A Joint Less Ordinary: Intriguing Roles for Hedgehog Signalling in the Development of the Temporomandibular Synovial Joint
Abstract
:1. Joint Types: An Overview
- (A)
- Immovable, such as the fibrous skull sutures.
- (B)
- Capable of limited movement, such as the cartilaginous pubic symphysis.
- (C)
- Full movement, such as the synovial knee and temporomandibular joints.
- (A)
- Fibrous joints that unite through thick connective tissue, such as the interosseous membrane between the tibia and fibula.
- (B)
- Cartilaginous joints that are united through cartilage, such as the pubic symphysis.
- (C)
- Synovial joints that have an articular capsule between two interconnected bones, which are themselves protected by articular cartilage and frequently reinforced by ligaments. These synovial joints can be further subdivided into:
- (i)
- Gliding joints that articulate on a single plane (e.g., intervertebral joints).
- (ii)
- Hinge joints move on a single axis (e.g., knee joint).
- (iii)
- Hinge and sliding joints that articulate on a single plane and axis (e.g., temporomandibular joint).
- (iv)
- Pivot joints provide rotation (e.g., atlas and axis joint).
- (v)
- Condyloid joints permit circular motions, extension and flexion (e.g., radiocarpal joint).
- (vi)
- Saddle joints allow extension and flexion, but no rotation (e.g., carpometacarpal joint).
- (vii)
- Ball and socket joints exhibit free movement on any axis (e.g., shoulder joint).
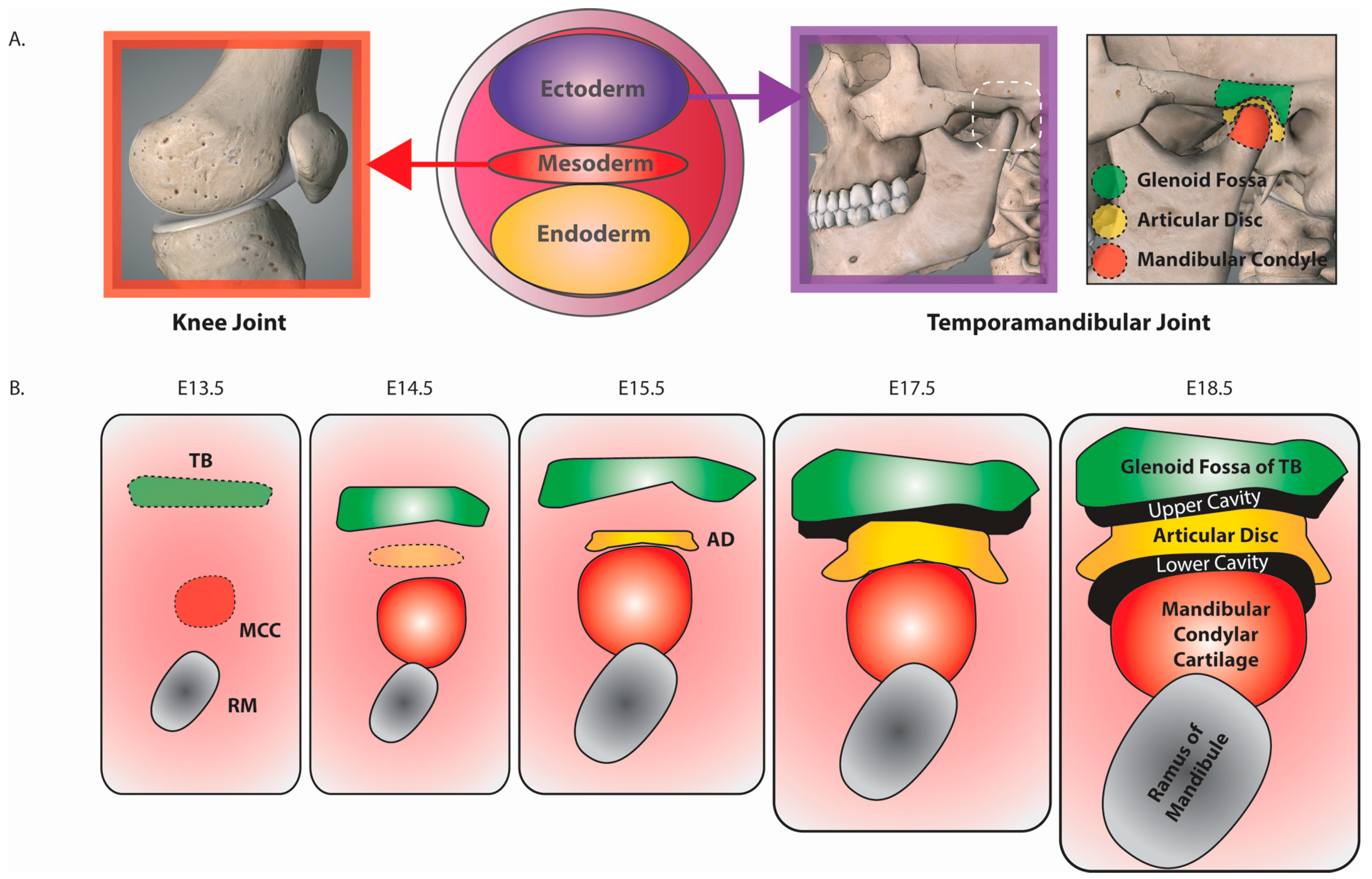
2. The Temporomandibular Joint
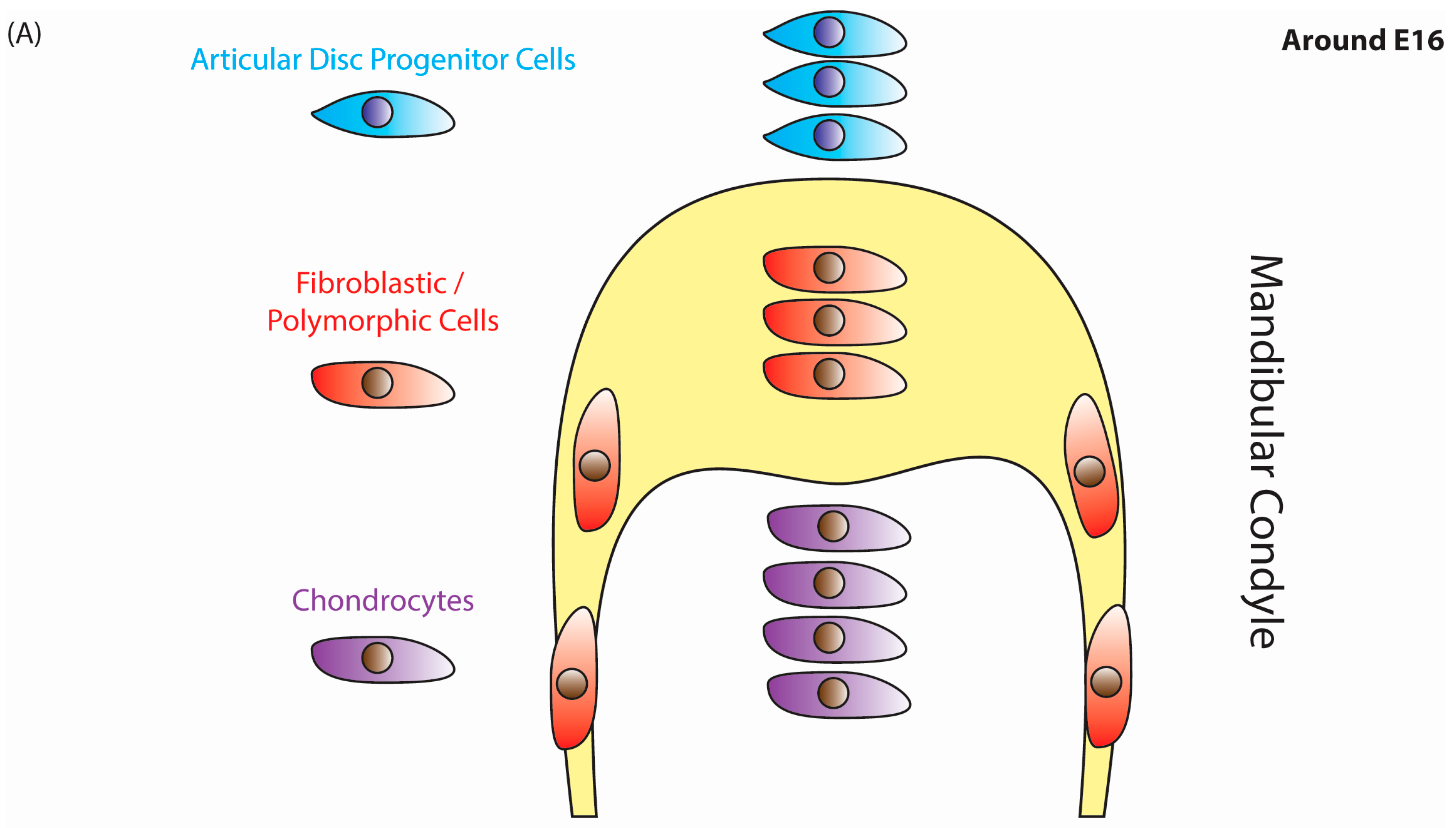
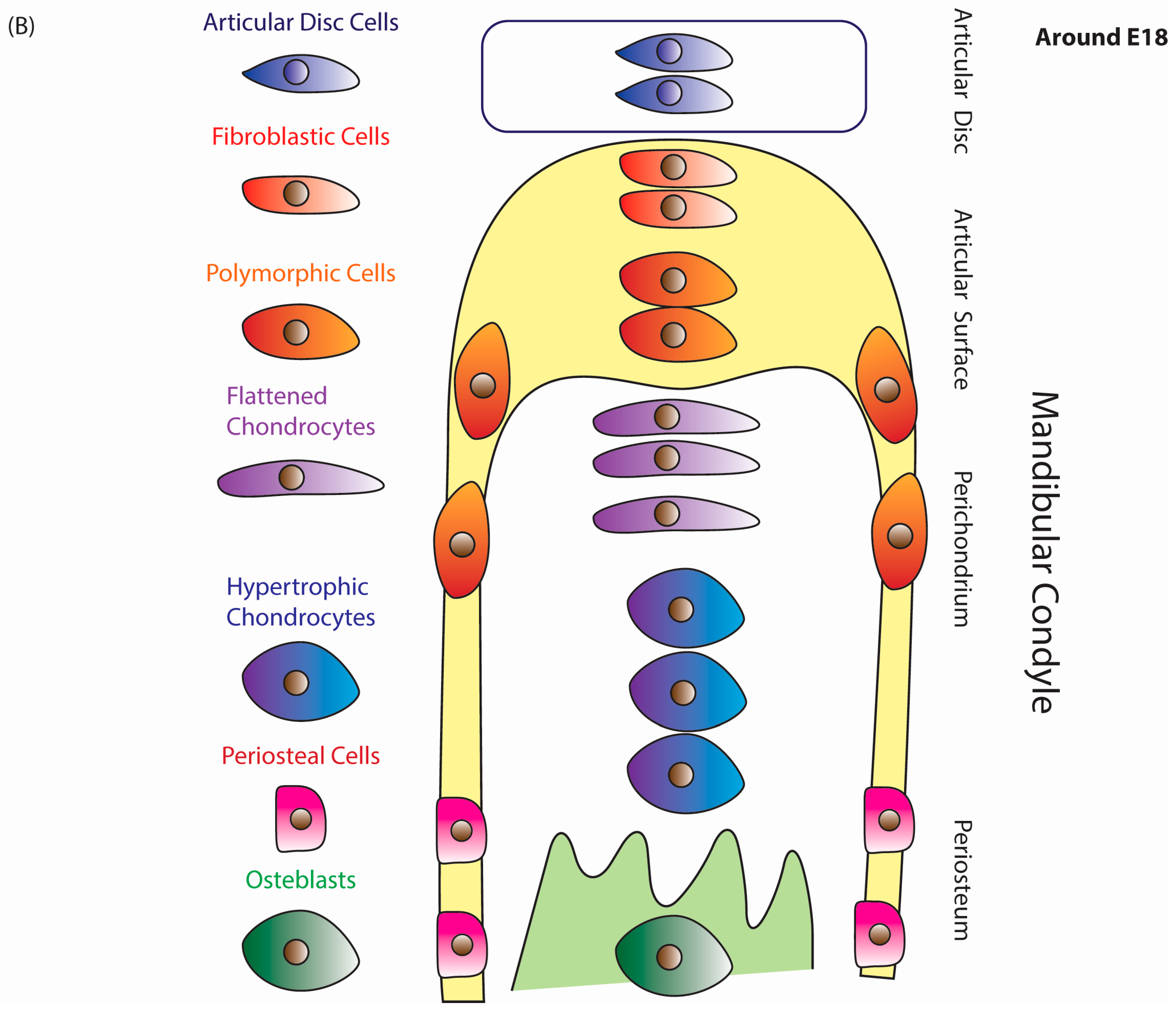
3. Murine TMJ Development
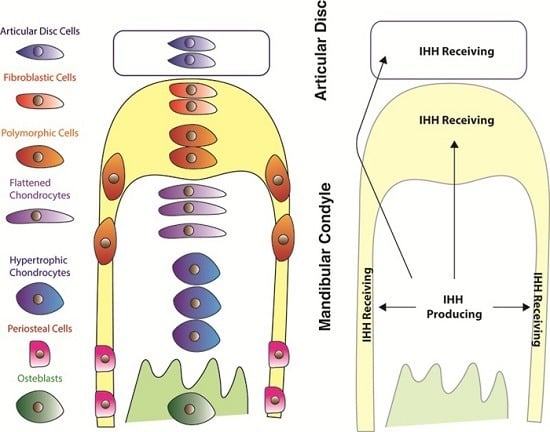
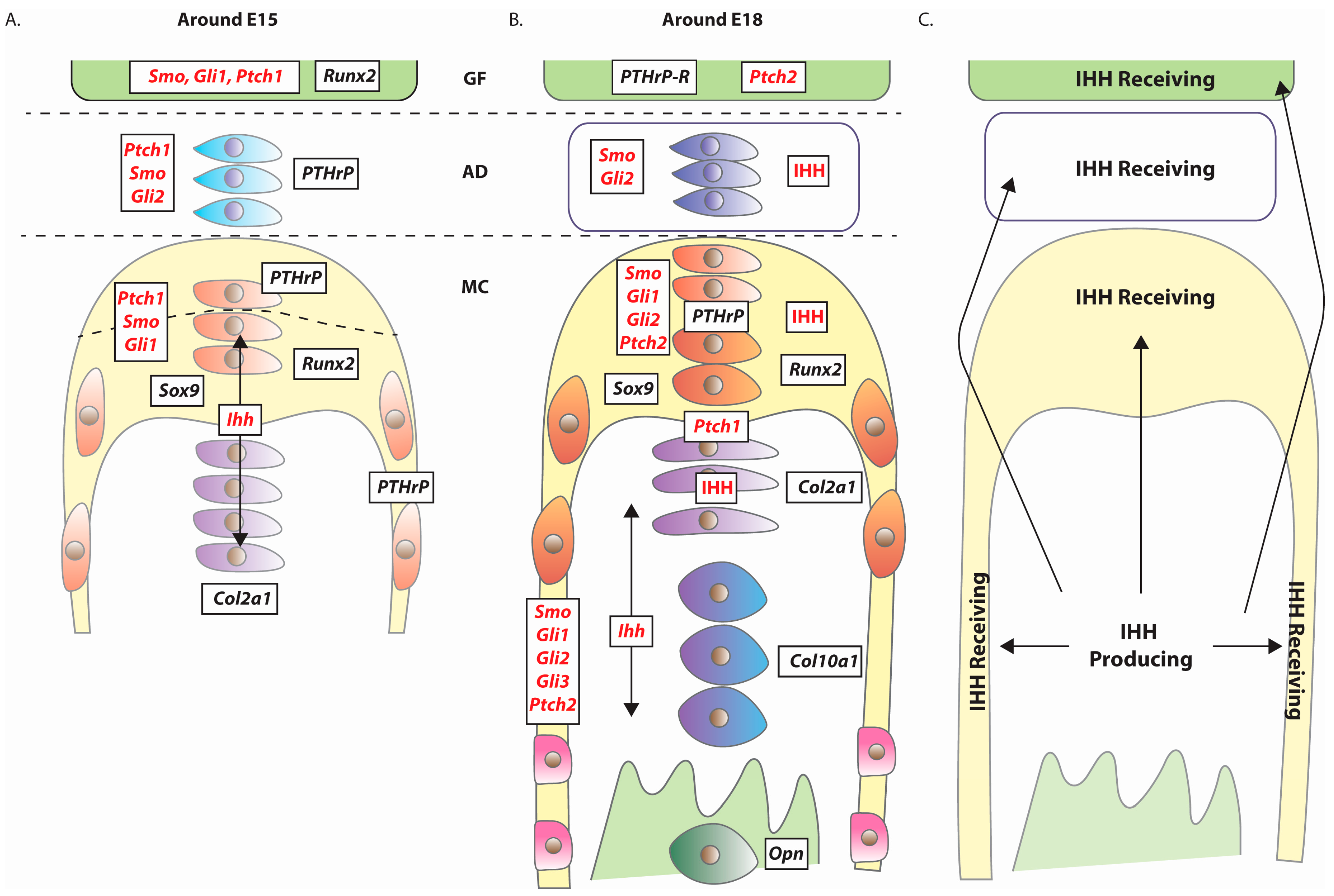
4. Expression of Hedgehog Pathway Components
5. TMJ Defects Associated with Loss of Hedgehog Signalling
5.1. Introduction
5.2. Mandibular Condyle Development
5.2.1. Indian Hedgehog (Ihh)
5.2.2. Glioma-Associated Oncogene-2 (Gli2)
5.2.3. Indian Hedgehog (Ihh) and Glioma-Associated Oncogene-3 (Gli3)
5.2.4. Smoothened (Smo)
5.2.5. Summary
5.3. Articular Disc Development
5.3.1. Indian Hedgehog (Ihh) and Glioma-associated Oncogene-3 (Gli3)
5.3.2. Glioma-Associated Oncogene-2 (Gli2)
5.3.3. Smoothened (Smo)
5.3.4. Summary
