Use of Farnesyl Transferase Inhibitors in an Ageing Model in Drosophila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Generating a Stable HeLa Cell Line with Inducible GFP-kuk Expression
2.3. Immunohistochemistry
2.4. Microscopy
2.5. Drosophila Strains
2.6. Lifespan Assays, Preparation of Muscle Tissue
3. Results
3.1. The Farnesyl Transferase Inhibitor ABT-100 Suppressed Ageing-like Phenotypes in Cultured Cells
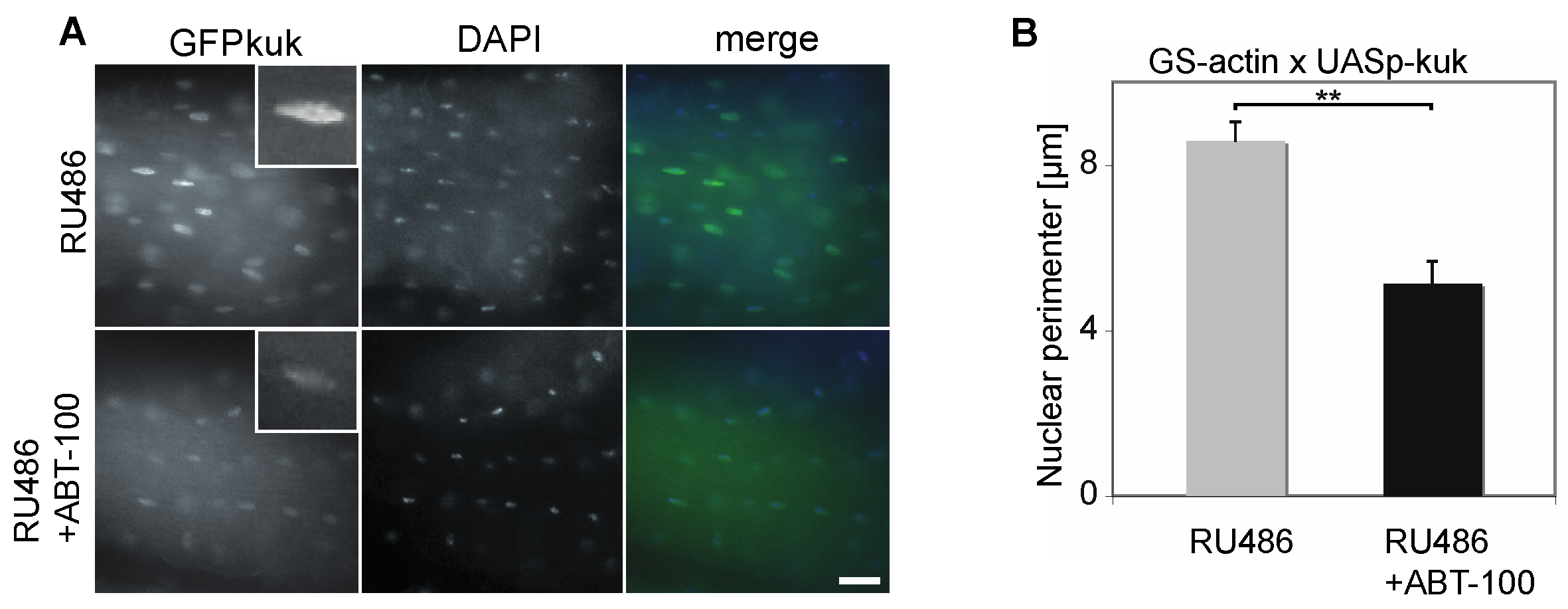
3.2. Suppression of Kuk-Induced Nuclear Phenotypes by ABT-100 in HeLa Cells with Inducible kuk Expression
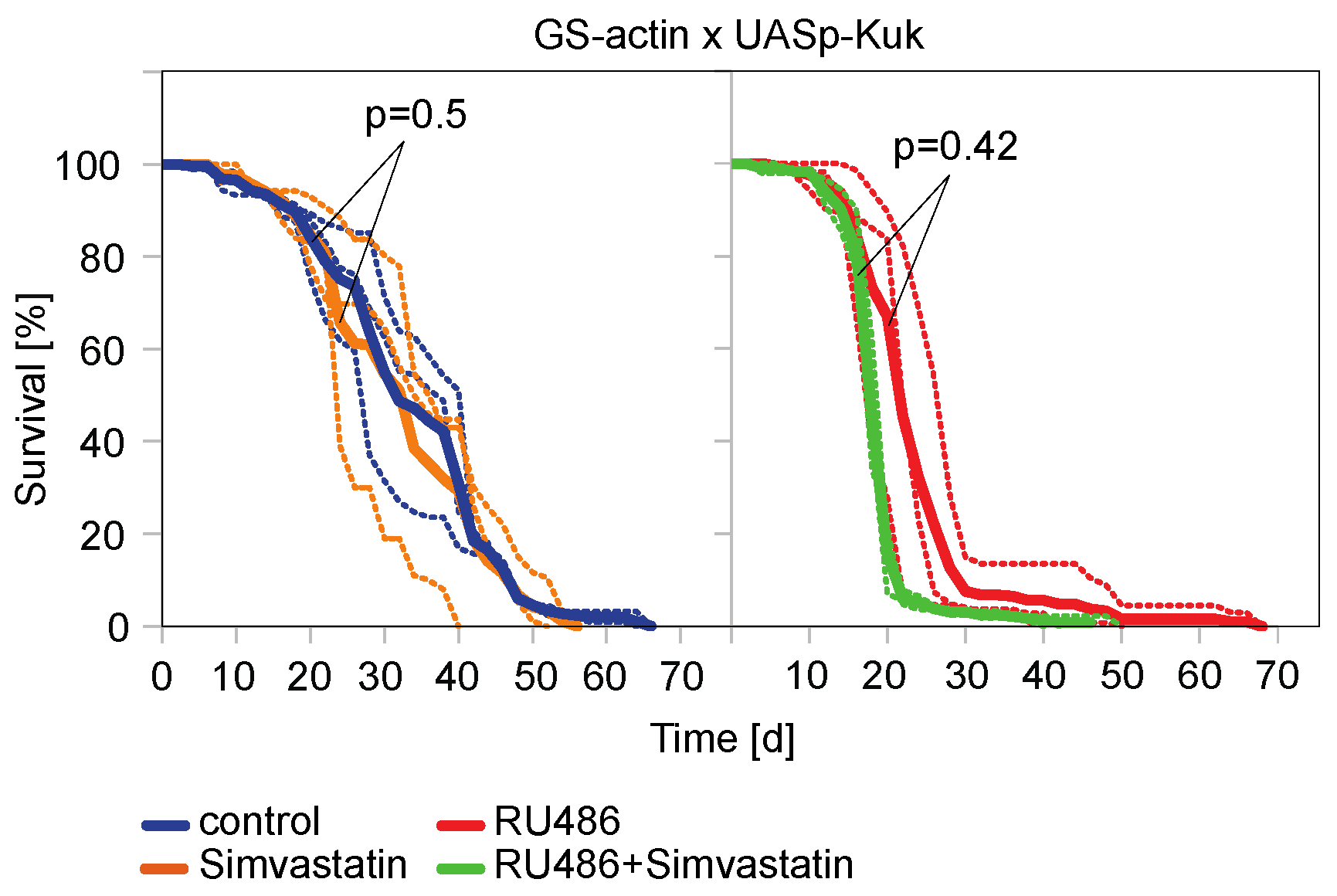
3.3. The Farnesyl Transferase Inhibitor ABT-100 Reverses the Shortened Lifespan Induced by Kuk
4. Discussion
4.1. Premature Ageing Models
4.2. Assay for Lifespan Altering Interventions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burke, B.; Stewart, C.L. The nuclear lamins: Flexibility in function. Nat. Rev. Mol. Cell Biol. 2013, 14, 13–24. [Google Scholar] [CrossRef]
- Gruenbaum, Y.; Foisner, R. Lamins: Nuclear Intermediate Filament Proteins with Fundamental Functions in Nuclear Mechanics and Genome Regulation. Annu. Rev. Biochem. 2015, 84, 131–164. [Google Scholar] [CrossRef]
- Zuela, N.; Bar, D.Z.; Gruenbaum, Y. Lamins in development, tissue maintenance and stress. EMBO Rep. 2012, 13, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 2006, 312, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- McClintock, D.; Ratner, D.; Lokuge, M.; Owens, D.M.; Gordon, L.B.; Collins, F.S.; Djabali, K. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS ONE 2007, 2, e1269. [Google Scholar] [CrossRef] [PubMed]
- Espada, J.; Varela, I.; Flores, I.; Ugalde, A.P.; Cadin, J.; Penda, A.M.; Stewart, C.L.; Tryggvason, K.; Blasco, M.A.; Freije, J.M.P.; et al. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J. Cell Biol. 2008, 181, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L.; et al. Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, S.; Kreienkamp, R.; Askjaer, P. Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res. Rev. 2017, 33, 18–29. [Google Scholar] [CrossRef]
