Cost-Effective Cosmetic-Grade Hyaluronan Hydrogels for ReNcell VM Human Neural Stem Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
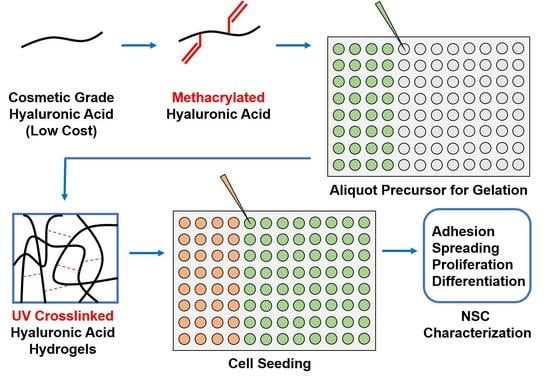
2.1. Synthesis of Methacrylated Hyaluronic Acid (MeHA)
2.2. Crosslinked Hydrogel Fabrication
2.3. Rheology
2.4. Scanning Electron Microscopy (SEM)
2.5. Stem Cell Culture
2.6. Analysis of Cell Adhesion and Spreading
2.7. Proliferation and Differentiation
2.8. Statistical Analysis
3. Results
3.1. Synthesis of Cosmetic-Grade MeHA and 1H-NMR Characterization
3.2. MeHA Hydrogel Fabrication and Physical Characterization
3.3. ReNcell VM Adhesion and Spreading on x-MeHA Hydrogels
3.4. ReNcell VM Proliferation on x-MeHA Hydrogels
3.5. ReNcell VM Differentiation on Crosslinked x-MeHA Hydrogels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruoslahti, E. Brain extracellular matrix. Glycobiology 1996, 6, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidlits, S.; Khaing, Z.; Petersen, R.; Nickels, J.; Vanscoy, J.; Shear, J.; Schmidt, C. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ren, Y.; Cui, F.; Xu, Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J. Neurosci. Res. 2009, 87, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.N.; Freitas, B.C.; Qian, H.; Lux, J.; Acab, A.; Trujillo, C.A.; Herai, R.H.; Nguyen Huu, V.A.; Wen, J.H.; Joshi-Barr, S.; et al. Layered hydrogels accelerate ipsc-derived neuronal maturation and reveal migration defects caused by mecp2 dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, 3185–3190. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.W. Three-dimensional electroconductive hyaluronic acid hydrogels incorporated with carbon nanotubes and polypyrrole by catechol-mediated dispersion enhance neurogenesis of human neural stem cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, R.; Duan, B.; Jiang, P. Three-dimensional hyaluronic acid hydrogel-based models for in vitro human ipsc-derived npc culture and differentiation. J. Mater. Chem. B 2017, 5, 3870–3878. [Google Scholar] [CrossRef]
- Seidlits, S.; Liang, J.; Bierman, R.; Sohrabi, A.; Karam, J.; Holley, S.; Cepeda, C.; Walthers, C. Peptide-modified, hyaluronic acid-based hydrogels as a 3d culture platform for neural stem/progenitor cell engineering. J. Biomed. Mater. Res. A 2019, 107, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H. Use of hyaluronic acid fillers for the treatment of the aging face. Clin. Interv. Aging 2007, 2, 369–376. [Google Scholar] [CrossRef]
- Burdick, J.; Prestwich, G. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-controlled radical polymerization: Mechanisms, methods, and applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Irion, S.; Nostro, M.C.; Kattman, S.J.; Keller, G.M. Directed differentiation of pluripotent stem cells: From developmental biology to therapeutic applications. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, X.J.; Renier, N.; Wu, Z.; Atkin, T.; Sun, Z.; Ozair, M.Z.; Tchieu, J.; Zimmer, B.; Fattahi, F.; et al. Combined small-molecule inhibition accelerates the derivation of functional cortical neurons from human pluripotent stem cells. Nat. Biotechnol. 2017, 35, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M. Towards stem cell based therapies for parkinson’s disease. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar] [CrossRef] [PubMed]
- Georges, P.C.; Miller, W.J.; Meaney, D.F.; Sawyer, E.S.; Janmey, P.A. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 2006, 90, 3012–3018. [Google Scholar] [CrossRef]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [Green Version]
- Keung, A.J.; de Juan-Pardo, E.M.; Schaffer, D.V.; Kumar, S. Rho gtpases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 2011, 29, 1886–1897. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, R.; Miljan, E.A.; Hines, S.J.; Aouabdi, S.; Pollock, K.; Patel, S.; Edwards, F.A.; Sinden, J.D. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A three-dimensional human neural cell culture model of alzheimer’s disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef]
