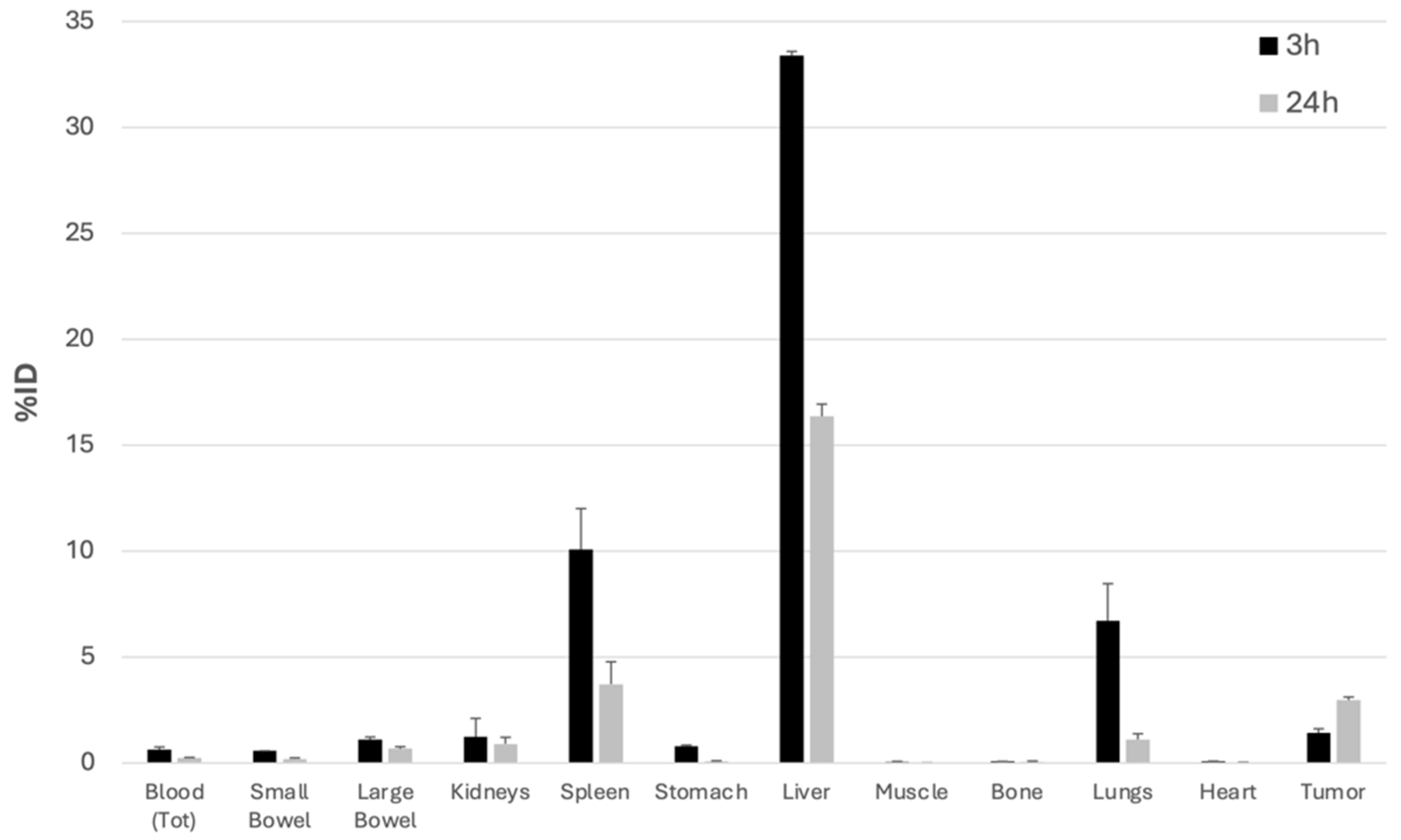
3.4. In Vivo and Ex Vivo Experiments in Mice
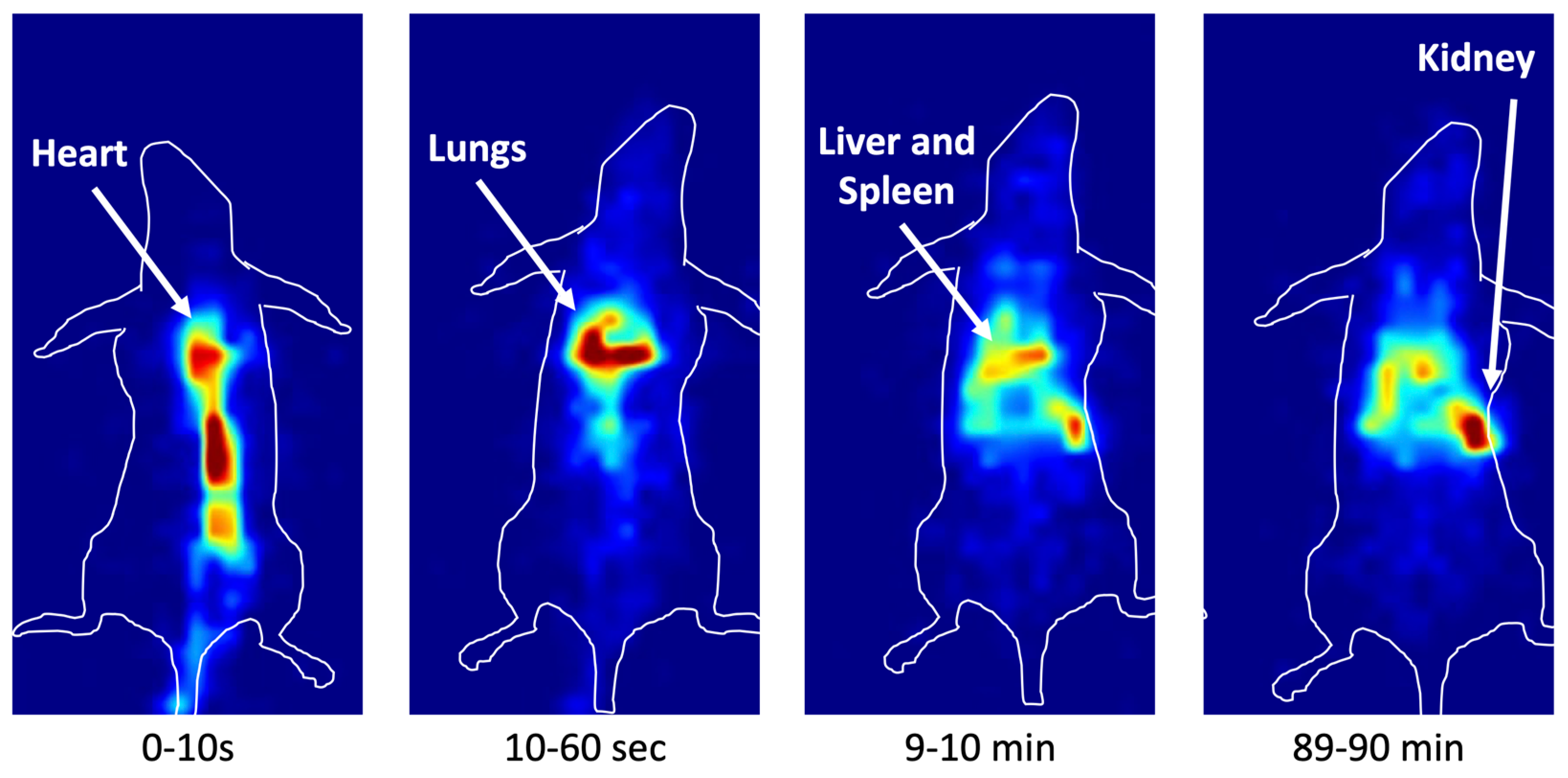
3.5. In Vivo Imaging in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zinkle, A.; Chen, L.; Mohammadi, M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat. Rev. Rheumatol. 2020, 16, 547–564. [Google Scholar] [CrossRef]
- Fang, B.; Lu, Y.; Li, X.; Wei, Y.; Ye, D.; Wei, G.; Zhu, Y. Targeting the tumor microenvironment, a new therapeutic approach for prostate cancer. Prostate Cancer Prostatic Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. The roles of FGFs in the early development of vertebrate limbs. Genes. Dev. 1998, 12, 1571–1586. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004, 20, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Couderc, B.; Prats, H.; Bayard, F.; Amalric, F. Potential oncogenic effects of basic fibroblast growth factor requires cooperation between CUG and AUG-initiated forms. Cell Regul. 1991, 2, 709–718. [Google Scholar] [CrossRef]