- Burke, B.; Stewart, C.L. Life at the edge: The nuclear envelope and human disease. Nat. Rev. Mol. Cell Biol. 2002, 3, 575–585. [Google Scholar] [CrossRef]
- Burke, B.; Stewart, C.L. The laminopathies: The functional architecture of the nucleus and its contribution to disease. Annu. Rev. Genom. Hum. Genet. 2006, 7, 369–405. [Google Scholar] [CrossRef]
- Wiesel, N.; Mattout, A.; Melcer, S.; Melamed-Book, N.; Herrmann, H.; Medalia, O.; Aebi, U.; Gruenbaum, Y. Laminopathic mutations interfere with the assembly, localization, and dynamics of nuclear lamins. Proc. Natl. Acad. Sci. USA 2008, 105, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Petillo, R.; D’Ambrosio, P.; Torella, A.; Taglia, A.; Picillo, E.; Testori, A.; Ergoli, M.; Nigro, G.; Piluso, G.; Nigro, V.; et al. Novel mutations in LMNA A/C gene and associated phenotypes. Acta Myol. 2015, 34, 116. [Google Scholar] [PubMed]
- De Sandre-Giovannoli, A.; Bernard, R.; Cau, P.; Navarro, C.; Amiel, J.; Boccaccio, I.; Lyonnet, S.; Stewart, C.L.; Munnich, A.; Le Merrer, M.; et al. Lamin a truncation in Hutchinson-Gilford progeria. Science 2003, 300, 2055. [Google Scholar] [CrossRef]
- Merideth, M.A.; Gordon, L.B.; Clauss, S.; Sachdev, V.; Smith, A.C.; Perry, M.B.; Brewer, C.C.; Zalewski, C.; Kim, H.J.; Solomon, B.; et al. Phenotype and course of Hutchinson—Gilford progeria syndrome. N. Engl. J. Med. 2008, 358, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Brown, W.T.; Gordon, L.B.; Glynn, M.W.; Singer, J.; Scott, L.; Erdos, M.R.; Robbins, C.M.; Moses, T.Y.; Berglund, P.; et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 2003, 423, 293–298. [Google Scholar] [CrossRef]
- Hennekam, R.C.M. Hutchinson-Gilford progeria syndrome: Review of the phenotype. Am. J. Med. Genet. A 2006, 140, 2603–2624. [Google Scholar] [CrossRef]
- Goldman, R.D.; Shumaker, D.K.; Erdos, M.R.; Eriksson, M.; Goldman, A.E.; Gordon, L.B.; Gruenbaum, Y.; Khuon, S.; Mendez, M.; Varga, R.; et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 8963–8968. [Google Scholar] [CrossRef]
- Pacheco, L.M.; Gomez, L.A.; Dias, J.; Ziebarth, N.M.; Howard, G.A.; Schiller, P.C. Progerin expression disrupts critical adult stem cell functions involved in tissue repair. Aging 2014, 6, 1049–1063. [Google Scholar] [CrossRef]