5.4. Other Genes Associated with Aberrant Hedgehog Signalling and TMJ Defects
5.4.1. Intraflagellar Transport (IFT) Genes
5.4.2. Sry-related HMG box 9 (Sox9) and Runt-related Transcription Factor-2 (Runx2)
5.4.3. Short Stature Homeobox Gene 2 (Shox2)
5.4.4. Trichorhinophalangeal Syndrome 1 (Trps1)
5.4.5. Summary
6. Perspectives
Acknowledgments
Conflicts of Interest
References
- Decker, R.S.; Koyama, E.; Pacifici, M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014, 39, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, L.; Li, T.; Tagliafierro, L.; Temple, J.D.; Willcockson, H.H.; Ye, P.; Esposito, A.; Xu, F.; Spagnoli, A. Synovial joints: From development to homeostasis. Curr. Osteoporos. Rep. 2015, 13, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.J.; Lewthwaite, J.C.; Bastow, E.R.; Pitsillides, A.A. Defining boundaries during joint cavity formation: Going out on a limb. Int. J. Exp. Pathol. 2003, 84, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Koyama, E.; Shibukawa, Y.; Nagayama, M.; Sugito, H.; Young, B.; Yuasa, T.; Okabe, T.; Ochiai, T.; Kamiya, N.; Rountree, R.B.; et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 2008, 316, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Alman, B.A. The role of hedgehog signalling in skeletal health and disease. Nat. Rev. Rheumatol. 2015, 11, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Ehlen, H.W.; Buelens, L.A.; Vortkamp, A. Hedgehog signaling in skeletal development. Birth Defects. Res. C Embryo. Today 2006, 78, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.J. Genes that regulate morphogenesis and growth of the temporomandibular joint: A review. Dev. Dyn. 2014, 243, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Iwata, J. Mouse genetic models for temporomandibular joint development and disorders. Oral. Dis. 2016, 22, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.; Newton-John, T.R.; Zakrzewska, J.M. Temporomandibular disorders. BMJ 2015, 350, h1154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, I.; Nakamura, M.; Takahashi, I.; Kagayama, M.; Mitani, H. An immunohistochemical study of localization of type I and type II collagens in mandibular condylar cartilage compared with tibial growth plate. Histochemistry 1990, 93, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Symons, N.B. A histochemical study of the secondary cartilage of the mandibular condyle in the rat. Arch. Oral Biol. 1965, 10, 579–584. [Google Scholar] [CrossRef]