- Shirakihara, T.; Horiguchi, K.; Miyazawa, K.; Ehata, S.; Shibata, T.; Morita, I.; Miyazono, K.; Saitoh, M. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 2011, 30, 783–795. [Google Scholar] [CrossRef]
- Katoh, M.; Loriot, Y.; Brandi, G.; Tavolari, S.; Wainberg, Z.A.; Katoh, M. FGFR-targeted therapeutics: Clinical activity, mechanisms of resistance and new directions. Nat. Rev. Clin. Oncol. 2024, 4, 312–329. [Google Scholar] [CrossRef]
- Babina, I.S.; Turner, N.C. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 2017, 17, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Massagué, J. Roles of TGFbeta in metastasis. Cell Res. 2009, 19, 89–102. [Google Scholar] [CrossRef]
- Brahimi, O.A.; Yeh, B.K.; Eliseenkova, A.V.; Zhang, F.; Olsen, S.K.; Igarashi, M.; Aaronson, S.A.; Linhardt, R.J.; Mohammadi, M. Analysis of mutations in fibroblast growth factor (FGF) and a pathogenic mutation in FGF receptor (FGFR) provides direct evidence for the symmetric two-end model for FGFR dimerization. Mol. Cell. Biol. 2005, 25, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Greulich, H.; Pollock, P.M. Targeting mutant fibroblast growth factor receptors in cancer. Trends Mol. Med. 2011, 17, 283–292. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hagio, M.; Seya, T.; Ishiwata, T. Fibroblast growth factor receptor 2 IIIc as a therapeutic target for colorectal cancer cells. Mol. Cancer Ther. 2012, 11, 2010–2020. [Google Scholar] [CrossRef]
- Gatius, S.; Velasco, A.; Azueta, A.; Santacana, M.; Pallares, J.; Valls, J.; Dolcet, X.; Prat, J.; Matias-Guiu, X. FGFR2 alterations in endometrial carcinoma. Mod. Pathol. 2011, 24, 1500–1510. [Google Scholar] [CrossRef]
- Im, J.H.; Buzzelli, J.N.; Jones, K.; Franchini, F.; Gordon-Weeks, A.; Markelc, B.; Chen, J.; Kim, J.; Cao, Y.; Muschel, R.J. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat. Commun. 2020, 11, 4064. [Google Scholar] [CrossRef] [PubMed]
- S-HyNic Bioconjugation Technical Manual. Available online: https://search.cosmobio.co.jp/cosmo_search_p/search_gate2/docs/SLK_/S90021.20090617.pdf (accessed on 27 May 2023).