- Yang, S.H.; Bergo, M.O.; Toth, J.I.; Qiao, X.; Hu, Y.; Sandoval, S.; Meta, M.; Bendale, P.; Gelb, M.H.; Young, S.G.; et al. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc. Natl. Acad. Sci. USA 2005, 102, 10291–10296. [Google Scholar] [CrossRef]
- Mallampalli, M.P.; Huyer, G.; Bendale, P.; Gelb, M.H.; Michaelis, S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 14416–14421. [Google Scholar] [CrossRef]
- Capell, B.C.; Erdos, M.R.; Madigan, J.P.; Fiordalisi, J.J.; Varga, R.; Conneely, K.N.; Gordon, L.B.; Der, C.J.; Cox, A.D.; Collins, F.S. Inhibiting farnesylation of Progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 12879–12884. [Google Scholar] [CrossRef] [PubMed]
- Glynn, M.W.; Glover, T.W. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 2005, 14, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.I.; Yang, S.H.; Qiao, X.; Beigneux, A.P.; Gelb, M.H.; Moulson, C.L.; Miner, J.H.; Young, S.G.; Fong, L.G. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. USA 2005, 102, 12873–12878. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Andres, D.A.; Spielmann, H.P.; Young, S.G.; Fong, L.G. Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J. Clin. Investig. 2008, 118, 3291–3300. [Google Scholar] [CrossRef]
- Yang, S.H.; Chang, S.Y.; Andres, D.A.; Spielmann, H.P.; Young, S.G.; Fong, L.G. Assessing the efficacy of protein farnesyltransferase inhibitors in mouse models of progeria. J. Lipid Res. 2010, 51, 400–405. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Miller, D.T.; Neuberg, D.S.; Giobbie-Hurder, A.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.; Snyder, B.D.; et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 16666–16671. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Kieran, M.W.; Miller, D.T.; Gordon, L.B.; Cho, Y.-J.; Silvera, V.M.; Giobbie-Hurder, A.; Neuberg, D.; Kleinman, M.E. Neurologic features of Hutchinson-Gilford progeria syndrome after lonafarnib treatment. Neurology 2013, 81, 427–430. [Google Scholar] [CrossRef]
