Development of Improved Spectrophotometric Assays for Biocatalytic Silyl Ether Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equpment
2.2. Hydrolysis Assays with TF-Silα-Strep
2.3. General Procedure for the Synthesis of Substrates 4–10
2.3.1. tert-Butyldimethyl(2-methyl-4-nitrophenoxy)silane, 4
2.3.2. tert-Butyldimethyl(3-methyl-4-nitrophenoxy)silane, 5
2.3.3. tert-Butyldimethyl(3-methoxy-4-nitrophenoxy)silane, 6
2.3.4. tert-Butyldimethyl(4-cyanophenoxy)silane, 7
2.3.5. tert-Butyldimethyl(2-methyl-4-cyanophenoxy)silane, 8
2.3.6. tert-Butyldimethyl(3-methyl-4-cyanophenoxy)silane, 9
2.3.7. (E)-1-(4-((tert-butyldimethylsilyl)oxy)phenyl)-2-(4-nitrophenyl)diazene, 10
3. Results and Discussion
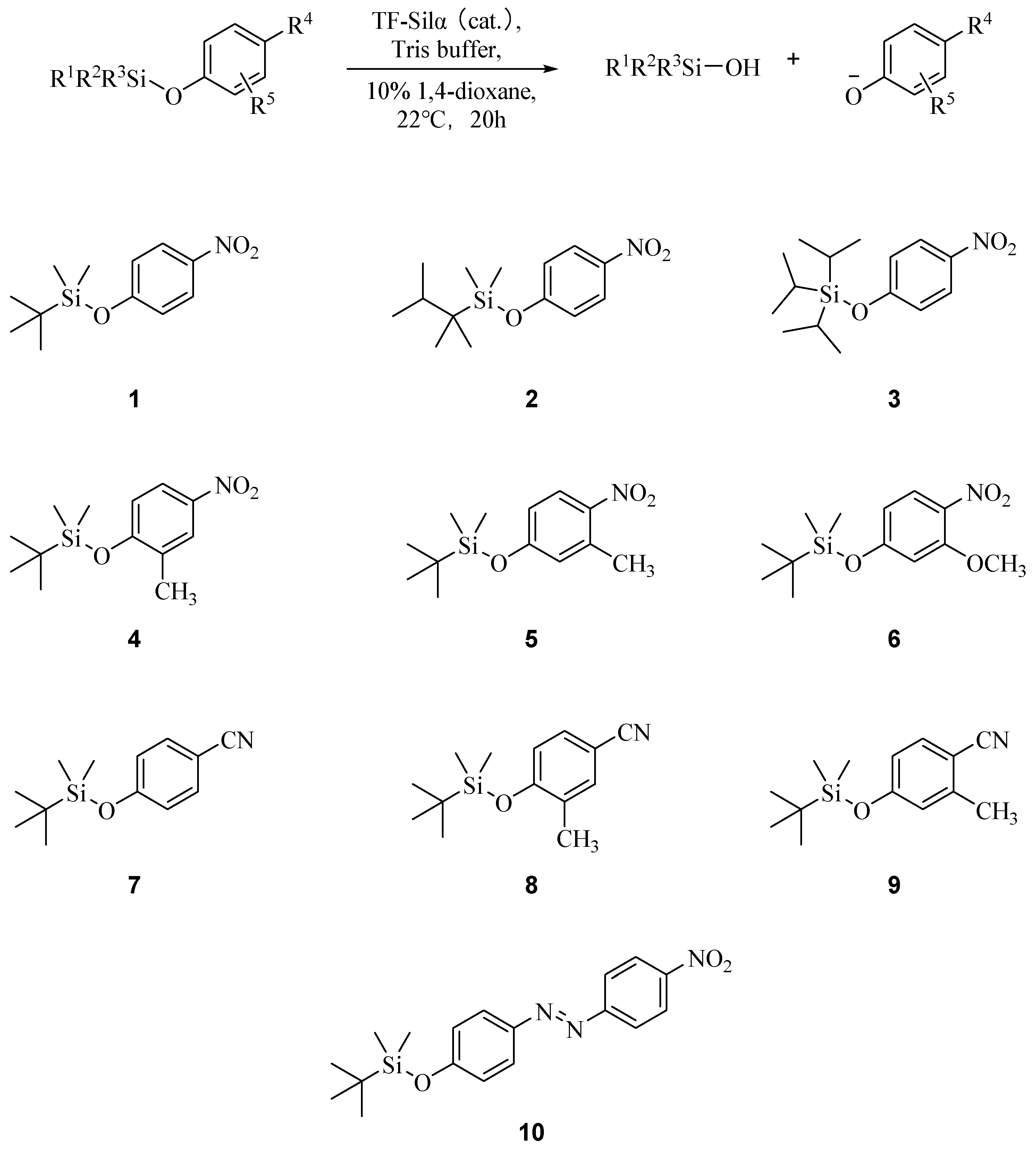
3.1. Design and Synthesis of Substrates
3.2. pH Optimisation of Assay
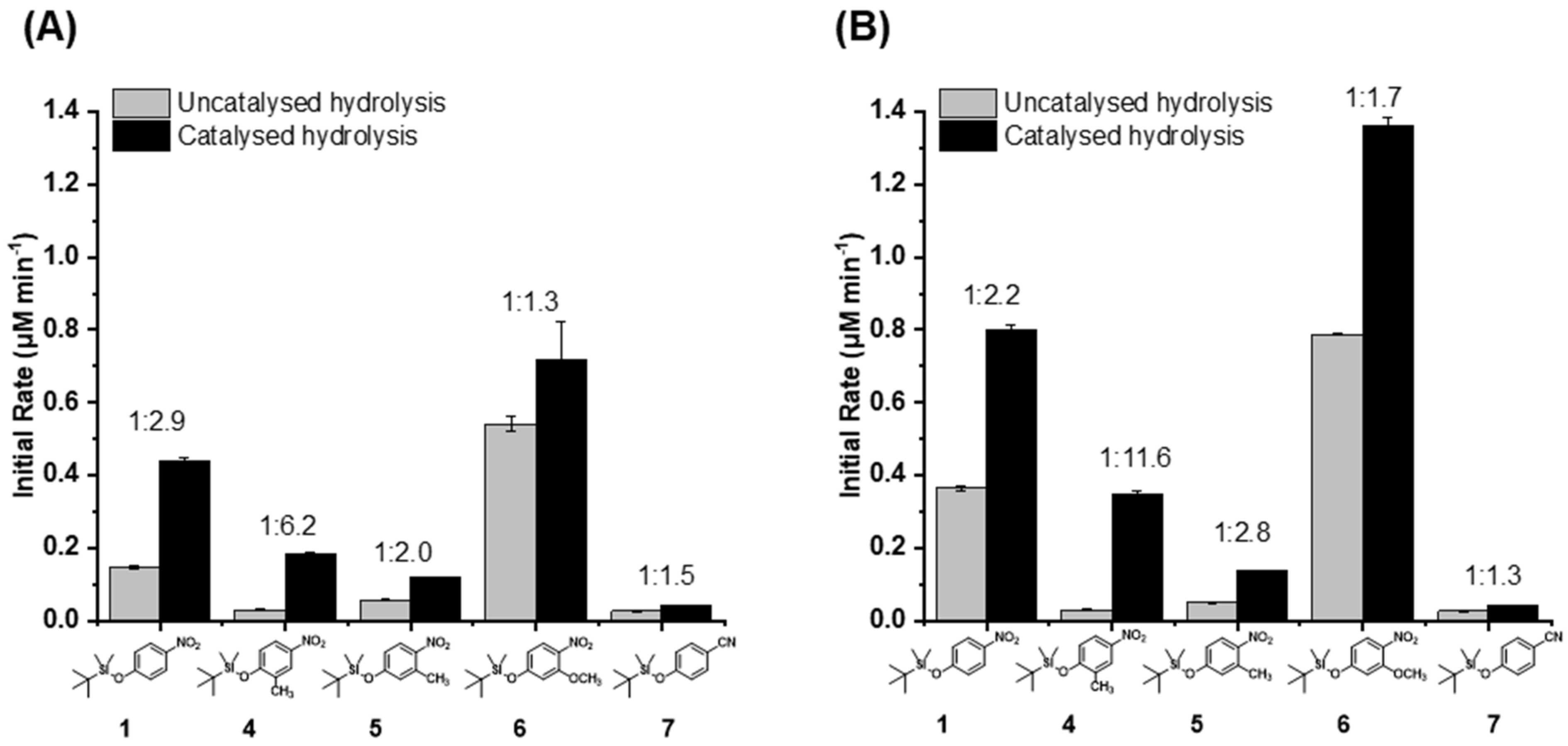
3.3. Substrate Screening
3.4. Kinetic Analysis of TF-Silα-Strep on Silyl Ether Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andriot, M.; DeGroot, J.V.; Meeks, R.; Wolf, A.T.; Leadley, S.; Garaud, J.L.; Gubbels, F.; Lecomte, J.P.; Lenoble, B.; Stassen, S.; et al. Silicones in Industrial Applications. In Inorganic Polymers; Nova Science Pub Inc.: Hauppauge, NY, USA, 2007; pp. 61–161. [Google Scholar]
- Corey, E.J.; Venkateswarlu, A. Protection of Hydroxyl Groups as Tert-Butyldimethylsilyl Derivatives. J. Am. Chem. Soc. 1972, 94, 6190–6191. [Google Scholar] [CrossRef]
- D’Sa, B.A.; McLeod, D.; Verkade, J.G. Nonionic Superbase-Catalyzed Silylation of Alcohols. J. Org. Chem. 1997, 62, 5057–5061. [Google Scholar] [CrossRef]
