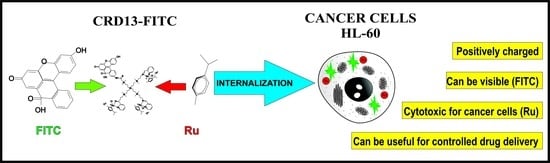
Synthesis and Characterization of FITC Labelled Ruthenium Dendrimer as a Prospective Anticancer Drug
Abstract
:1. Introduction
2. Materials and Methods
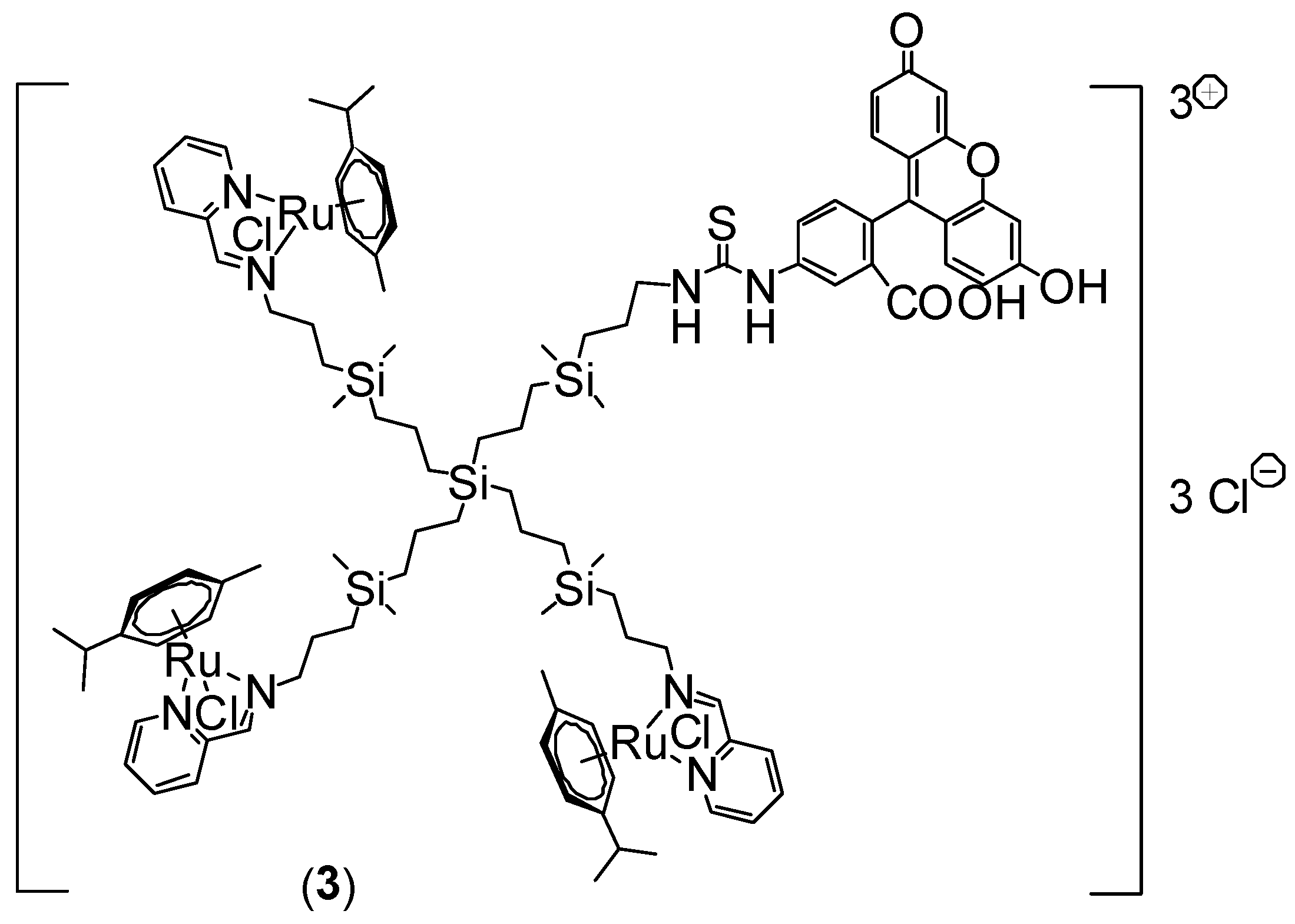
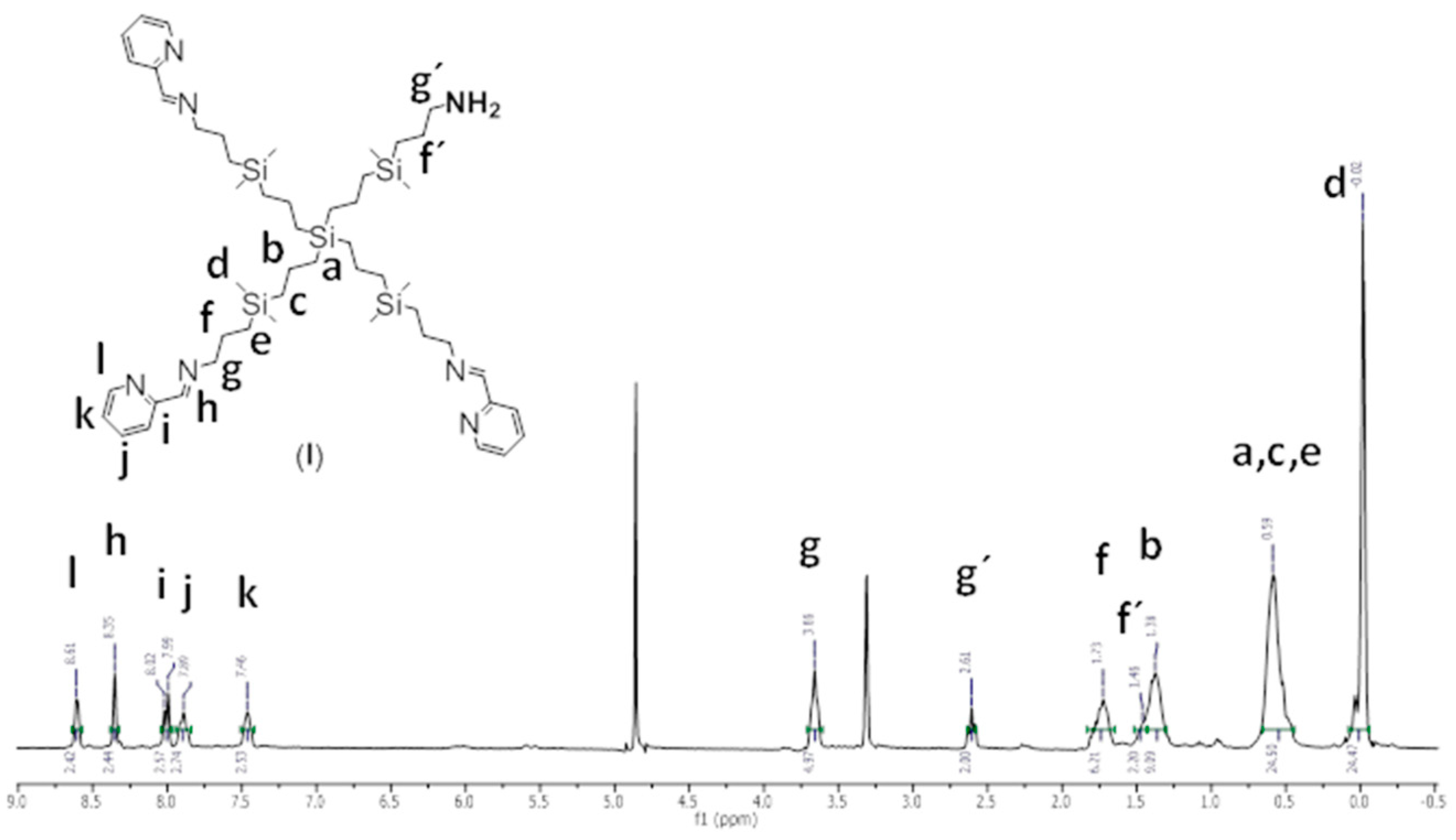
2.1. Ruthenium-Terminated Carbosilane Dendrimer with FITC (CRD13-FITC)
2.2. Zeta Potential
2.3. Measurement of Particle Size
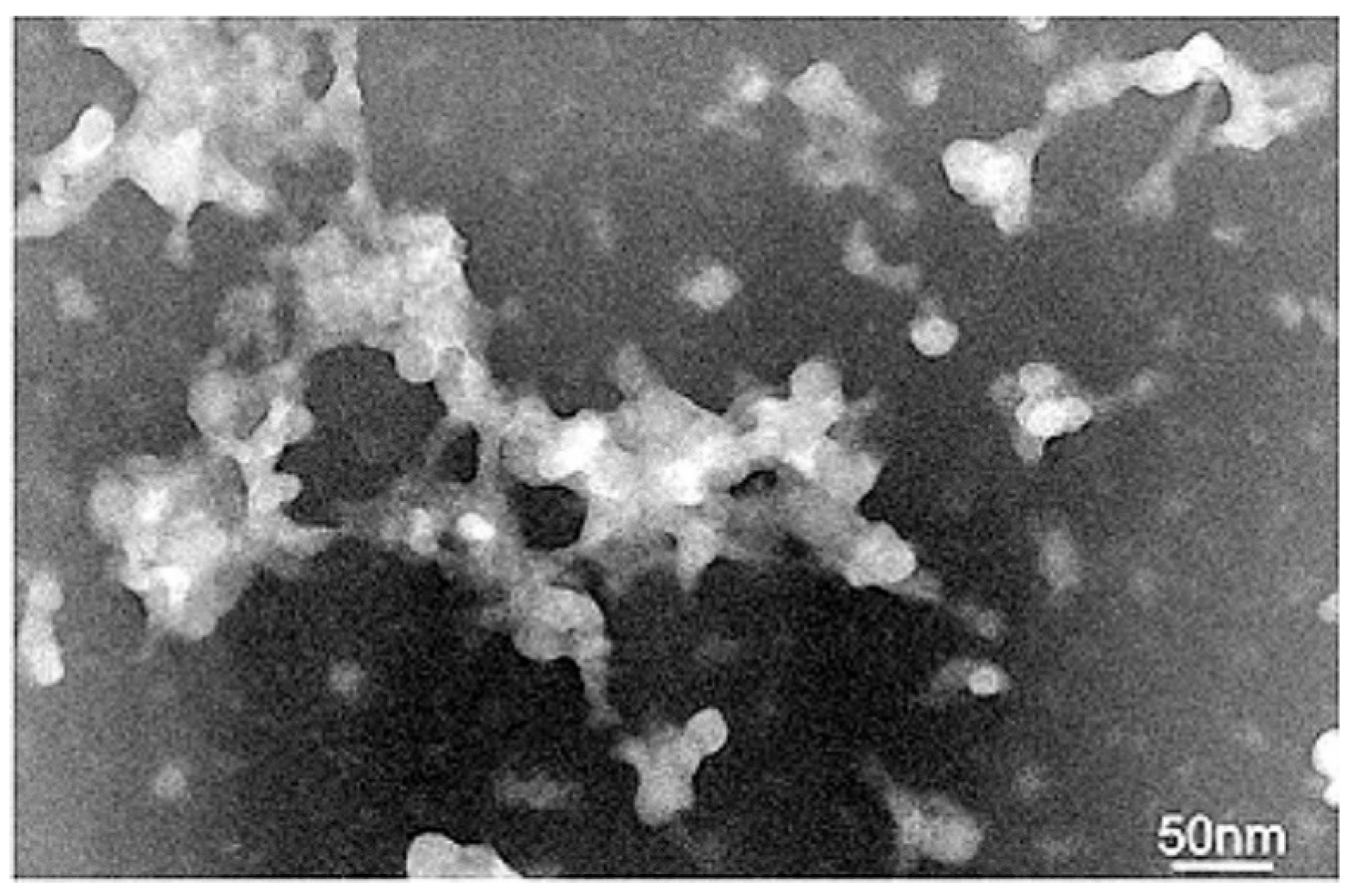
2.4. Transmission Electron Microscopy
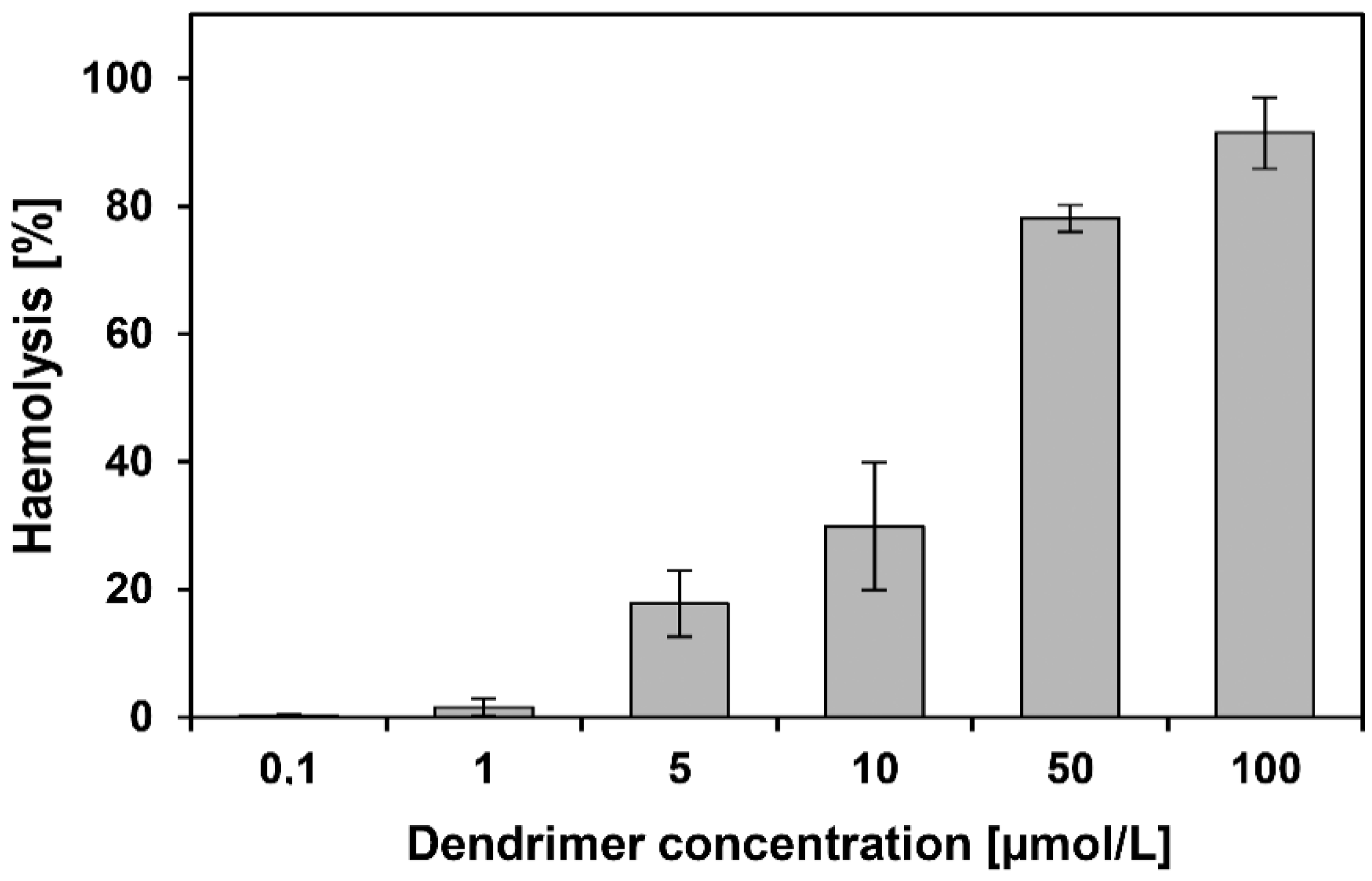
2.5. Haemotoxicity
2.6. Fluorescence Anisotropy
2.7. Cells
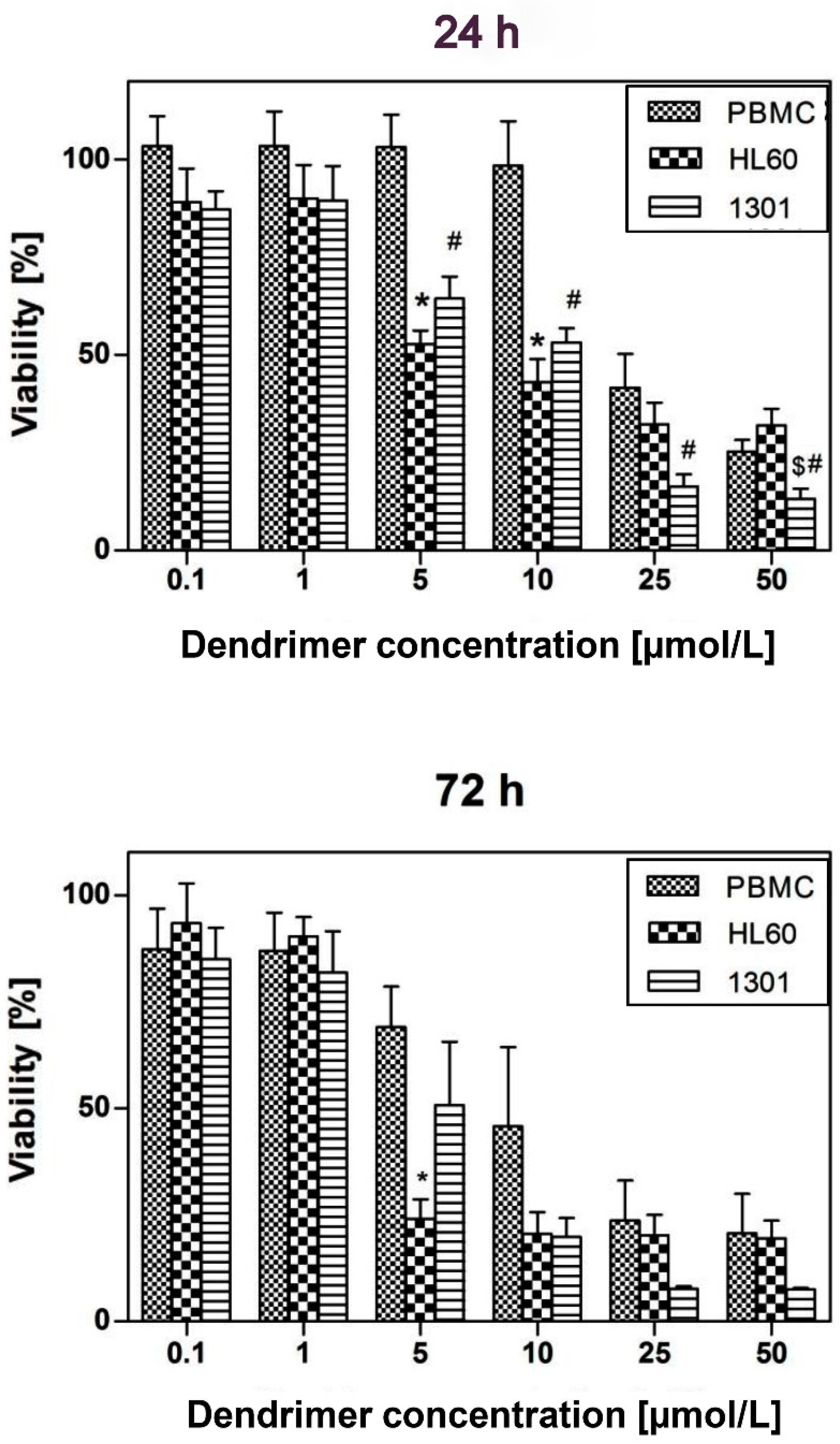
2.8. Cytotoxicity
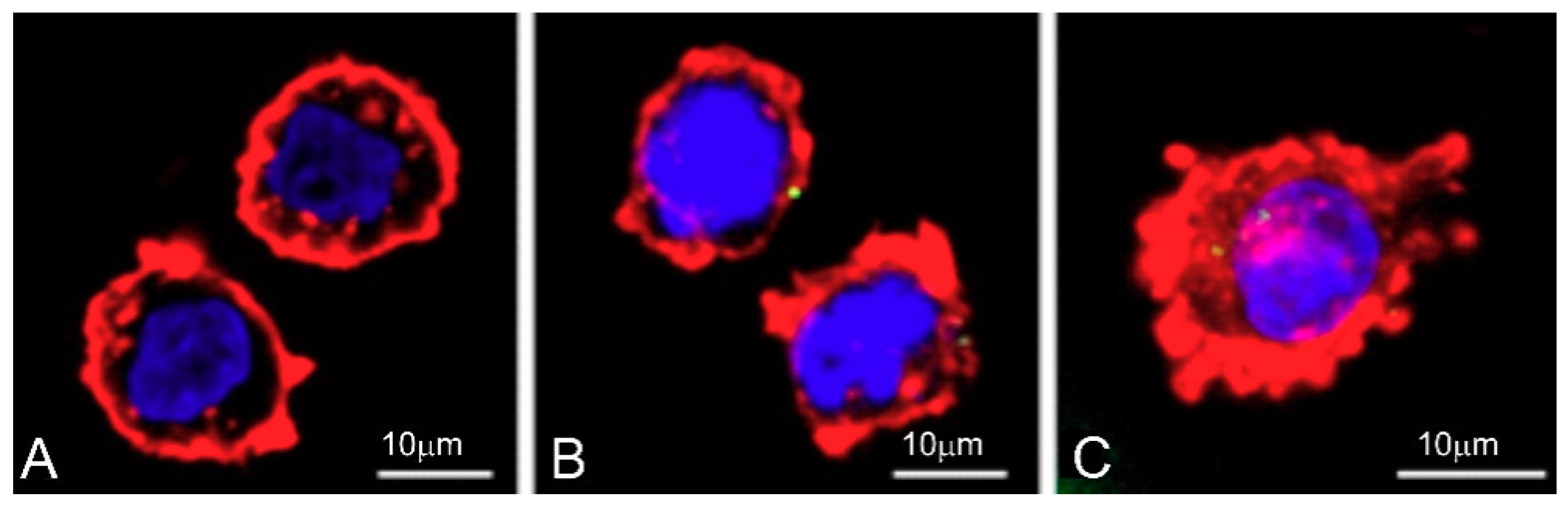
2.9. Cellular Uptake
2.10. Statistical Analysis
3. Results
3.1. Synthesis of Ruthenium-Terminated Carbosilane Dendrimer with FITC (CRD-FITC)
Structural Characterization


3.2. Zeta Potential and Particle Size
3.3. Transmission Electron Microscopy
3.4. Haemotoxicity
3.5. Fluorescence Anisotropy
3.6. Viability
3.7. Cellular Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vankayala, R.; Hwang, K.C. Near-Infrared-Light-Activatable Nanomaterial-Mediated Phototheranostic Nanomedicines: An Emerging Paradigm for Cancer Treatment. Adv. Mater. 2018, 30, 1706320. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Tang, B.; Wang, Y.-J.; Guo, B.-H.; Yin, H.; Yi, Q.-Y.; Liu, Y.-J. Synthesis and anticancer properties of ruthenium (II) complexes as potent apoptosis inducers through mitochondrial disruption. Eur. J. Med. Chem. 2017, 139, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-Y.; Xi, Q.-Y.; Huang, K.-B.; Tang, X.