- Koshimizu, E.; Imamura, S.; Qi, J.; Toure, J.; Valdez, D.M., Jr.; Carr, C.E.; Hanai, J.; Kishi, S. Embryonic senescence and laminopathies in a progeroid zebrafish model. PLoS ONE 2011, 6, e17688. [Google Scholar] [CrossRef]
- Brandt, A.; Krohne, G.; Grosshans, J. The farnesylated nuclear proteins KUGELKERN and LAMIN B promote aging-like phenotypes in Drosophila flies. Aging Cell 2008, 7, 541–551. [Google Scholar] [CrossRef]
- Beard, G.S.; Bridger, J.M.; Kill, I.R.; Tree, D.R.P. Towards a Drosophila model of Hutchinson-Gilford progeria syndrome. Biochem. Soc. Trans. 2008, 36, 1389–1392. [Google Scholar] [CrossRef]
- Haithcock, E.; Dayani, Y.; Neufeld, E.; Zahand, A.J.; Feinstein, N.; Mattout, A.; Gruenbaum, Y.; Liu, J. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2005, 102, 16690–16695. [Google Scholar] [CrossRef] [PubMed]
- Rzepecki, R.; Gruenbaum, Y. Invertebrate models of lamin diseases. Nucleus 2018, 9, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Pałka, M.; Tomczak, A.; Grabowska, K.; Machowska, M.; Piekarowicz, K.; Rzepecka, D.; Rzepecki, R. Laminopathies: What can humans learn from fruit flies. Cell Mol. Biol. Lett. 2018, 23, 32. [Google Scholar] [CrossRef]
- Duan, T.; Thyagarajan, S.; Amoiroglou, A.; Rogers, G.C.; Geyer, P.K. Analysis of a rare progeria variant of Barrier-to-autointegration factor in Drosophila connects centromere function to tissue homeostasis. Cell Mol. Life Sci. 2023, 2023 80, 73. [Google Scholar] [CrossRef]
- Tsurumi, A.; Li, W.X. Aging mechanisms—A perspective mostly from Drosophila. Adv. Genet. 2020, 1, e1002. [Google Scholar] [CrossRef] [PubMed]
- Prüfert, K.; Vogel, A.; Krohne, G. The lamin CxxM motif promotes nuclear membrane growth. J. Cell Sci. 2004, 117, 6105–6116. [Google Scholar] [CrossRef] [PubMed]
- Ralle, T.; Grund, C.; Franke, W.W.; Stick, R. Intranuclear membrane structure formations by CaaX-containing nuclear proteins. J. Cell Sci. 2004, 117, 6095–6104. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Papagiannouli, F.; Wagner, N.; Wilsch-Bräuninger, M.; Braun, M.; Furlong, E.E.; Loserth, S.; Wenzl, C.; Pilot, F.; Vogt, N.; et al. Developmental control of nuclear size and shape by Kugelkern and Kurzkern. Curr. Biol. 2006, 16, 543–552. [Google Scholar] [CrossRef]
