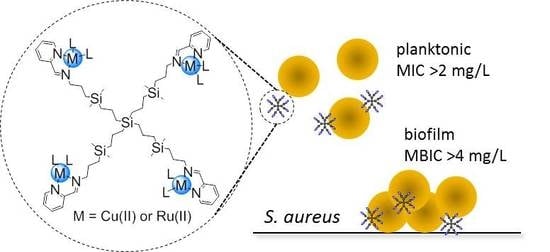
Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metal Complexes and Metallodendrimers
2.2. Bacterial Strains
2.3. Zeta Potential Evaluation
2.4. In Vitro Antibacterial Activity Tests against Planktonic Cells
2.5. In Vitro Antibacterial Activity Tests to Prevent S. aureus Biofilm Formation
2.6. Hemolysis Evaluation
3. Results
3.1. Surface Charge of Metal Complexes and Metallodendrimers
3.2. Antibacterial Activity of Carbosilane Metallodendrimers on Planktonic Cells
3.3. Antibacterial Activity of Carbosilane Metallodendrimers on Preventing S. aureus Biofilm Formation
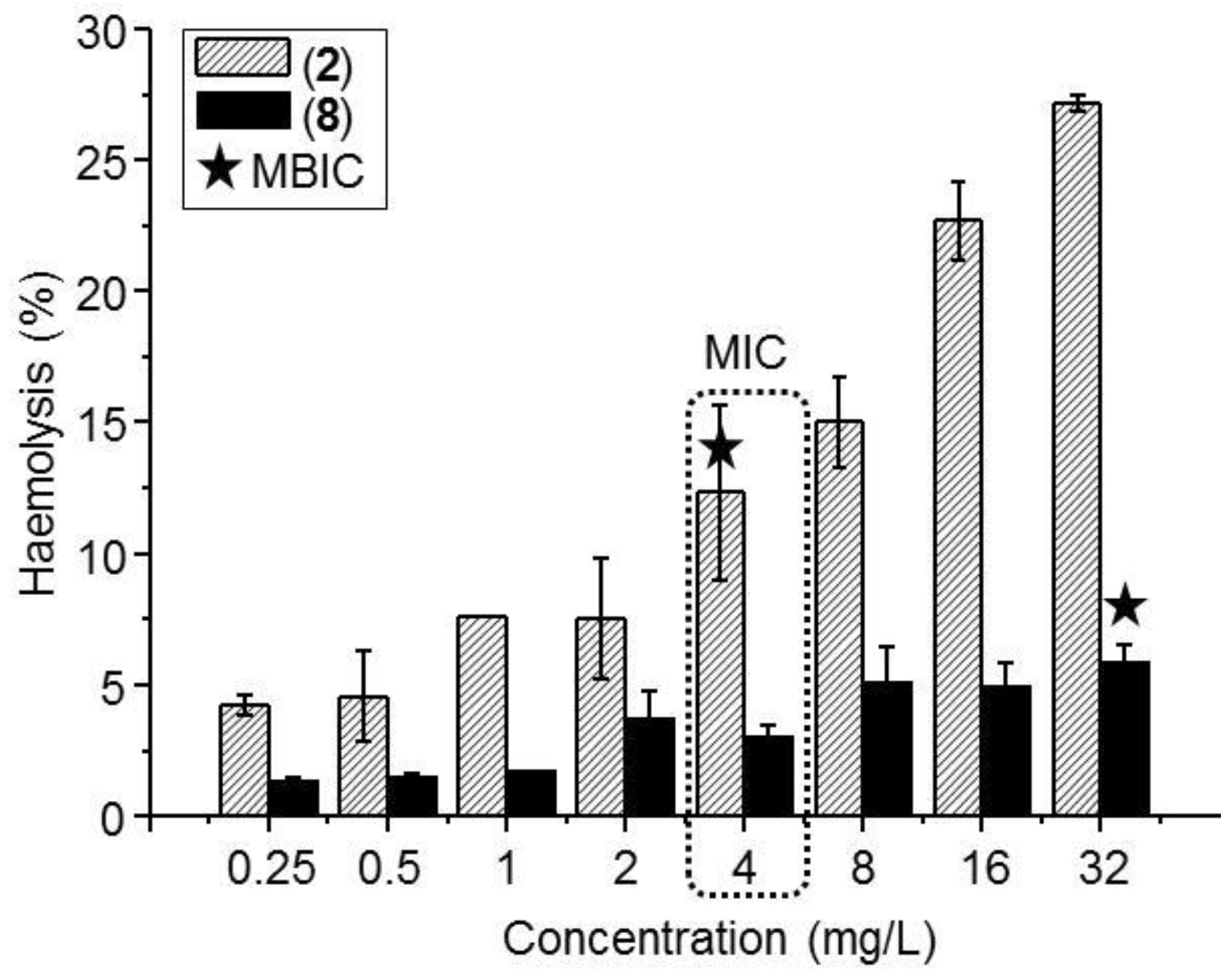
3.4. Hemolysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kloss, W.E.; Schleir, K.H.; Goirtz, F. The genus Staphylococcus. In The Prokaryotes, 2nd ed.; Balows, A., Truper, H.G., Dwoekin, M., Eds.; Springer: New York, NY, USA, 1992. [Google Scholar]
- Vivoni, A.M.; Moreira, B.M. Application of molecular techniques in the study of Staphylococcus aureus clonal evolution—A review. Mem. Inst. Oswaldo Cruz. 2005, 100, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Compernolle, V.; Verschraegen, G.; Claeys, G. Combined use of Pastorex Staph-Plus and either of two new chromogenic agars, MRSA ID and CHROMagar MRSA, for detection of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2007, 45, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Kloss, W.E.; Bamerman, T.L. Staphylococcus and Micrococcus. In Manual of Clinical Microbiology, 6th ed.; Murra, P.R., Baron, E.J., Pfaller, M.A., Eds.; ASM Press: Washington DC, USA, 1995. [Google Scholar]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, P.; Que, Y.; Glauser, M. Staphylococcus aureus. In Principles and Practice of Infectious Diseases, 6th ed.; Mandell, G.L., Bennett, J.E., Olin, R., Eds.; Churchill Livingston: Philadelphia, PA, USA, 2005. [Google Scholar]
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Dall’Antonia, M.; Coen, P.G.; Wilks, M.; Whiley, A.; Millar, M. Competition between methicillin-sensitive and resistant Staphylococcus aureus in the anterior nares. J. Hosp. Infect. 2005, 61, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, P.O.; Gagnaire, J.; Botelho-Nevers, E.; Grattard, F.; Carricajo, A.; Lucht, F.; Pozzetto, B.; Berthelot, P. Detection and clinical relevance of Staphylococcus aureus nasal carriage: An update. Expert Rev. Anti-infect. Ther. 2014, 12, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Cui, L.; Kuroda, M.; Ito, T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001, 9, 486–493. [Google Scholar] [CrossRef]
- Crossley, K.B.; Jefferson, K.K.; Archer, G.; Fouler, V.G. Staphylococci in Human Disease, 2nd ed.; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet, 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Anwar, S.; Prince, L.R.; Foster, S.J.; Whyte, M.K.; Sabroe, I. The rise and rise of Staphylococcus aureus: Laughing in the face of granulocytes. Clin. Exp. Immunol. 