-M.; Chen, Z.-F.; Liu, Y.-C.; Liang, H. Crystal structure, cytotoxicity and action mechanism of Zn(II)/Mn(II) complexes with isoquinoline ligands. J. Inorg. Biochem. 2017, 169, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Xiong, Y.-H.; Fu, Y.-L.; Zhang, C.-L.; Wang, B.-F.; Mao, Z.-W.; Le, X.-Y. Two new mixed copper(ii)–dipeptide complexes of N,N-donor heterocycle ligands: studies on their non-covalent DNA binding, chemical nuclease, antioxidant and anticancer activities. RSC Adv. 2016, 6, 35952–35965. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Shcharbin, D.; Maroto-Díaz, M.; Ramirez, R.G.; De La Mata, F.J.; Bryszewska, M. Ruthenium metallodendrimers with anticancer potential in an acute promyelocytic leukemia cell line (HL60). Eur. Polym. J. 2017, 87, 39–47. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Hartinger, C.G. Anticancer metallodrugs: where is the next cisplatin? Futur. Med. Chem. 2018, 10, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Shamsi, F.; Azam, A. Ruthenium Complexes: An Emerging Ground to the Development of Metallopharmaceuticals for Cancer Therapy. Min. Rev. Med. Chem. 2015. [CrossRef]
- Hamid, S.; Donaldson, I.A.; Hu, Y.; Rodell, R.; Villarini, B.; Bonmati, E.; Tranter, P.; Punwani, S.; Sidhu, H.S.; Willis, S.; et al. The SmartTarget Biopsy Trial: A Prospective, Within-person Randomised, Blinded Trial Comparing the Accuracy of Visual-registration and Magnetic Resonance Imaging/Ultrasound Image-fusion Targeted Biopsies for Prostate Cancer Risk Stratification. Eur. Urol. 2019, 75, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Kim, J.H.; Park, S.J.; Han, J.K. Prediction of the therapeutic response after FOLFOX and FOLFIRI treatment for patients with liver metastasis from colorectal cancer using computerized CT texture analysis. Eur. J. Radiol. 