- Petrovsky, R.; Krohne, G.; Großhans, J. Overexpression of the lamina proteins Lamin and Kugelkern induces specific ultrastructural alterations in the morphology of the nuclear envelope of intestinal stem cells and enterocytes. Eur. J. Cell Biol. 2018, 97, 102–113. [Google Scholar] [CrossRef]
- Bar, D.Z.; Neufeld, E.; Feinstein, N.; Gruenbaum, Y. Gliotoxin reverses age-dependent nuclear morphology phenotypes, ameliorates motility, but fails to affect lifespan of adult Caenorhabditis elegans. Cell Motil. Cytoskelet. 2009, 66, 791–797. [Google Scholar] [CrossRef]
- Wessells, R.J.; Bodmer, R. Age-related cardiac deterioration: Insights from Drosophila. Front. Biosci. 2007, 12, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Rodriguez, L.E.; Palma, J.P.; Refici, M.; Jarvis, K.; O’Connor, J.; Sullivan, G.M.; Frost, D.; Marsh, K.; Bauch, J.; et al. Antitumor activity of orally bioavailable farnesyltransferase inhibitor, ABT-100, is mediated by antiproliferative, proapoptotic, and antiangiogenic effects in xenograft models. Clin. Cancer Res. 2005, 11, 3045–3054. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowiak, J.W.; Gibbs, R.A.; Mattingly, R.R. Working together: Farnesyl transferase inhibitors and statins block protein prenylation. Mol. Cell Pharmacol. 2009, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mans, R.A.; McMahon, L.L.; Li, L. Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience 2012, 202, 1–9. [Google Scholar] [CrossRef]
- Weidenfeld, I.; Gossen, M.; Löw, R.; Kentner, D.; Berger, S.; Görlich, D.; Bartsch, D.; Bujard, H.; Schönig, K. Inducible expression of coding and inhibitory RNAs from retargetable genomic loci. Nucleic Acids Res. 2009, 37, e50. [Google Scholar] [CrossRef]
- Whitworth, C. The Biological Resources of Model Organisms; Jarret, R.L., McCluskey, K., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Chapter 8; pp. 145–162. [Google Scholar]
- Thurmond, J.; Goodman, J.L.; Strelets, V.B.; Attrill, H.; Gramates, L.S.; Marygold, S.J.; Matthews, B.B.; Millburn, G.; Antonazzo, G.; Trovisco, V.; et al. FlyBase 2.0: The next generation. Nucleic Acids Res. 2019, 47, D759–D765. [Google Scholar] [CrossRef]
