Fish Oil, but Not Olive Oil, Ameliorates Depressive-Like Behavior and Gut Microbiota Dysbiosis in Rats under Chronic Mild Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
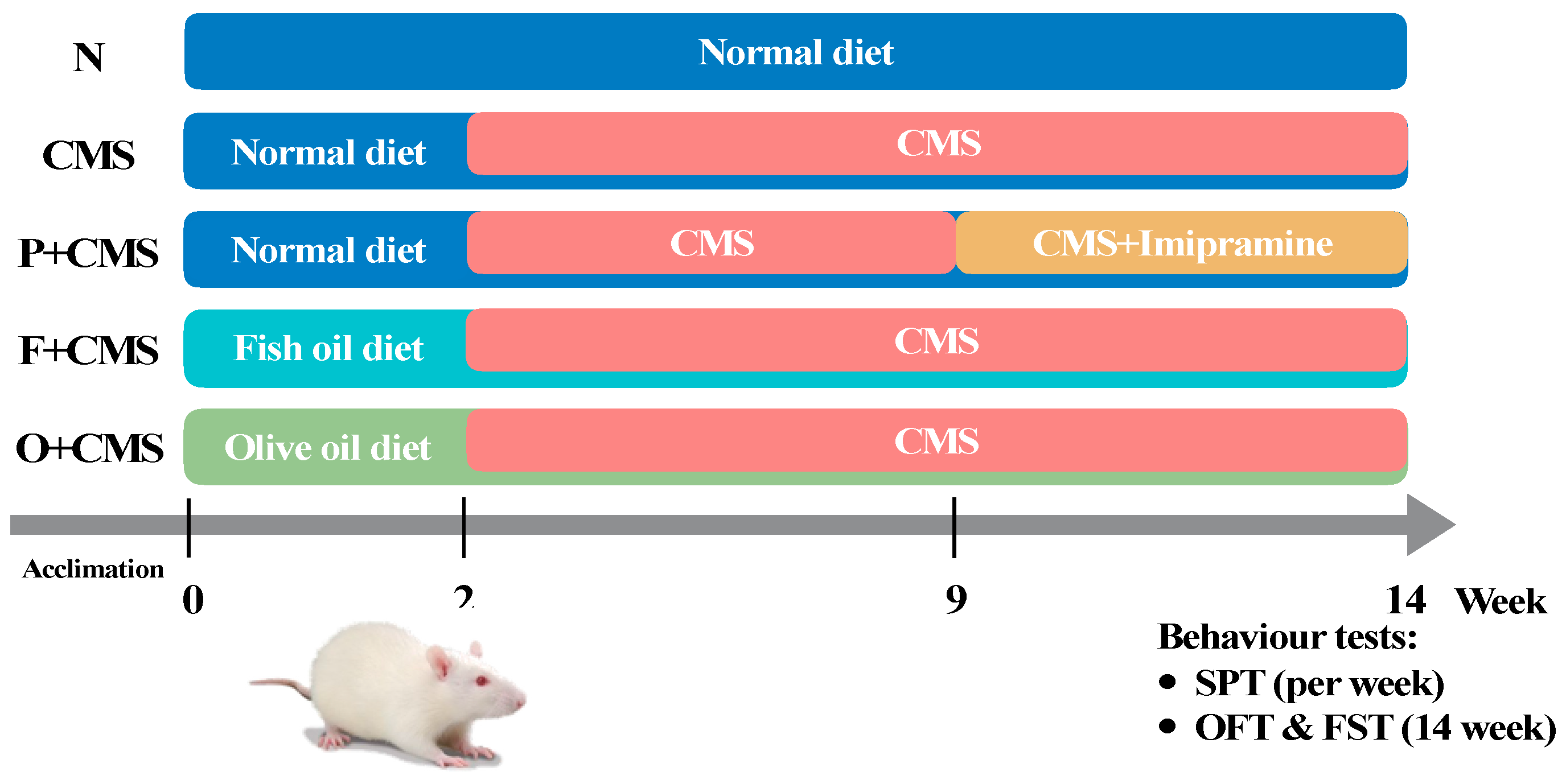
2.2. Experimental Protocols
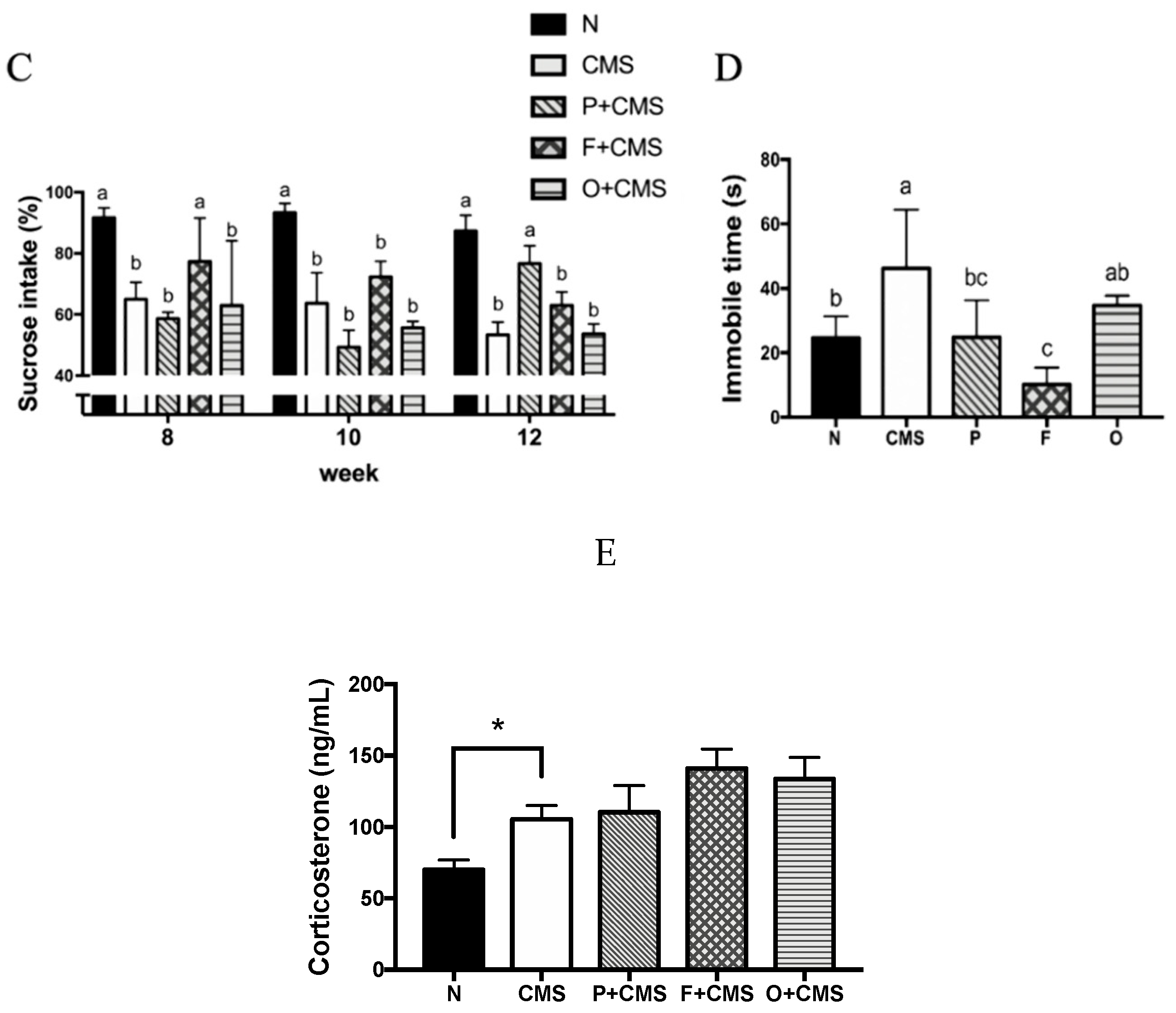
2.3. Sucrose Preference Test
2.4. Open Field Test and Forced Swimming Test
2.5. Corticosterone Assay
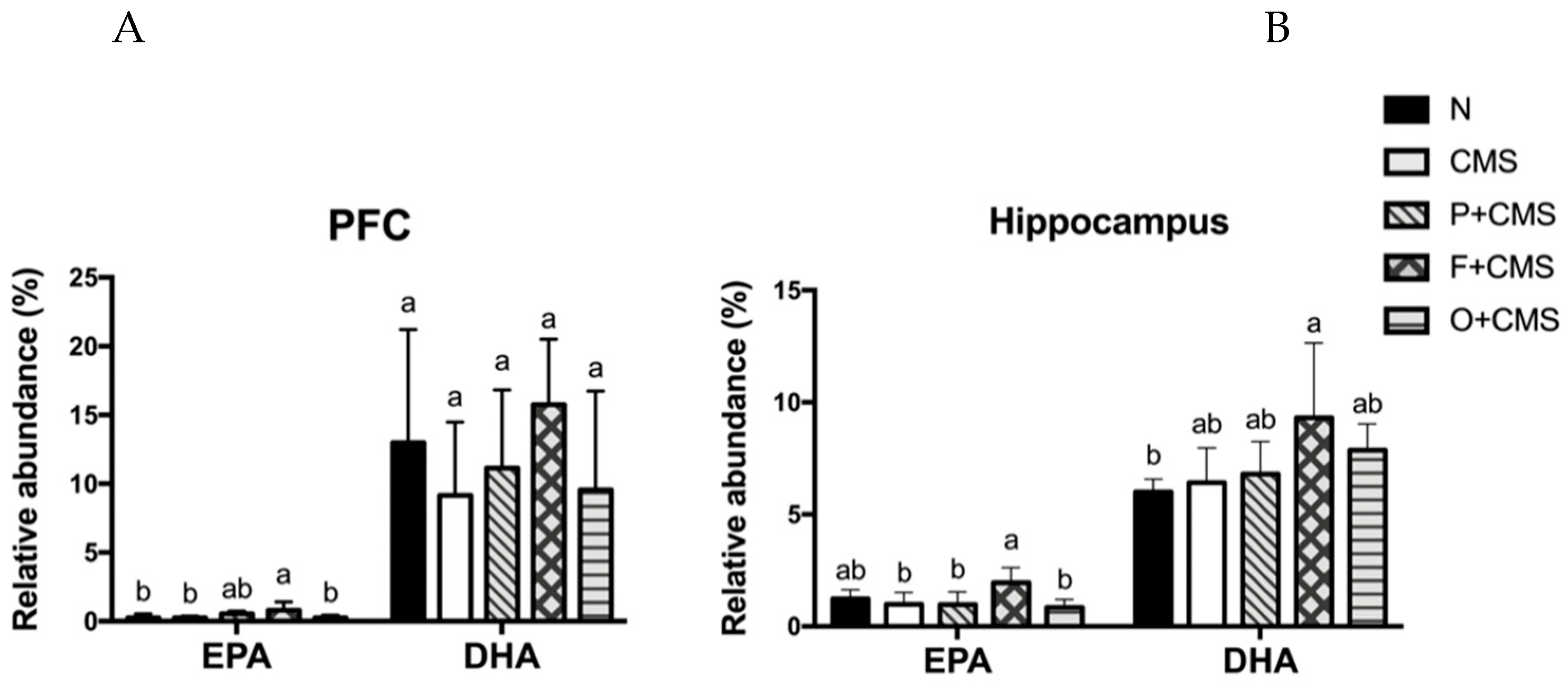
2.6. Lipid Extraction and Fatty Acid Profile Analysis
2.7. DNA Extraction, Amplification, and Sequencing
2.8. Statistics
3. Results
3.1. Weight Change under CMS
3.2. Anxiety-Like Behavior Test
3.3. Depressive-Like Behavior Tests
3.4. Fatty Acid Profiles of Brain and Red Blood Cells
3.5. Microbiota Alteration after Different Dietary Lipid Interventions
3.6. Correlation between Fatty Acid Profiles and Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.Y.; Guo, Y.J.; Han, W.X.; Yang, M.Q.; Wen, L.P.; Wang, K.Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El, A.S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Frohlich, E.E.; Matak, A.; Mayerhofer, R.; Reichmann, F.; Olivares, M.; Neyrinck, A.M.; Delzenne, N.M.; et al. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Winpenny, E.M.; van Harmelen, A.L.; White, M.; van Sluijs, E.M.; Goodyer, I.M. Diet quality and depressive symptoms in adolescence: No cross-sectional or prospective associations following adjustment for covariates. Public Health Nutr. 2018, 21, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Khayyatzadeh, S.S.; Mehramiz, M.; Mirmousavi, S.J.; Mazidi, M.; Ziaee, A.; Kazemi-Bajestani, S.M.R.; Ferns, G.A.; Moharreri, F.; Ghayour-Mobarhan, M. Adherence to a Dash-style diet in relation to depression and aggression in adolescent girls. Psychiatry Res. 2018, 259, 104–109. [Google Scholar] [CrossRef]