2016, 85, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Tanzina, H.S.; Chowdhury, E. Recent progress in delivery of therapeutic and imaging agents utilizing organic-inorganic hybrid nanoparticles. Curr. Drug Deliv. 2018, 15, 485–496. [Google Scholar] [CrossRef]
- A Tomalia, D.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Venyaminova, A.; Miłowska, K.; Nowacka, O.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (A). Mechanisms of interaction. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Govender, P.; Renfrew, A.K.; Clavel, C.M.; Dyson, P.J.; Therrien, B.; Smith, G.S. Antiproliferative activity of chelating N,O- and N,N-ruthenium(ii) arene functionalised poly(propyleneimine) dendrimer scaffolds. Dalton Trans. 2011, 40, 1158–1167. [Google Scholar] [CrossRef]
- Heffeter, P.; Riabtseva, A.; Senkiv, Y.; Kowol, C.R.; Körner, W.; Jungwith, U.; Mitina, N.; Keppler, B.K.; Konstantinova, T.; Yanchuk, I.; et al. Nanoformulation Improves Activity of the (pre)Clinical Anticancer Ruthenium Complex KP1019. J. Biomed. Nanotechnol. 2014, 10, 877–884. [Google Scholar] [CrossRef]
- Fischer, B.; Heffeter, P.; Kryeziu, K.; Gille, L.; Meier, S.M.; Berger, W.; Kowol, C.R.; Keppler, B.K.; Meier-Menches, S.M. Poly(lactic acid) nanoparticles of the lead anticancer ruthenium compound KP1019 and its surfactant-mediated activation. Dalton Trans. 2014, 43, 1096–1104. [Google Scholar] [CrossRef]