- Frampton, M.B.; Zelisko, P.M. Biocatalysis in Silicon Chemistry. Chem. Asian J. 2017, 12, 1153–1167. [Google Scholar] [CrossRef]
- Sarai, N.S.; Levin, B.J.; Roberts, J.M.; Katsoulis, D.E.; Arnold, F.H. Biocatalytic Transformations of Silicon—The Other Group 14 Element. ACS Cent. Sci. 2021, 7, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Bassindale, A.R.; Brandstadt, K.F.; Lane, T.H.; Taylor, P.G. Enzyme-Catalysed Siloxane Bond Formation. J. Inorg. Biochem. 2003, 96, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.B.; Simionescu, R.; Dudding, T.; Zelisko, P.M. The Enzymatic Cleavage of Si–O Bonds: A Kinetic Analysis of the Biocatalyzed Hydrolysis of Phenyltrimethoxysilane. J. Mol. Catal. B Enzym. 2010, 66, 105–112. [Google Scholar] [CrossRef]
- Abbate, V.; Bassindale, A.R.; Brandstadt, K.F.; Taylor, P.G. A Large Scale Enzyme Screen in the Search for New Methods of Silicon–Oxygen Bond Formation. J. Inorg. Biochem. 2011, 105, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Dakhili, S.Y.T.; Caslin, S.A.; Faponle, A.S.; Quayle, P.; De Visser, S.P.; Wong, L.S. Recombinant Silicateins as Model Biocatalysts in Organosiloxane Chemistry. Proc. Natl. Acad. Sci. USA 2017, 114, E5285–E5291. [Google Scholar] [CrossRef]
- Sparkes, E.I.; Kettles, R.A.; Egedeuzu, C.S.; Stephenson, N.L.; Caslin, S.A.; Dakhili, S.Y.T.; Wong, L.S. Improved Production and Biophysical Analysis of Recombinant Silicatein-α. Biomolecules 2020, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