- Matta, J.; Hoertel, N.; Kesse-Guyot, E.; Plesz, M.; Wiernik, E.; Carette, C.; Czernichow, S.; Limosin, F.; Goldberg, M.; Zins, M.; et al. Diet and physical activity in the association between depression and metabolic syndrome: Constances study. J. Affect. Disord. 2019, 244, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef]
- Masana, M.F.; Haro, J.M.; Mariolis, A.; Piscopo, S.; Valacchi, G.; Bountziouka, V.; Anastasiou, F.; Zeimbekis, A.; Tyrovola, D.; Gotsis, E.; et al. Mediterranean diet and depression among older individuals: The multinational MEDIS study. Exp. Gerontol. 2018, 110, 67–72. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, A.C.; Loughman, A.; Bernard, A.; Raipuria, M.; Abbott, K.N.; Dachtler, J.; Van, T.T.H.; Moore, R.J. An intermittent hypercaloric diet alters gut microbiota, prefrontal cortical gene expression and social behaviours in rats. Nutr. Neurosci. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ishida, H.; Kaneko, K.; Shirayama, Y. Learned helplessness activates hippocampal microglia in rats: A potential target for the antidepressant imipramine. Pharmacol. Biochem. Behav. 2016, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; Lee, H.C.; Lo, Y.C.; Yu, S.C.; Huang, S.Y. N-3 polyunsaturated fatty acids prevent d-galactose-induced cognitive deficits in prediabetic rats. Food Funct. 2018, 9, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, R.J.; Roth, K.A.; Carroll, B.J. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci. Biobehav. Rev. 1981, 5, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef]
- Calabrese, F.; Brivio, P.; Gruca, P.; Lason-Tyburkiewicz, M.; Papp, M.; Riva, M.A. Chronic, Mild Stress-Induced Alterations of Local Protein Synthesis: A Role for Cognitive Impairment. ACS Chem. Neurosci. 2017, 8, 817–825. [Google Scholar] [CrossRef]