- Lange, C.; Mix, E.; Frahm, J.; Glass, A.; Müller, J.; Schmitt, O.; Schmöle, A.C.; Klemm, K.; Ortinau, S.; Hübner, R.; et al. Small molecule gsk-3 inhibitors increase neurogenesis of human neural progenitor cells. Neurosci. Lett. 2011, 488, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Jin, G.W.; Gehret, P.M.; Chada, N.C.; Suh, W.H. A novel cell penetrating peptide for the differentiation of human neural stem cells. Biomolecules 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; Amicis, C.V.D.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. Nmr chemical shifts of trace impurities: Industrially preferred solvents used in process and green chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Brigham, M.D.; Bick, A.; Lo, E.; Bendali, A.; Burdick, J.A.; Khademhosseini, A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng. Part A 2009, 15, 1645–1653. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef]
- Messager, L.; Portecop, N.; Hachet, E.; Lapeyre, V.; Pignot-Paintrand, I.; Catargi, B.; Auzely-Velty, R.; Ravaine, V. Photochemical crosslinking of hyaluronic acid confined in nanoemulsions: Towards nanogels with a controlled structure. J. Mater. Chem. B 2013, 1, 3369–3379. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Jaggi, B.; Palcic, B. Measurement of cell motility and morphology with an automated microscope system. Cytometry 1988, 9, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Henley-Smith, C.J.; De Canha, M.N.; Oosthuizen, C.B.; Berrington, D. Viability reagent, prestoblue, in comparison with other available reagents, utilized in cytotoxicity and antimicrobial assays. Int. J. Microbiol. 2013, 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; McCanna, D.J.; Sivak, J.G. Use of the viability reagent prestoblue in comparison with alamarblue and mtt to assess the viability of human corneal epithelial cells. J. Pharmacol. Toxicol. Methods 2015, 71, 1–7. [Google Scholar] [CrossRef]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grutzmacher, H.; Liska, R. Highly efficient water-soluble visible light photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Walters, B.; Uynuk-Ool, T.; Rothdiener, M.; Palm, J.; Hart, M.L.; Stegemann, J.P.; Rolauffs, B. Engineering the geometrical shape of mesenchymal stromal cells through defined cyclic stretch regimens. Sci. Rep. 2017, 7, 6640. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.B.; Parmar, M. Strengths and limitations of the neurosphere culture system. Mol. Neurobiol. 2006, 34, 153–161. [Google Scholar] [CrossRef]
- Naruse, M.; Shibasaki, K.; Yokoyama, S.; Kurachi, M.; Ishizaki, Y. Dynamic changes of cd44 expression from progenitors to subpopulations of astrocytes and neurons in developing cerebellum. PLoS ONE 2013, 8, e53109. [Google Scholar] [CrossRef]
- Khaing, Z.; Seidlits, S. Hyaluronic acid and neural stem cells: Implications for biomaterial design. J. Mater. Chem. B 2015, 3, 7850–7866. [Google Scholar] [CrossRef]
- Yousefi, F.; Kandel, S.; Pleshko, N. Infrared spectroscopic quantification of methacrylation of hyaluronic acid: A scaffold for tissue engineering applications. Appl. Spectrosc. 2018, 72, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Tous, E.; Ifkovits, J.L.; Koomalsingh, K.J.; Shuto, T.; Soeda, T.; Kondo, N.; Gorman, J.H.; Gorman, R.C.; Burdick, J.A. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules 2011, 12, 4127–4135. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell. Fact. 2011, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Baeva, L.F.; Sarkar Das, S.; Hitchins, V.M. Bacterial endotoxin detection in hyaluronic acid-based medical devices. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of peg-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Mistry, P.; Herbert, K.E.; Lunec, J. Molecular and cellular effects of ultraviolet light-induced genotoxicity. Crit. Rev. Clin. Lab. Sci. 1998, 35, 189–237. [Google Scholar] [CrossRef] [PubMed]