- Lievre, C.L. Role of mesectodermal cells arising from the cephalic neural crest in the formation of the branchial arches and visceral skeleton. J. Embryol. Exp. Morphol. 1974, 31, 453–477. [Google Scholar] [PubMed]
- Shen, G.; Darendeliler, M.A. The adaptive remodeling of condylar cartilage—A transition from chondrogenesis to osteogenesis. J. Dent. Res. 2005, 84, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.H.; Bard, J. The Anatomical Basis of Mouse Development; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Shibata, S.; Sato, S.; Murakami, G.; Fukuoka, H.; Rodríguez-Vázquez, J.F. Origin of mandibular condylar cartilage in mice, rats, and humans: Periosteum or separate blastema? J. Oral Biosci. 2013, 55, 208–216. [Google Scholar] [CrossRef]
- Purcell, P.; Joo, B.W.; Hu, J.K.; Tran, P.V.; Calicchio, M.L.; O’Connell, D.J.; Maas, R.L.; Tabin, C.J. Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc. Natl. Acad. Sci. USA 2009, 106, 18297–18302. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K. Mandibular morphogenesis and craniofacial malformations. J. Craniofac. Genet. Dev. Biol. 1982, 2, 309–322. [Google Scholar] [PubMed]
- Percival, C.J.; Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 2013, 242, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Koyama, E.; Shimazu, A.; Leatherman, J.L.; Golden, E.B.; Nah, H.D.; Pacifici, M. Expression of syndecan-3 and tenascin-C: Possible involvement in periosteum development. J. Orthop. Res. 1996, 14, 403–412. [Google Scholar] [CrossRef] [PubMed]
- von der Mark, K.; von der Mark, H. The role of three genetically distinct collagen types in endochondral ossification and calcification of cartilage. J. Bone Joint Surg. Br. 1977, 59, 458–464. [Google Scholar] [PubMed]
- Petrovic, A.G. Mechanisms and regulation of mandibular condylar growth. Acta Morphol. Neerl. Scand. 1972, 10, 25–34. [Google Scholar] [PubMed]
- Duben, W.; Gelbke, H. Animal experiments relating to the question of epiphyseal versus interstitial longitudinal bone growth. Acta Orthop. Scand. 1955, 25, 3–25. [Google Scholar] [PubMed]