- Xu, M.; Wen, Y.; Liu, Y.; Tan, X.; Chen, X.; Zhu, X.; Wei, C.; Chen, L.; Wang, Z.; Liu, J.; et al. Hollow mesoporous ruthenium nanoparticles conjugated bispecific antibody for targeted anti-colorectal cancer response of combination therapy. Nanoscale 2019, 11, 9661–9678. [Google Scholar] [CrossRef]
- Dou, Y.-K.; Shang, Y.; He, X.-W.; Li, W.-Y.; Li, Y.-H.; Zhang, Y.-K. Preparation of a Ruthenium-Complex-Functionalized Two-Photon-Excited Red Fluorescence Silicon Nanoparticle Composite for Targeted Fluorescence Imaging and Photodynamic Therapy in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 13954–13963. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar]
- Martinho, N.; Florindo, H.; Silva, L.; Brocchini, S.; Zloh, M.; Barata, T. Molecular Modeling to Study Dendrimers for Biomedical Applications. Molecules 2014, 19, 20424–20467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionov, M.; Ciepluch, K.; Garaiova, Z.; Melikishvili, S.; Michlewska, S.; Balcerzak, Ł.; Glińska, S.; Miłowska, K.; Gomez-Ramirez, R.; De La Mata, F.J.; et al. Dendrimers complexed with HIV-1 peptides interact with liposomes and lipid monolayers. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionov, M.; Ciepluch, K.; Klajnert, B.; Glińska, S.; Gomez-Ramirez, R.; De La Mata, F.J.; Muñoz-Fernández, M.Á.; Bryszewska, M.; Klajnert-Maculewicz, B. Complexation of HIV derived peptides with carbosilane dendrimers. Colloids Surf. B 2013, 101, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Ihnatsyeu-Kachan, A.; Michlewska, S.; Shcharbina, N.; Shcharbin, D.; Majoral, J.