- Boiko, A.; Kulik, V.; Seoudi, B.; Chun, H.; Lee, I. Measurement method of complex viscoelastic material properties. Int. J. Solids Struct. 2010, 47, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Naficy, S.; Kawakami, S.; Sadegholvaad, S.; Wakisaka, M.; Spinks, G. Mechanical properties of interpenetrating polymer network hydrogels based on hybrid ionically and covalently crosslinked networks. J. Appl. Polym. Sci. 2013, 130, 2504–2513. [Google Scholar] [CrossRef]
- Ahearne, M.; Yang, Y.; El Haj, A.J.; Then, K.Y.; Liu, K.K. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2005, 2, 455–463. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-gag scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Nazarov, R.; Jin, H.J.; Kaplan, D.L. Porous 3-d scaffolds from regenerated silk fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Weska, R.; Vieira, W.; Nogueira, G.; Beppu, M. Effect of freezing methods on the properties of lyophilized porous silk fibroin membranes. Mater. Res.-IBERO-Am. J. 2009, 12, 233–237. [Google Scholar] [CrossRef]
- Lai, J.Y.; Ma, D.H.; Lai, M.H.; Li, Y.T.; Chang, R.J.; Chen, L.M. Characterization of cross-linked porous gelatin carriers and their interaction with corneal endothelium: Biopolymer concentration effect. PLoS ONE 2013, 8, e54058. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.Z.; Duan, Z.; Wang, Y.; Feng, R.R. A novel human-like collagen hydrogel scaffold with porous structure and sponge-like properties. Polymers 2017, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Foster, S.C.; Xing, R.; Feistel, K.; Olsen, R.H.; Acevedo, S.F.; Raber, J.; Sherman, L.S. Cd44 transmembrane receptor and hyaluronan regulate adult hippocampal neural stem cell quiescence and differentiation. J. Biol. Chem. 2017, 292, 4434–4445. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, A.; Ortega, F.; Fan, W.; Marichal, N.; Paez, J.I.; Berninger, B.; Campo, A.D.; Salierno, M.J. Bifunctional hydrogels containing the laminin motif ikvav promote neurogenesis. Stem Cell Reports 2017, 9, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.; Romanyuk, N.; Jirakova, K.; Hejcl, A.; Janouskova, O.; Machova, L.U.; Bochin, M.; Pradny, M.; Vargova, L.; Jendelova, P. The effect of ips-derived neural progenitors seeded on laminin-coated phema-moetacl hydrogel with dual porosity in a rat model of chronic spinal cord injury. Cell Transplant. 2019, 963689718823705. [Google Scholar] [CrossRef] [PubMed]
- Mercier, F. Fractones: Extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell. Mol. Life Sci. 2016, 73, 4661–4674. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.A.; Rebaza, L.M.; Derzic, S.; Schwartz, P.H.; Monuki, E.S. Regulation of human neural precursor cells by laminin and integrins. J. Neurosci. Res. 2006, 83, 845–856. [Google Scholar] [CrossRef] [Green Version]
- Hall, P.E.; Lathia, J.D.; Caldwell, M.A.; Ffrench-Constant, C. Laminin enhances the growth of human neural stem cells in defined culture media. BMC Neurosci. 2008, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Hernández, J.C.; Salmerón Sánchez, M.; Soria, J.M.; Gómez Ribelles, J.L.; Monleón Pradas, M. Substrate chemistry-dependent conformations of single laminin molecules on polymer surfaces are revealed by the phase signal of atomic force microscopy. Biophys. J. 2007, 93, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lee-Thedieck, C.; Rauch, N.; Fiammengo, R.; Klein, G.; Spatz, J.P. Impact of substrate elasticity on human hematopoietic stem and progenitor cell adhesion and motility. J. Cell. Sci. 2012, 125, 3765–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalali, S.; Tafazzoli-Shadpour, M.; Haghighipour, N.; Omidvar, R.; Safshekan, F. Regulation of endothelial cell adherence and elastic modulus by substrate stiffness. Cell Commun. Adhes. 2015, 22, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Li, L.; Li, Y. Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef]
- Suslov, O.N.; Kukekov, V.G.; Ignatova, T.N.; Steindler, D.A. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc. Natl. Acad. Sci. USA 2002, 99, 14506–14511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Szczesna, K.; Ochalek, A.; Kobolák, J.; Varga, E.; Nemes, C.; Chandrasekaran, A.; Rasmussen, M.; Cirera, S.; Hyttel, P.; et al. Neurosphere based differentiation of human ipsc improves astrocyte differentiation. Stem Cells Int. 2016, 2016, 4937689. [Google Scholar] [CrossRef]