- Cavigelli, S.A.; Bao, A.D.; Bourne, R.A.; Caruso, M.J.; Caulfield, J.I.; Chen, M.; Smyth, J.M. Timing matters: The interval between acute stressors within chronic mild stress modifies behavioral and physiologic stress responses in male rats. Stress 2018, 21, 453–463. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, X.Y.; Zhang, Q.Y.; Kong, L.D. Microglial, NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain, Behav. Immun. 2014, 41, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, G.C.; Cohn, J.B.; Fabre, L.F.; Feighner, J.P.; Fieve, R.R.; Mendels, J.; Shrivastava, R.K. A comparison of paroxetine, imipramine and placebo in depressed out-patients. Br. J. Psychiatry 1991, 159, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, D.F.; Campion, J.; Milagro, F.I.; Lomba, A.; Marzo, F.; Martinez, J.A. Chronic mild stress induces variations in locomotive behavior and metabolic rates in high fat fed rats. J. Physiol. Biochem. 2007, 63, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Chanana, P.; Kumar, A. GABA-BZD Receptor Modulating Mechanism of Panax quinquefolius against 72-h Sleep Deprivation Induced Anxiety like Behavior: Possible Roles of Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation. Front. Neurosci. 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Reus, G.Z.; Maciel, A.L.; Abelaira, H.M.; de Moura, A.B.; de Souza, T.G.; Dos Santos, T.R.; Darabas, A.C.; Parzianello, M.; Matos, D.; Abatti, M.; et al. Omega-3 and folic acid act against depressive-like behavior and oxidative damage in the brain of rats subjected to early- or late-life stress. Nutrition 2018, 53, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Aguerre, S.; Fernandez, F.; Edno, L.; Combourieu, I.; Mormede, P.; Chaouloff, F. Strain-dependent neurochemical and neuroendocrine effects of desipramine, but not fluoxetine or imipramine, in spontaneously hypertensive and Wistar-Kyoto rats. Neuropharmacology 2000, 39, 2464–2477. [Google Scholar] [CrossRef]