-P.; Bryszewska, M. Effect of dendrimers on selected enzymes—Evaluation of nano carriers. Int. J. Pharm. 2016, 499, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Pedziwiatr-Werbicka, E.; Fuentes, E.; Dzmitruk, V.; Sánchez-Nieves, J.; Sudas, M.; Drozd, E.; Shakhbazau, A.; Shcharbin, D.; De La Mata, F.J.; Gomez-Ramirez, R.; et al. Novel ‘SiC’ carbosilane dendrimers as carriers for anti-HIV nucleic acids: Studies on complexation and interaction with blood cells. Colloids Surf. B 2013, 109, 183–189. [Google Scholar] [CrossRef]
- Shcharbin, D.; Pedziwiatr, E.; Nowacka, O.; Kumar, M.; Zaborski, M.; Ortega, P.; Javier de la Mata, F.; Gómez, R.; Muñoz-Fernandez, M.A.; Bryszewska, M. Carbosilane dendrimers NN8 and NN16 form a stable complex with siGAG1. Colloids Surf. B 2011, 83, 388–391. [Google Scholar] [CrossRef]
- Szwed, A.; Fuentes-Paniagua, E.; Pedziwiatr-Werbicka, E.; Kurowska, J.; Gabryelak, T.; Gomez-Ramirez, R.; Moreno, S.; El Brahmi, N.; Milowska, K.; Katir, N.; et al. Synthesis, characterization and biological properties of new hybrid carbosilane–viologen–phosphorus dendrimers. RSC Adv. 2015, 5, 25942–25958. [Google Scholar]
- Ziemba, B.; Halets, I.; Shcharbin, D.; Appelhans, D.; Voit, B.; Pieszyński, I.; Bryszewska, M.; Klajnert, B.; Klajnert-Maculewicz, B. Influence of fourth generation poly(propyleneimine) dendrimers on blood cells. J. Biomed. Mater. Res. Part A 2012, 100, 2870–2880. [Google Scholar] [CrossRef]