- Mukai, T.; Nagamura-Inoue, T.; Shimazu, T.; Mori, Y.; Takahashi, A.; Tsunoda, H.; Yamaguchi, S.; Tojo, A. Neurosphere formation enhances the neurogenic differentiation potential and migratory ability of umbilical cord-mesenchymal stromal cells. Cytotherapy 2016, 18, 229–241. [Google Scholar] [CrossRef]
- Mori, H.; Ninomiya, K.; Kino-oka, M.; Shofuda, T.; Islam, M.O.; Yamasaki, M.; Okano, H.; Taya, M.; Kanemura, Y. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J. Neurosci. Res. 2006, 84, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Gao, H.; Zhen, Y.; Chen, X.; Lin, W.; Shen, J.; Yan, Y.; Wang, X.; Liu, M.; Gao, Y. Optimal time for passaging neurospheres based on primary neural stem cell cultures. Cytotechnology 2011, 63, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musah, S.; Wrighton, P.J.; Zaltsman, Y.; Zhong, X.; Zorn, S.; Parlato, M.B.; Hsiao, C.; Palecek, S.P.; Chang, Q.; Murphy, W.L.; et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator yap is a potent regulator of neuronal specification. Proc. Natl. Acad. Sci. USA 2014, 111, 13805–13810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deo, M.; Thompson, R.C.; Uhler, M.D.; Turner, D.L. Negative regulation of yap during neuronal differentiation. Dev. Biol. 2012, 361, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of yap/taz in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Byun, S.H.; Park, S.; Kim, J.; Kim, I.; Ha, S.; Kwon, M.; Yoon, K. Yap/taz enhance mammalian embryonic neural stem cell characteristics in a tead-dependent manner. Biochem. Biophys. Res. Commun. 2015, 458, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. Yap regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.F.; Ye, F.; Wang, Y.B.; Feng, D.X. Rock inhibition enhances neurite outgrowth in neural stem cells by upregulating yap expression in vitro. Neural Regen. Res. 2016, 11, 983–987. [Google Scholar] [CrossRef]
- Fujikake, K.; Sawada, M.; Hikita, T.; Seto, Y.; Kaneko, N.; Herranz-Pérez, V.; Dohi, N.; Homma, N.; Osaga, S.; Yanagawa, Y.; et al. Detachment of chain-forming neuroblasts by fyn-mediated control of cell-cell adhesion in the postnatal brain. J. Neurosci. 2018, 38, 4598–4609. [Google Scholar] [CrossRef]
- Conover, J.; Notti, R. The neural stem cell niche. Cell Tissue Res. 2008, 331, 211–224. [Google Scholar] [CrossRef]
- Miller, F.D.; Gauthier-Fisher, A. Home at last: Neural stem cell niches defined. Cell Stem Cell 2009, 4, 507–510. [Google Scholar] [CrossRef]
- Madl, C.M.; LeSavage, B.L.; Dewi, R.E.; Lampe, K.J.; Heilshorn, S.C. Matrix remodeling enhances the differentiation capacity of neural progenitor cells in 3d hydrogels. Adv. Sci. 2019, 6, 1801716. [Google Scholar] [CrossRef]







| Peaks of Interest | Proton Ratio (Methacrylate to Reference) | Methacrylation % (Mean ± S.D.) | Data Boxplot |
|---|---|---|---|
| δ 6.2 ppm, δ 5.8 ppm, δ 3.0–4.2 ppm [28] | 2:10 | 58.2 ± 19.5% |  |
| δ 6.2 ppm, δ 5.8 ppm, δ 2.0–2.1 ppm, δ 1.9–2.0 ppm [29] | 5:3 | 59.6 ± 16.6% | |
| δ 6.2 ppm, δ 5.8 ppm, δ 4.2–4.8 ppm [30] | 1:1 | 54.4 ± 4.7% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Suh, W.H. Cost-Effective Cosmetic-Grade Hyaluronan Hydrogels for ReNcell VM Human Neural Stem Cell Culture. Biomolecules 2019, 9, 515. https://doi.org/10.3390/biom9100515
Ma W, Suh WH. Cost-Effective Cosmetic-Grade Hyaluronan Hydrogels for ReNcell VM Human Neural Stem Cell Culture. Biomolecules. 2019; 9(10):515. https://doi.org/10.3390/biom9100515
Chicago/Turabian StyleMa, Weili, and Won Hyuk Suh. 2019. "Cost-Effective Cosmetic-Grade Hyaluronan Hydrogels for ReNcell VM Human Neural Stem Cell Culture" Biomolecules 9, no. 10: 515. https://doi.org/10.3390/biom9100515
APA StyleMa, W., & Suh, W. H. (2019). Cost-Effective Cosmetic-Grade Hyaluronan Hydrogels for ReNcell VM Human Neural Stem Cell Culture. Biomolecules, 9(10), 515. https://doi.org/10.3390/biom9100515