- Brondani, P.; Mittersteiner, M.; Voigt, M.; Klinkowski, B.; Riva Scharf, D.; de Jesus, P. Synthetic Versatility of Lipases: Application for Si–O Bond Formation and Cleavage. Synthesis 2019, 51, 477–485. [Google Scholar] [CrossRef]
- Sparkes, E.I.; Egedeuzu, C.S.; Lias, B.; Sung, R.; Caslin, S.A.; Tabatabaei Dakhili, S.Y.; Taylor, P.G.; Quayle, P.; Wong, L.S. Biocatalytic Silylation: The Condensation of Phenols and Alcohols with Triethylsilanol. Catalysts 2021, 11, 879. [Google Scholar] [CrossRef]
- Dorval, C.; Tricoire, M.; Begouin, J.-M.; Gandon, V.; Gosmini, C. Cobalt-Catalyzed C(Sp 2)–CN Bond Activation: Cross-Electrophile Coupling for Biaryl Formation and Mechanistic Insight. ACS Catal. 2020, 10, 12819–12827. [Google Scholar] [CrossRef]
- Carvalho, E.; Francisco, A.P.; Iley, J.; Rosa, E. The Mechanism of Hydrolysis of Aryl Ether Derivatives Of3-Hydroxymethyltriazenes. Eur. J. Org. Chem. 2005, 2005, 2056–2063. [Google Scholar] [CrossRef]
- Onyido, I.; Swierczek, K.; Purcell, J.; Hengge, A.C. A Concerted Mechanism for the Transfer of the Thiophosphinoyl Group from Aryl Dimethylphosphinothioate Esters to Oxyanionic Nucleophiles in Aqueous Solution. J. Am. Chem. Soc. 2005, 127, 7703–7711. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, A.A.; Sokolova, I.V. Development of Method for Destruction of 4-Cyanophenol Using Photolysis and Activated Oxidative Processes. J. Appl. Spectrosc. 2023, 90, 825–829. [Google Scholar] [CrossRef]
- Yu, Z.-C.; Lu, Y.; Zhao, J.; Dai, J.-J.; Chen, G.-R.; Shan, P.-H.; Redshaw, C.; Tao, Z.; Xiao, X. A Study of the Supramolecular Assembly Formed by Cucurbit[7]Uril and 4-Cyanophenol. J. Mol. Struct. 2023, 1278, 134969. [Google Scholar] [CrossRef]