- Klajnert, B.; Walach, W.; Bryszewska, M.; Dworak, A.; Shcharbin, D.; Klajnert-Maculewicz, B. Cytotoxicity, haematotoxicity and genotoxicity of high molecular mass arborescent polyoxyethylene polymers with polyglycidol-block-containing shells. Cell Boil. Int. 2006, 30, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, B.; Matuszko, G.; Bryszewska, M.; Klajnert, B. Influence of Dendrimers on Red Blood Cells Cell. Mol. Biol. Lett. 2011, 17, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Shcharbina, N.; Milowska, K.; De La Mata, F.J.; Muñoz-Fernández, M.Á.; Mignani, S.; Gomez-Ramirez, R.; Majoral, J.-P.; Bryszewska, M. Interference of cationic polymeric nanoparticles with clinical chemistry tests—Clinical relevance. Int. J. Pharm. 2014, 473, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A.K.; Gupta, U.; Gupta, D.U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today 2017, 22, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Lazniewska, J.; Milowska, K.; Katir, N.; El Kadib, A.; Bryszewska, M.; Majoral, J.-P.; Gabryelak, T. Viologen-phosphorus dendrimers exhibit minor toxicity against a murine neuroblastoma cell line. Cell. Mol. Boil. Lett. 2013, 18, 459–478. [Google Scholar] [CrossRef] [Green Version]
- Michlewska, S.; Ionov, M.; Maroto-Díaz, M.; Szwed, A.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Dzmitruk, V.; Rogalska, A.; Denel, M.; Gapinska, M.; et al. Ruthenium dendrimers against acute promyelocytic leukemia: in vitro studies on HL-60 cells. Futur. Med. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Díaz, M.; Elie, B.T.; Gómez-Sal, P.; Pérez-Serrano, J.; Gómez, R.; Contel, M.; De La Mata, F.J. Synthesis and anticancer activity of carbosilane metallodendrimers based on arene ruthenium (II) complexes. Dalton Trans. 2016, 45, 7049–7066. [Google Scholar] [CrossRef]