- Rogulja, D.; Irvine, K.D. Regulation of cell proliferation by a morphogen gradient. Cell 2005, 123, 449–461. [Google Scholar] [CrossRef]
- Osterwalder, T.; Yoon, K.S.; White, B.H.; Keshishian, H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA 2001, 98, 12596–12601. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat. Med. 2005, 11, 440. [Google Scholar] [CrossRef]
- Fong, L.G.; Frost, D.; Meta, M.; Qiao, X.; Yang, S.H.; Coffinier, C.; Young, S.G. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 2006, 311, 1621–1623. [Google Scholar] [CrossRef]
- Yang, S.H.; Meta, M.; Qiao, X.; Frost, D.; Bauch, J.; Coffinier, C.; Majumdar, S.; Bergo, M.O.; Young, S.G.; Fong, L.G. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J. Clin. Investig. 2006, 116, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.-Z.; Joseph, I.; Wang, Y.-C.; Frost, D.; Sullivan, G.M.; Wang, L.; Lin, N.-H.; Cohen, J.; Stoll, V.S.; Jakob, C.G.; et al. A highly potent and selective farnesyltransferase inhibitor ABT-100 in preclinical studies. Anti-Cancer Drugs 2005, 16, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Polychronidou, M.; Hellwig, A.; Grosshans, J. Farnesylated nuclear proteins Kugelkern and lamin Dm0 affect nuclear morphology by directly interacting with the nuclear membrane. Mol. Biol. Cell 2010, 21, 3409–3420. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.; Endo, K.; Zong, L.; Davis, R.L. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2001, 98, 12602–12607. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.; Hoe, N.; Landis, G.N.; Tozer, K.; Luu, A.; Bhole, D.; Badrinath, A.; Tower, J. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycycline or RU486/Mifepristone. Exp. Gerontol. 2007, 242, 483–497. [Google Scholar] [CrossRef]
- Shen, J.; Curtis, C.; Tavaré, S.; Tower, J. A screen of apoptosis and senescence regulatory genes for life span effects when over-expressed in Drosophila. Aging 2009, 1, 191–211. [Google Scholar] [CrossRef]
- Poirier, L.; Shane, A.; Zheng, J.; Seroude, L. Characterization of the Drosophila gene-switch system in aging studies: A cautionary tale. Aging Cell 2008, 7, 758–770. [Google Scholar] [CrossRef]