- Simoes, L.R.; Netto, S.; Generoso, J.S.; Ceretta, R.A.; Valim, R.F.; Dominguini, D.; Michels, M.; Reus, G.Z.; Valvassori, S.S.; Dal-Pizzol, F.; et al. Imipramine treatment reverses depressive- and anxiety-like behaviors, normalize adrenocorticotropic hormone, and reduces interleukin-1beta in the brain of rats subjected to experimental periapical lesion. Pharmacol. Rep. 2019, 71, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Sheridan, J.F. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav. Immun. 2016, 57, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Nemets, B.; Stahl, Z.; Belmaker, R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatry 2002, 159, 477–479. [Google Scholar] [CrossRef]
- Su, K.P.; Huang, S.Y.; Chiu, T.H.; Huang, K.C.; Huang, C.L.; Chang, H.C.; Pariante, C.M. Omega-3 fatty acids for major depressive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2008, 69, 644–651. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira, O.C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Bastiaansen, J.A.; Munafo, M.R.; Appleton, K.M.; Oldehinkel, A.J. The efficacy of fish oil supplements in the treatment of depression: Food for thought. Transl. Psychiatry 2016, 6, 975. [Google Scholar] [CrossRef] [PubMed]
- Touma, C. Stress and affective disorders: Animal models elucidating the molecular basis of neuroendocrine-behavior interactions. Pharmacopsychiatry 2011, 44, 15–26. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Doolin, K.; Farrell, C.; Tozzi, L.; Harkin, A.; Frodl, T.; O’Keane, V. Diurnal Hypothalamic-Pituitary-Adrenal Axis Measures and Inflammatory Marker Correlates in Major Depressive Disorder. Int J. Mol. Sci. 2017, 18, 2206. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.G. EPA but Not, DHA Appears To Be Responsible for the Efficacy of Omega-3 Long Chain Polyunsaturated Fatty Acid Supplementation in Depression: Evidence from a Meta-Analysis of Randomized Controlled Trials. J. Am. Coll. Nutr. 2009, 28, 525–542. [Google Scholar] [CrossRef]