- Milowska, K.; Rybczyńska, A.; Mosiolek, J.; Durdyn, J.; Szewczyk, E.M.; Katir, N.; Brahmi, Y.; Majoral, J.-P.; Bousmina, M.; Bryszewska, M.; et al. Biological Activity of Mesoporous Dendrimer-Coated Titanium Dioxide: Insight on the Role of the Surface–Interface Composition and the Framework Crystallinity. ACS Appl. Mater. Interfaces 2015, 7, 199994–200003. [Google Scholar] [CrossRef]
- Koceva-Chyła, A.; Matczak, K.; Hikisz, M.P.; Durka, M.K.; Kochel, M.K.; Süss-Fink, G.; Furrer, J.; Kowalski, K. Insights into the in vitro Anticancer Effects of Diruthenium-1. ChemMedChem 2016, 11, 2171–2187. [Google Scholar] [CrossRef]
- Dickerson, M.; Sun, Y.; Howerton, B.; Glazer, E.C. Modifying Charge and Hydrophilicity of Simple Ru(II) Polypyridyl Complexes Radically Alters Biological Activities: Old Complexes, Surprising New Tricks. Inorg. Chem. 2014, 53, 10370–10377. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Luo, Z.; Wu, Q.; Zheng, W.; Feng, Y.; Chen, T. Mixed-ligand ruthenium polypyridyl complexes as apoptosis inducers in cancer cells, the cellular translocation and the important role of ROS-mediated signaling. Dalton Trans. 2014, 43, 17017–17028. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Lopalco, A.; Iacobazzi, R.M.; Lopedota, A.; Cutrignelli, A.; Iacobellis, G.; Annese, C.; Cascione, M.; Leporatti, S.; et al. In vitro targeting and imaging the translocator protein TSPO 18-kDa through G(4)-PAMAM–FITC labeled dendrimer. J. Control. Release 2013, 172, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Michlewska, S.; Ionov, M.; Maroto-Diaz, M.; Szwed, A.; Ihnatsyeu-Kachan, A.; Loznikova, S.; Shcharbin, D.; Malý, M.; Ramírez, R.G.; De La Mata, F.J.; et al. Ruthenium dendrimers as carriers for anticancer siRNA. J. Inorg. Biochem. 