- Vinci, G.; Xia, X.; Veitia, R.A. Preservation of genes involved in sterol metabolism in cholesterol auxotrophs: Facts and hypotheses. PLoS ONE 2008, 3, e2883. [Google Scholar] [CrossRef]
- Hieb, W.F.; Rothstein, M. Sterol requirement for reproduction of a free-living nematode. Science 1968, 160, 778–780. [Google Scholar] [CrossRef]
- Rosenfeld, J.M.; Osborne, T.F. HLH106, a Drosophila sterol regulatory element-binding protein in a natural cholesterol auxotroph. J. Biol. Chem. 1998, 273, 16112–16121. [Google Scholar] [CrossRef]
- Meta, M.; Yang, S.H.; Bergo, M.O.; Fong, L.G.; Young, S.G. Protein farnesyltransferase inhibitors and progeria. Trends Mol. Med. 2006, 12, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Helfand, S.L.; Rogina, B. Genetics of aging in the fruit fly, Drosophila melanogaster. Annu. Rev. Genet. 2003, 37, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Lees, H.; Walters, H.; Cox, L.S. Animal and human models to understand ageing. Maturitas 2016, 93, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Wang, A.M.; Bennett, C.F.; Kaeberlein, M. Biochemical Genetic Pathways that Modulate Aging in Multiple Species. Cold Spring Harb. Perspect. Med. 2015, 5, a025114. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.N.; Shaposhnikov, M.V.; Sadritdinova, A.F.; Kudryavtseva, A.V.; Moskalev, A.A. Basic mechanisms of longevity: A case study of Drosophila pro-longevity genes. Ageing Res. Rev. 2015, 24, 218–231. [Google Scholar] [CrossRef]
- Castillo-Quan, J.I.; Kinghorn, K.J.; Bjedov, I. Genetics and pharmacology of longevity: The road to therapeutics for healthy aging. Adv. Genet. 2015, 90, 1–101. [Google Scholar]
- Nicholson, L.; Singh, G.K.; Osterwalder, T.; Roman, G.W.; Davis, R.L.; Keshishian, H. Spatial and temporal control of gene expression in Drosophila using the inducible GeneSwitch GAL4 system. I. Screen for larval nervous system drivers. Genetics 2008, 178, 215–234. [Google Scholar] [CrossRef]
- Yamada, R.; Deshpande, S.A.; Keebaugh, E.S.; Ehrlich, M.R.; Soto Obando, A.; Ja, W.W. Mifepristone Reduces Food Palatability and Affects Drosophila Feeding and Lifespan. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 173–180. [Google Scholar] [CrossRef]