- Rauch, F. Bone growth in length and width: The Yin and Yang of bone stability. J. Musculoskelet. Neuronal Interact. 2005, 5, 194–201. [Google Scholar] [PubMed]
- Kozhemyakina, E.; Zhang, M.; Ionescu, A.; Ayturk, U.M.; Ono, N.; Kobayashi, A.; Kronenberg, H.; Warman, M.L.; Lassar, A.B. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015, 67, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Kantomaa, T.; Tuominen, M.; Pirttiniemi, P. Effect of mechanical forces on chondrocyte maturation and differentiation in the mandibular condyle of the rat. J. Dent. Res. 1994, 73, 1150–1156. [Google Scholar] [PubMed]
- Silbermann, M.; Frommer, J. The nature of endochondral ossification in the mandibular condyle of the mouse. Anat Rec. 1972, 172, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Glineburg, R.W.; Laskin, D.M.; Blaustein, D.I. The effects of immobilization on the primate temporomandibular joint: A histologic and histochemical study. J. Oral Maxillofac. Surg. 1982, 40, 3–8. [Google Scholar] [CrossRef]
- Lydiatt, D.D.; Davis, L.F. The effects of immobilization on the rabbit temporomandibular joint. J. Oral Maxillofac. Surg. 1985, 43, 188–193. [Google Scholar] [CrossRef]
- Shibata, S.; Suda, N.; Suzuki, S.; Fukuoka, H.; Yamashita, Y. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J. Anat. 2006, 208, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mori-Akiyama, Y.; Akiyama, H.; Rowitch, D.H.; de Crombrugghe, B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. USA 2003, 100, 9360–9365. [Google Scholar] [CrossRef] [PubMed]
- Shibukawa, Y.; Young, B.; Wu, C.; Yamada, S.; Long, F.; Pacifici, M.; Koyama, E. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev. Dyn. 2007, 236, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Li, X.; Gao, B.; Gan, H.; Lin, X.; Liao, L.; Li, C. Observing the development of the temporomandibular joint in embryonic and post-natal mice using various staining methods. Exp. Ther. Med. 2016, 11, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wei, N.; Yu, L.; Fei, J.; Chen, Y. Shox2-deficiency leads to dysplasia and ankylosis of the temporomandibular joint in mice. Mech. Dev. 2008, 125, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Luder, H.U.; Leblond, C.P.; von der Mark, K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am. J. Anat. 1988, 182, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Petrova, R.; Joyner, A.L. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 2014, 141, 3445–3457. [Google Scholar] [CrossRef] [PubMed]