- Jazayeri, S.; Tehrani-Doost, M.; Keshavarz, S.A.; Hosseini, M.; Djazayery, A.; Amini, H.; Jalali, M.; Peet, M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Z. J. Psychiatry 2008, 42, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Yalagala, P.C.; Sugasini, D.; Dasarathi, S.; Pahan, K.; Subbaiah, P.V. Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: Potential treatment for depression. J. Lipid Res. 2019, 60, 566–578. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burgos, A.; Wang, B.; Mao, Y.K.; Mistry, B.; McVey Neufeld, K.A.; Bienenstock, J.; Kunze, W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G211–G220. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.H.; Duan, J.A.; Jiang, S.; Feng, N.N.; Qiu, W.Q.; Ling, Y. Polysaccharides from Chrysanthemum morifolium Ramat ameliorate colitis rats by modulating the intestinal microbiota community. Oncotarget 2017, 8, 80790–80803. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, T.-H.; Tung, Y.-T.; Lin, I.-H.; Shih, C.-K.; Nguyen, N.T.K.; Shabrina, A.; Huang, S.-Y. Fish Oil, but Not Olive Oil, Ameliorates Depressive-Like Behavior and Gut Microbiota Dysbiosis in Rats under Chronic Mild Stress. Biomolecules 2019, 9, 516. https://doi.org/10.3390/biom9100516
Tung T-H, Tung Y-T, Lin I-H, Shih C-K, Nguyen NTK, Shabrina A, Huang S-Y. Fish Oil, but Not Olive Oil, Ameliorates Depressive-Like Behavior and Gut Microbiota Dysbiosis in Rats under Chronic Mild Stress. Biomolecules. 2019; 9(10):516. https://doi.org/10.3390/biom9100516
Chicago/Turabian StyleTung, Te-Hsuan, Yu-Tang Tung, I-Hsuan Lin, Chun-Kuang Shih, Ngan Thi Kim Nguyen, Amalina Shabrina, and Shih-Yi Huang. 2019. "Fish Oil, but Not Olive Oil, Ameliorates Depressive-Like Behavior and Gut Microbiota Dysbiosis in Rats under Chronic Mild Stress" Biomolecules 9, no. 10: 516. https://doi.org/10.3390/biom9100516