- Cheng, X.; Li, S.; Xu, G.; Li, C.; Qin, J.; Li, Z. A Reaction-Based Colorimetric Fluoride Probe: Rapid “Naked-Eye” Detection and Large Absorption Shift. Chempluschem 2012, 77, 908–913. [Google Scholar] [CrossRef]
- Kirschke, H.; Langer, J.; Wiederanders, B.; Ansorge, S.; Bohley, P.; Cathepsin, L. A new proteinase from rat-liver lysosomes. Eur. J. Biochem. 1977, 74, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Sanatan, P.T.; Lomate, P.R.; Giri, A.P.; Hivrale, V.K. Characterization of a Chemostable Serine Alkaline Protease from Periplaneta Americana. BMC Biochem. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]

| Substrate | KM (µM) | kcat (min−1) |
|---|---|---|
| 1 | 44.4 ± 11.9 | 0.0858 ± 0.0102 |
| 2 | 24.1 ± 5.00 | 0.0136 ± 0.0010 |
| 3 | 20.4 ± 8.13 | 0.0023 ± 0.0003 |
| 4 | 72.5 ± 15.3 | 0.0577 ± 0.0101 |
| 5 | 34.6 ± 4.11 | 0.0247 ± 0.0013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Egedeuzu, C.S.; Taylor, P.G.; Wong, L.S. Development of Improved Spectrophotometric Assays for Biocatalytic Silyl Ether Hydrolysis. Biomolecules 2024, 14, 492. https://doi.org/10.3390/biom14040492
Lu Y, Egedeuzu CS, Taylor PG, Wong LS. Development of Improved Spectrophotometric Assays for Biocatalytic Silyl Ether Hydrolysis. Biomolecules. 2024; 14(4):492. https://doi.org/10.3390/biom14040492
Chicago/Turabian StyleLu, Yuqing, Chisom S. Egedeuzu, Peter G. Taylor, and Lu Shin Wong. 2024. "Development of Improved Spectrophotometric Assays for Biocatalytic Silyl Ether Hydrolysis" Biomolecules 14, no. 4: 492. https://doi.org/10.3390/biom14040492