- Towers, M.; Tickle, C. Growing models of vertebrate limb development. Development 2009, 136, 179–190. [Google Scholar] [CrossRef] [PubMed]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Vortkamp, A.; Lee, K.; Lanske, B.; Segre, G.V.; Kronenberg, H.M.; Tabin, C.J. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 1996, 273, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Shibukawa, Y.; Nagayama, M.; Mundy, C.; Yasuda, T.; Okabe, T.; Shimono, K.; Kanyama, M.; Hasegawa, H.; Maeda, Y.; et al. Indian hedgehog roles in post-natal TMJ development and organization. J. Dent. Res. 2010, 89, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, T.E.; Saunders, C.; Decker, R.S.; Um, H.B.; Cottingham, N.; Salhab, I.; Kurio, N.; Billings, P.C.; Pacifici, M.; Nah, H.D.; et al. Osteophyte formation and matrix mineralization in a TMJ osteoarthritis mouse model are associated with ectopic hedgehog signaling. Matrix Biol. 2016, 52, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Gradilla, A.C.; González, E.; Seijo, I.; Andrés, G.; Bischoff, M.; González-Mendez, L.; Sánchez, V.; Callejo, A.; Ibáñez, C.; Guerra, M.; et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rios, P.; Guerrero, I.; Gonzalez-Reyes, A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012, 10, e1001298. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hsiung, F.; Kornberg, T.B. Specificity of Drosophila cytonemes for distinct signaling pathways. Science 2011, 332, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, T.B.; Roy, S. Cytonemes as specialized signaling filopodia. Development 2014, 141, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Rodenfels, J.; Lavrynenko, O.; Ayciriex, S.; Sampaio, J.L.; Carvalho, M.; Shevchenko, A.; Eaton, S. Production of systemically circulating Hedgehog by the intestine couples nutrition to growth and development. Genes Dev. 2014, 28, 2636–2651. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Therond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Danielian, P.S.; Muccino, D.; Rowitch, D.H.; Michael, S.K.; McMahon, A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998, 8, 1323–1326. [Google Scholar] [CrossRef]