- Björke, H.; Andersson, K. Automated, high-resolution cellular retention and uptake studies in vitro. Appl. Radiat. Isot. 2006, 64, 901–905. [Google Scholar] [CrossRef]
- Galli, F.; Artico, M.; Taurone, S.; Manni, I.; Bianchi, E.; Piaggio, G.; Weintraub, B.D.; Szkudlinski, M.W.; Agostinelli, E.; Dierckx, R.A.J.O.; et al. Radiolabeling of VEGF165 with 99mTc to evaluate VEGFR expression in tumor angiogenesis. Int. J. Oncol. 2017, 50, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Auletta, S.; Galli, F.; Varani, M.; Campagna, G.; Conserva, M.; Martinelli, D.; Santino, I.; Signore, A. In Vitro and In Vivo Evaluation of 99mTc-Polymyxin B for Specific Targeting of Gram-Bacteria. Biomolecules 2021, 11, 232. [Google Scholar] [CrossRef]
- Ranieri, D.; Rosato, B.; Nanni, M.; Magenta, A.; Belleudi, F.; Torrisi, M.R. Expression of the FGFR2 mesenchymal splicing variant in epithelial cells drives epithelial-mesenchymal transition. Oncotarget 2016, 7, 5440–5460. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Bonfiglio, R.; Varani, M.; Galli, F.; Campagna, G.; Desco, M.; Cussó, L.; Mattei, M.; Wunder, A.; Borri, F.; et al. Radioimmune Imaging of α4β7 Integrin and TNFα for Diagnostic and Therapeutic Applications in Inflammatory Bowel Disease. Pharmaceutics 2023, 15, 817. [Google Scholar] [CrossRef] [PubMed]
- Massari, R.; Ucci, A.; Campisi, C.; Scopinaro, F.; Soluri, A. A novel fully integrated handheld gamma camera. Nucl. Instrum. Methods Phys. Res. 2016, 832, 271–278. [Google Scholar] [CrossRef]
- Massari, R.; Caputo, D.; Ronchi, S.; Soluri, A. Low-Power Charge Division Circuits for Wireless Applications Based on Silicon Photomultipliers. IEEE Sens. J. 2016, 16, 8214–8219. [Google Scholar] [CrossRef]
- Ranieri, D.; Nanni, M.; Persechino, F.; Torrisi, M.R.; Belleudi, F. Role of PKCϵ in the epithelial-mesenchymal transition induced by FGFR2 isoform switch. Cell Commun. Signal. 2020, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2—A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Kletting, S.; Barthold, S.; Repnik, U.; Griffiths, G.; Loretz, B.; Schneider-Daum, N.; de Souza Carvalho-Wodarz, C.; Lehr, C.M. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX 2018, 35, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Franken, P.R.; Guglielmi, J.; Vanhove, C.; Koulibaly, M.; Defrise, M.; Darcourt, J.; Pourcher, T. Distribution and dynamics of (99m)Tc-pertechnetate uptake in the thyroid and other organs assessed by single-photon emission computed tomography in living mice. Thyroid 2010, 20, 519–526. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Zboralski, D.; Hoehne, A.; Bredenbeck, A.; Schumann, A.; Nguyen, M.; Schneider, E.; Ungewiss, J.; Paschke, M.; Haase, C.; von Hacht, J.L.; et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3651–3667. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Finck, R.; Mokoala, K.M.G.; Staudinger, F.; Schillings, L.; Heger, U.; Röhrich, M.; Kratochwil, C.; Sathekge, M.; et al. 68Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4089–4100. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Rekabpour, S.J.; Jafari, E.; Divband, G.; Nikkholgh, B.; Amini, H.; Kamali, H.; Ebrahimi, S.; Shakibazad, N.; Jokar, N.; et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients with Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021, 46, e523–e530. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, G.; Fan, M.; Jia, H.; Xiao, L.; Lang, J. Anti-tumour effects of a xenogeneic fibroblast activation protein-based whole cell tumour vaccine in murine tumour models. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4182–4193. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Yang, G.; Zhao, M.; Liu, Y.; Ge, X.; Jia, B.; Gao, S. Synthesis and Preliminary Study of 99mTc-Labeled HYNIC-FAPi for Imaging of Fibroblast Activation Proteins in Tumors. Mol. Pharm. 2024, 21, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ou, L.; Zhang, C. Comparison of 68Ga-FAPI and 18F-FDG PET/CT in Metastases of Papillary Thyroid Carcinoma. Endocrine 2021, 73, 767–768. [Google Scholar] [CrossRef]