- Landis, G.N.; Salomon, M.P.; Keroles, D.; Brookes, N.; Sekimura, T.; Tower, J. The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila. Aging 2015, 7, 53–69. [Google Scholar] [CrossRef]
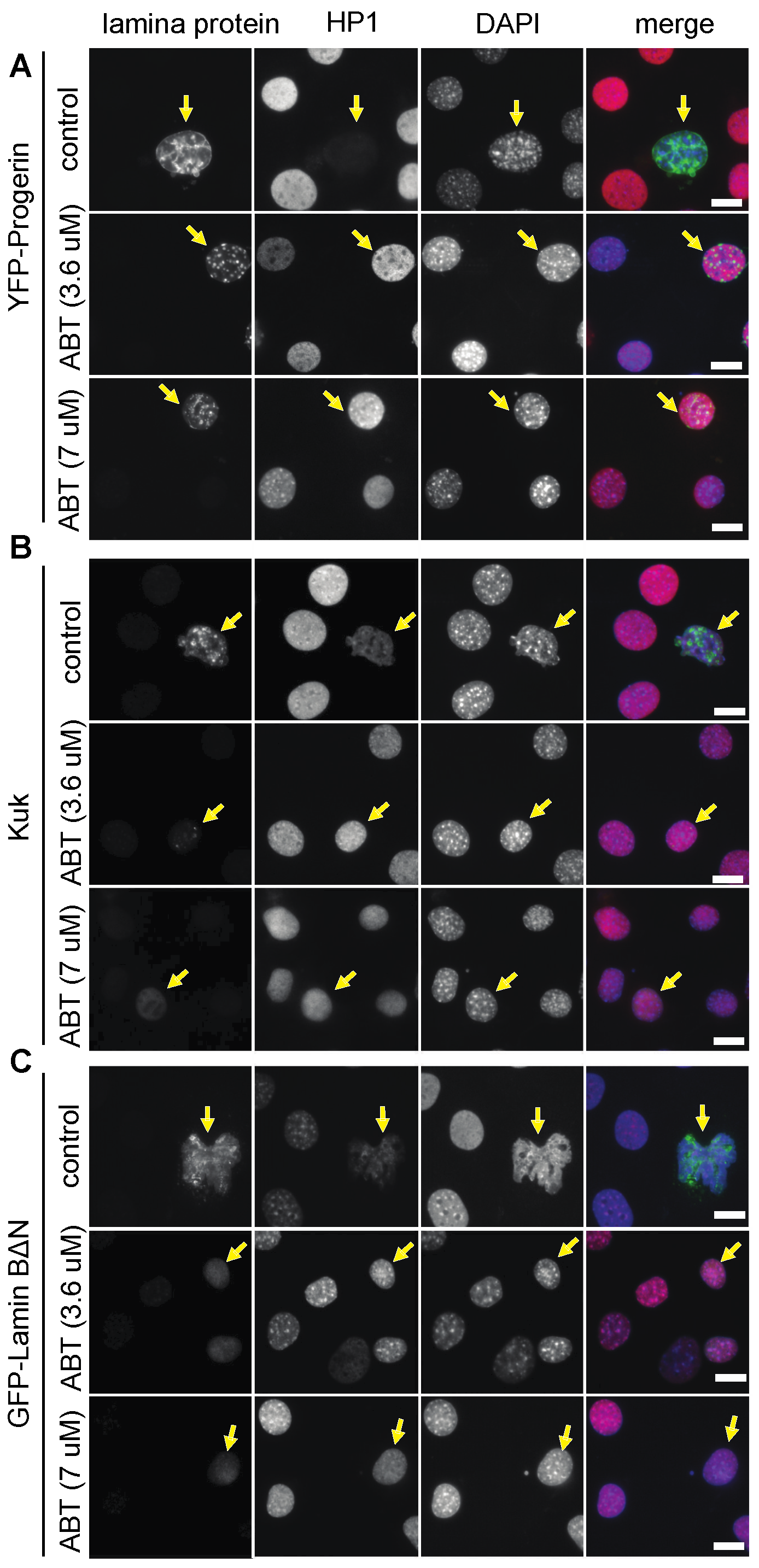



| Nuclear Perimeter (µm) | |||||
|---|---|---|---|---|---|
| YFP-Progerin | Transfected | S.E.M. | Non-Transfected | S.E.M. (2) | p-Value (1) |
| control | 61.79 | ±1.64 | 39.33 | ±1.26 | <0.00001 |
| ABT (3.6 µM) | 39.05 | ±1.06 | 38.02 | ±0.87 | n.s. (3) |
| ABT (7 µM) | 35.95 | ±1.19 | 36.00 | ±0.87 | n.s. |
| Kuk | |||||
| control | 42.17 | ±2.85 | 33.44 | ±2.85 | 0.021 |
| ABT (3.6 µM) | 30.94 | ±0.68 | 33.73 | ±0.65 | n.s. |
| ABT (7 µM) | 32.41 | ±0.72 | 33.04 | ±0.61 | n.s. |
| GFP-LaminBDN | |||||
| control | 60.13 | ±5.33 | 34.76 | ±1.27 | 0.00003 |
| ABT (3.6 µM) | 27.07 | ±1.14 | 31.75 | ±0.99 | 0.00032 |
| ABT (7 µM) | 33.49 | ±0.67 | 35.71 | ±1.10 | n.s. |
| Nuclear Perimeter (µm) | |||||
|---|---|---|---|---|---|
| GFP-Progerin | Transfected | S.E.M. | Non-Transfected | S.E.M. (2) | p-Value (1) |
| control | 53.48 | ±1.19 | 33.19 | ±0.82 | <0.00001 |
| Sim | 38.69 | ±1.10 | 36.00 | ±0.85 | 0.043 |
| FTI-277 | 42.66 | ±0.98 | 37.40 | ±1.44 | n.s. (3) |
| GFP-LaminBDN | |||||
| control | 58.96 | ±18.19 | 34.83 | ±0.99 | 0.005 |
| Sim | 37.62 | ±2.67 | 33.57 | ±0.88 | 0.017 |
| FTI-277 | 38.31 | ±4.83 | 36.63 | ±0.78 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt, A.; Petrovsky, R.; Kriebel, M.; Großhans, J. Use of Farnesyl Transferase Inhibitors in an Ageing Model in Drosophila. J. Dev. Biol. 2023, 11, 40. https://doi.org/10.3390/jdb11040040
Brandt A, Petrovsky R, Kriebel M, Großhans J. Use of Farnesyl Transferase Inhibitors in an Ageing Model in Drosophila. Journal of Developmental Biology. 2023; 11(4):40. https://doi.org/10.3390/jdb11040040
Chicago/Turabian StyleBrandt, Annely, Roman Petrovsky, Maria Kriebel, and Jörg Großhans. 2023. "Use of Farnesyl Transferase Inhibitors in an Ageing Model in Drosophila" Journal of Developmental Biology 11, no. 4: 40. https://doi.org/10.3390/jdb11040040