- Fukada, K.; Shibata, S.; Suzuki, S.; Ohya, K.; Kuroda, T. In situ hybridisation study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J. Anat. 1999, 195, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell. Biol. 2012, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Ornitz, D.M. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013, 5, a008334. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhang, X.M.; Karp, S.; Yang, Y.; McMahon, A.P. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 2001, 128, 5099–5108. [Google Scholar] [PubMed]
- Kobayashi, T.; Soegiarto, D.W.; Yang, Y.; Lanske, B.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J. Clin. Investig. 2005, 115, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gu, S.; Ye, W.; Song, Y.; Chen, Y. Augmented Indian hedgehog signaling in cranial neural crest cells leads to craniofacial abnormalities and dysplastic temporomandibular joint in mice. Cell. Tissue Res. 2016, 364, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Bertolacini, C.D.; Ribeiro-Bicudo, L.A.; Petrin, A.; Richieri-Costa, A.; Murray, J.C. Clinical findings in patients with GLI2 mutations-phenotypic variability. Clin. Genet. 2012, 81, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Freer, A.M.; Zinyk, D.L.; Crackower, M.A.; Michaud, J.; Heng, H.H.; Chik, K.W.; Shi, X.M.; Tsui, L.C.; Cheng, S.H.; et al. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development 1997, 124, 113–123. [Google Scholar] [PubMed]
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 2015, 142, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wynshaw-Boris, A.; Zhou, F.; Kuo, A.; Leder, P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 1996, 84, 911–921. [Google Scholar] [CrossRef]
- Purcell, P.; Jheon, A.; Vivero, M.P.; Rahimi, H.; Joo, A.; Klein, O.D. Spry1 and spry2 are essential for development of the temporomandibular joint. J. Dent. Res. 2012, 91, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.J.; Wheatley, S.; Muscat, G.E.; Conway-Campbell, J.; Bowles, J.; Wright, E.; Bell, D.M.; Tam, P.P.; Cheah, K.S.; Koopman, P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997, 183, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.C.; Joyner, A.L. A mouse model of greig cephalopolysyndactyly syndrome: The extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet. 1993, 3, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Arensdorf, A.M.; Marada, S.; Ogden, S.K. Smoothened Regulation: A Tale of Two Signals. Trends Pharmacol. Sci. 2016, 37, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Ditzel, M. A prickly subject: Apoptotic regulation by hedgehog morphogens. Open Cell Signal. J. 2011, 3. [Google Scholar] [CrossRef]
- Robbins, D.J.; Fei, D.L.; Riobo, N.A. The Hedgehog signal transduction network. Sci. Signal. 2012, 5, re6. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Liu, H.; Plut, P.; Niu, M.; Huo, R.; Goltzman, D.; Henderson, J.E. Impaired endochondral bone development and osteopenia in Gli2-deficient mice. Exp. Cell Res. 2004, 294, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Liu, A.; Rakeman, A.S.; Murcia, N.S.; Niswander, L.; Anderson, K.V. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 2003, 426, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, Y.I.; Lin, C.; Chuang, P.T. Hedgehog signaling from the primary cilium to the nucleus: An emerging picture of ciliary localization, trafficking and transduction. Curr. Opin. Genet. Dev. 2013, 23, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kinumatsu, T.; Shibukawa, Y.; Yasuda, T.; Nagayama, M.; Yamada, S.; Serra, R.; Pacifici, M.; Koyama, E. TMJ development and growth require primary cilia function. J. Dent. Res. 2011, 90, 988–994. [Google Scholar] [CrossRef] [PubMed]
- May, S.R.; Ashique, A.M.; Karlen, M.; Wang, B.; Shen, Y.; Zarbalis, K.; Reiter, J.; Ericson, J.; Peterson, A.S. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 2005, 287, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, B.; Niswander, L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 2005, 132, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Rohr, J.; Liu, H.; He, F.; Yu, J.; Sun, C.; Li, L.; Gu, S.; Chen, Y. Tissue interaction is required for glenoid fossa development during temporomandibular joint formation. Dev. Dyn. 2011, 240, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Suda, N.; Yoda, S.; Fukuoka, H.; Ohyama, K.; Yamashita, Y.; Komori, T. Runx2-deficient mice lack mandibular condylar cartilage and have deformed Meckel’s cartilage. Anat. Embryol. 