2009, 157, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Torigoe, R.; Arata, J. Interaction of Staphylococcus aureus cells and silk threads in vitro and in mouse skin. J. Dermatol. Sci. 1993, 6, 247–257. [Google Scholar] [CrossRef]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontology 2000, 53, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Dastgheyb, S.; Parvizi, J.; Shapiro, I.M.; Hickok, N.J.; Otto, M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J. Infect. Dis. 2015, 211, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Winn, W.; Allen, S.; Janda, W.; Koneman, E.; Procop, G. Schreckenberger, P. and Woods, G. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Gorman, S.P.; Sussman, M. Microbial Biofilms: Formation and Control; Wiley-Blackwell Scientific Publications LTD: London, UK, 1993. [Google Scholar]
- Proctor, R.A.; Balwit, J.M.; Vesga, O. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect. Agents Dis. 1994, 3, 302–312. [Google Scholar]
- Kraus, D.; Peschel, A. Staphylococcus aureus evasion of innate antimicrobial defense. Future Microbiol. 2008, 3, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Stryjewski, M.E.; Corey, G.R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014, 58, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.L. , Prentice, T. and Reinders, L.T. The World Health Report 2007: A Safer Future: Global Public Health Security in The 21st Century; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Liu, Y.; Shi, L.; Su, L.; van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428–446. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-K.; Lam, S.J.; Ho, K.K.K.; Kumar, N.; Qiao, G.G.; Egan, S.; Boyer, C.; Wong, E.H.H. Rational design of single-chain polymeric nanoparticles that kill planktonic and biofilm bacteria. ACS Infect. Dis. 2017, 3, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Webster, T.J. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int. J. Nanomed. 2009, 4, 145–152. [Google Scholar] [Green Version]
- Chung, P.Y.; Toh, Y.S. Anti-biofilm agents: Recent breakthrough against multi-drug resistant Staphylococcus aureus. Pathog. Dis. 2014, 70, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons and Dendritic Polymers: Discovery, Applications and the Future; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Zarena, A.S.; Shubha, G. Dendrimer a new dimension in targeting biofilms. Mini-Rev. Med. Chem. 2013, 13, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Swanson, D.R.; Tomalia, D.A.; Hagnauer, G.L.; McManus, A.T. Dendrimer−silver complexes and nanocomposites as antimicrobial agents. Nano Lett. 2001, 1, 18–21. [Google Scholar] [CrossRef]
- Grabchev, I.; Vasileva-Tonkova, E.; Staneva, D.; Bosch, P.; Kukeva, R.; Stoyanova, R. Impact of Cu(II) and Zn(II) ions on the functional properties of new PAMAM metallodendrimers. New J. Chem. 2018, 42, 7853–7862. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Carloni, R.; Bajo, A.M.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Insight into the antitumor activity of carbosilane Cu(II)–metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Maroto-Díaz, M.; Gómez, R.; Ortega, P.; Cangiotti, M.; Ottaviani, M.F.; de la Mata, F.J. Carbosilane metallodendrimers based on copper (II) complexes: Synthesis, EPR characterization and anticancer activity. J. Inorg. Biochem. 2017, 177, 211–218. [Google Scholar] [CrossRef]