- Fu, H.; Fu, J.; Huang, J.; Su, X.; Chen, H. 68Ga-FAPI PET/CT in Thyroid Cancer with Thyroglobulin Elevation and Negative Iodine Scintigraphy. Clin. Nucl. Med. 2021, 46, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Tatar, G.; Alçın, G.; Erol Fenercioğlu, Ö.; ¸Sahin, R.; Çermik, T.F. Findings of I-131 SPECT/CT, 18F-FDG, and 68Ga-FAPI-04 PET/CT Imaging in a Patient Treated with Radioiodine Therapy for Metastatic Papillary Thyroid Carcinoma. Mol. Imaging Radionucl. Ther. 2023, 32, 57–61. [Google Scholar] [CrossRef]
- Kaghazchi, F.; Aghdam, R.A.; Haghighi, S.; Vali, R.; Adinehpour, Z. 177Lu-FAPI Therapy in a Patient with End-Stage Metastatic Pancreatic Adenocarcinoma. Clin. Nucl. Med. 2022, 47, e243–e245. [Google Scholar] [CrossRef]
- Liu, L.; Zhong, J.; Zhang, Z.; Ye, X.; Wang, X.; Liu, S.; Zhang, Z. Preclinical study and first-in-human imaging of [18F]FAP-2286, and comparison with 2-[18F]FDG PET/CT in various cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2024. epub ahead of print. [Google Scholar] [CrossRef]
- Wegen, S.; Claus, K.; Linde, P.; Rosenbrock, J.; Trommer, M.; Zander, T.; Tuchscherer, A.; Bruns, C.; Schlößer, H.A.; Schröder, W.; et al. Impact of FAPI-46/dual-tracer PET/CT imaging on radiotherapeutic management in esophageal cancer. Radiat. Oncol. 2024, 19, 44. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Rösch, F.; ArunRaj, S.T.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Bal, C. First-in-Human Experience with 177Lu-DOTAGA.(SA.FAPi)2 Therapy in an Uncommon Case of Aggressive Medullary Thyroid Carcinoma Clinically Mimicking as Anaplastic Thyroid Cancer. Clin. Nucl. Med. 2022, 47, e444–e445. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Sonni, I.; Benz, M.R.; Gafita, A.; Bahri, S.; Shuch, B.M.; Yu, R.; Liu, S.T.; Czernin, J.; Calais, J. 68Ga-FAPI-46 and 18F-FDG PET/CT in a Patient with Immune-Related Thyroiditis Induced by Immune Checkpoint Inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3736–3737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, J.; Chen, Y. 68Ga-FAPI PET/CT Imaging in a Patient with Thyroiditis. Endocrine 2021, 73, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, L.; Lei, L.; Wang, L.; Chen, Y. Clinical Significance of Diffusely Increased Uptake of 68Ga-FAPI in Thyroid Gland. Front. Med. 2021, 8, 782231. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Wu, J.; Wu, J.; Mou, C.; Zhang, C. Follicular Thyroid Adenoma Showing Avid Uptake on 68Ga-DOTA-FAPI-04 PET/CT. Clin. Nucl. Med. 2021, 46, 840–841. [Google Scholar]
- Can, C.; Gündoğan, C.; Güzel, Y.; Kaplan, İ.; Kömek, H. 68Ga-FAPI Uptake of Thyroiditis in a Patient with Breast Cancer. Clin. Nucl. Med. 2021, 46, 683–685. [Google Scholar] [CrossRef]


| 3 h | 24 h | ||
|---|---|---|---|
| Tumor/Muscle | %ID/g | 4.0 | 151.6 |
| %ID | 20.7 | 341.8 | |
| Tumor/Blood | %ID/g | 4.1 | 26.1 |
| %ID | 2.3 | 12.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentivoglio, V.; Galli, F.; Varani, M.; Ranieri, D.; Nayak, P.; D’Elia, A.; Soluri, A.; Massari, R.; Lauri, C.; Signore, A. Radiolabelled FGF-2 for Imaging Activated Fibroblasts in the Tumor Micro-Environment. Biomolecules 2024, 14, 491. https://doi.org/10.3390/biom14040491
Bentivoglio V, Galli F, Varani M, Ranieri D, Nayak P, D’Elia A, Soluri A, Massari R, Lauri C, Signore A. Radiolabelled FGF-2 for Imaging Activated Fibroblasts in the Tumor Micro-Environment. Biomolecules. 2024; 14(4):491. https://doi.org/10.3390/biom14040491
Chicago/Turabian StyleBentivoglio, Valeria, Filippo Galli, Michela Varani, Danilo Ranieri, Pallavi Nayak, Annunziata D’Elia, Andrea Soluri, Roberto Massari, Chiara Lauri, and Alberto Signore. 2024. "Radiolabelled FGF-2 for Imaging Activated Fibroblasts in the Tumor Micro-Environment" Biomolecules 14, no. 4: 491. https://doi.org/10.3390/biom14040491