2004, 208, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Pratap, J.; Wixted, J.J.; Gaur, T.; Zaidi, S.K.; Dobson, J.; Gokul, K.D.; Hussain, S.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Runx2 transcriptional activation of Indian Hedgehog and a downstream bone metastatic pathway in breast cancer cells. Cancer Res. 2008, 68, 7795–7802. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.A.; Furuichi, T.; Fujita, T.; Fukuyama, R.; Kanatani, N.; Kobayashi, S.; Satake, M.; Takada, K.; Komori, T. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat. Genet. 2002, 32, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, H.; Yan, M.; Yang, J.; Long, F.; Muneoka, K.; Chen, Y. Shox2 is required for chondrocyte proliferation and maturation in proximal limb skeleton. Dev. Biol. 2007, 306, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Michikami, I.; Fukushi, T.; Honma, S.; Yoshioka, S.; Itoh, S.; Muragaki, Y.; Kurisu, K.; Ooshima, T.; Wakisaka, S.; Abe, M. Trps1 is necessary for normal temporomandibular joint development. Cell Tissue Res. 2012, 348, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, M.; Ditzel, M. A Joint Less Ordinary: Intriguing Roles for Hedgehog Signalling in the Development of the Temporomandibular Synovial Joint. J. Dev. Biol. 2016, 4, 25. https://doi.org/10.3390/jdb4030025
Kubiak M, Ditzel M. A Joint Less Ordinary: Intriguing Roles for Hedgehog Signalling in the Development of the Temporomandibular Synovial Joint. Journal of Developmental Biology. 2016; 4(3):25. https://doi.org/10.3390/jdb4030025
Chicago/Turabian StyleKubiak, Malgorzata, and Mark Ditzel. 2016. "A Joint Less Ordinary: Intriguing Roles for Hedgehog Signalling in the Development of the Temporomandibular Synovial Joint" Journal of Developmental Biology 4, no. 3: 25. https://doi.org/10.3390/jdb4030025
APA StyleKubiak, M., & Ditzel, M. (2016). A Joint Less Ordinary: Intriguing Roles for Hedgehog Signalling in the Development of the Temporomandibular Synovial Joint. Journal of Developmental Biology, 4(3), 25. https://doi.org/10.3390/jdb4030025