2018, 181, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Chattopadhyay, S.; Jen, Y.-J.; Peng, C.-Y.; Liu, T.-A.; Hsu, Y.-K.; Pan, C.-L.; Lo, H.-C.; Hsu, C.-H.; Chang, Y.-H.; et al. Improved broadband and quasi-omnidirectional anti-reflection properties with biomimetic silicon nanostructures. Nat. Nanotechnol. 2007, 2, 770–774. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michlewska, S.; Kubczak, M.; Maroto-Díaz, M.; Sanz del Olmo, N.; Ortega, P.; Shcharbin, D.; Gomez Ramirez, R.; Javier de la Mata, F.; Ionov, M.; Bryszewska, M. Synthesis and Characterization of FITC Labelled Ruthenium Dendrimer as a Prospective Anticancer Drug. Biomolecules 2019, 9, 411. https://doi.org/10.3390/biom9090411
Michlewska S, Kubczak M, Maroto-Díaz M, Sanz del Olmo N, Ortega P, Shcharbin D, Gomez Ramirez R, Javier de la Mata F, Ionov M, Bryszewska M. Synthesis and Characterization of FITC Labelled Ruthenium Dendrimer as a Prospective Anticancer Drug. Biomolecules. 2019; 9(9):411. https://doi.org/10.3390/biom9090411
Chicago/Turabian StyleMichlewska, Sylwia, Małgorzata Kubczak, Marta Maroto-Díaz, Natalia Sanz del Olmo, Paula Ortega, Dzmitry Shcharbin, Rafael Gomez Ramirez, Francisco Javier de la Mata, Maksim Ionov, and Maria Bryszewska. 2019. "Synthesis and Characterization of FITC Labelled Ruthenium Dendrimer as a Prospective Anticancer Drug" Biomolecules 9, no. 9: 411. https://doi.org/10.3390/biom9090411