- De la Mata, F.J.; Gómez, R.; Ortega, P.; García-Gallego, S.; Sanz del Olmo, N.; Triguero Rubio, A.; Maroto-Díaz, M. Metalodendrímeros de naturaleza carbosilano conteniendo rutenio y cobre coordinados a ligandos base de Schiff, su preparación y sus usos. PCT/ES2019/070416, 14 June 2019. [Google Scholar]
- Holota, M.; Magiera, J.; Michlewska, S.; Kubczak, M.; Del Olmo, N.S.; García-Gallego, S.; Ortega, P.; de la Mata, F.J.; Ionov, M.; Bryszewska, M. In vitro anticancer properties of copper metallodendrimers. Biomolecules 2019, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Tramer, F.; Da Ros, T.; Passamonti, S. Screening of fullerene toxicity by hemolysis assay. In Nanotoxicity Methods in Molecular Biology (Methods and Protocols); Reineke, J., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 926. [Google Scholar]
- Ahamad, T.; Mapolie, S.F.; Alshehri, S.M. Synthesis and characterization of polyamide metallodendrimers and their anti-bacterial and anti-tumor activities. Med. Chem. Res. 2012, 21, 2023–2031. [Google Scholar] [CrossRef]
- Wang, L.; Erasquin, U.J.; Zhao, M.; Ren, L.; Zhang, M.Y.; Cheng, G.J.; Wang, Y.; Cai, C. Stability, antimicrobial activity, and cytotoxicity of poly(amidoamine) dendrimers on titanium substrates. ACS Appl. Mater. Interfaces 2011, 3, 2885–2894. [Google Scholar] [CrossRef] [PubMed]


| Compound | Metal Atoms | Zeta Potential, [mV] | S. aureus | E. coli | ||
|---|---|---|---|---|---|---|
| MIC [mg/L] | MBC [mg/L] | MIC [mg/L] | MBC [mg/L] | |||
| Cu(NO3)2 | 1 | - | >512 | >512 | >512 | >512 |
| 1 | 1 | 14.79 ± 1.92 a | 128 | 128 | 128 | 256 |
| 2 | 4 | 25.90 ± 2.32 a | 4 | 8 | 4 | 4 |
| 3 | 8 | 39.23 ± 3.78 a | 256 | 256 | 256 | 256 |
| CuCl2 | 1 | - | >512 | >512 | >512 | >512 |
| 4 | 1 | 10.45 ± 1.25 a | 64 | 64 | 256 | 256 |
| 5 | 4 | 19.68 ± 1.78 a | 2 | 4 | 8 | 8 |
| 6 | 8 | 37.48 ± 3.09 a | 32 | 32 | 64 | 64 |
| RuCl3 | 1 | - | >512 | >512 | >512 | >512 |
| 7 | 1 | 18.70 ± 4.21 | 16 | 16 | 64 | 64 |
| 8 | 4 | 25.62 ± 4.70 | 4 | 4 | 16 | 16 |
| 9 | 8 | 44.90 ± 5.08 | 16 | 32 | 128 | 128 |
| Compound | Metal Atoms | S. aureus Biofilms | |
|---|---|---|---|
| MIC [mg/L] | MBIC [mg/L] | ||
| 2 | 4 | 8 | 4 |
| 5 | 4 | 8 | 8 |
| 8 | 4 | 32 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llamazares, C.; Sanz del Olmo, N.; Ortega, P.; Gómez, R.; Soliveri, J.; de la Mata, F.J.; García-Gallego, S.; Copa-Patiño, J.L. Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm. Biomolecules 2019, 9, 405. https://doi.org/10.3390/biom9090405
Llamazares C, Sanz del Olmo N, Ortega P, Gómez R, Soliveri J, de la Mata FJ, García-Gallego S, Copa-Patiño JL. Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm. Biomolecules. 2019; 9(9):405. https://doi.org/10.3390/biom9090405
Chicago/Turabian StyleLlamazares, Celia, Natalia Sanz del Olmo, Paula Ortega, Rafael Gómez, Juan Soliveri, F. Javier de la Mata, Sandra García-Gallego, and José Luis Copa-Patiño. 2019. "Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm" Biomolecules 9, no. 9: 405. https://doi.org/10.3390/biom9090405