Moving the Shh Source over Time: What Impact on Neural Cell Diversification in the Developing Spinal Cord?
Abstract
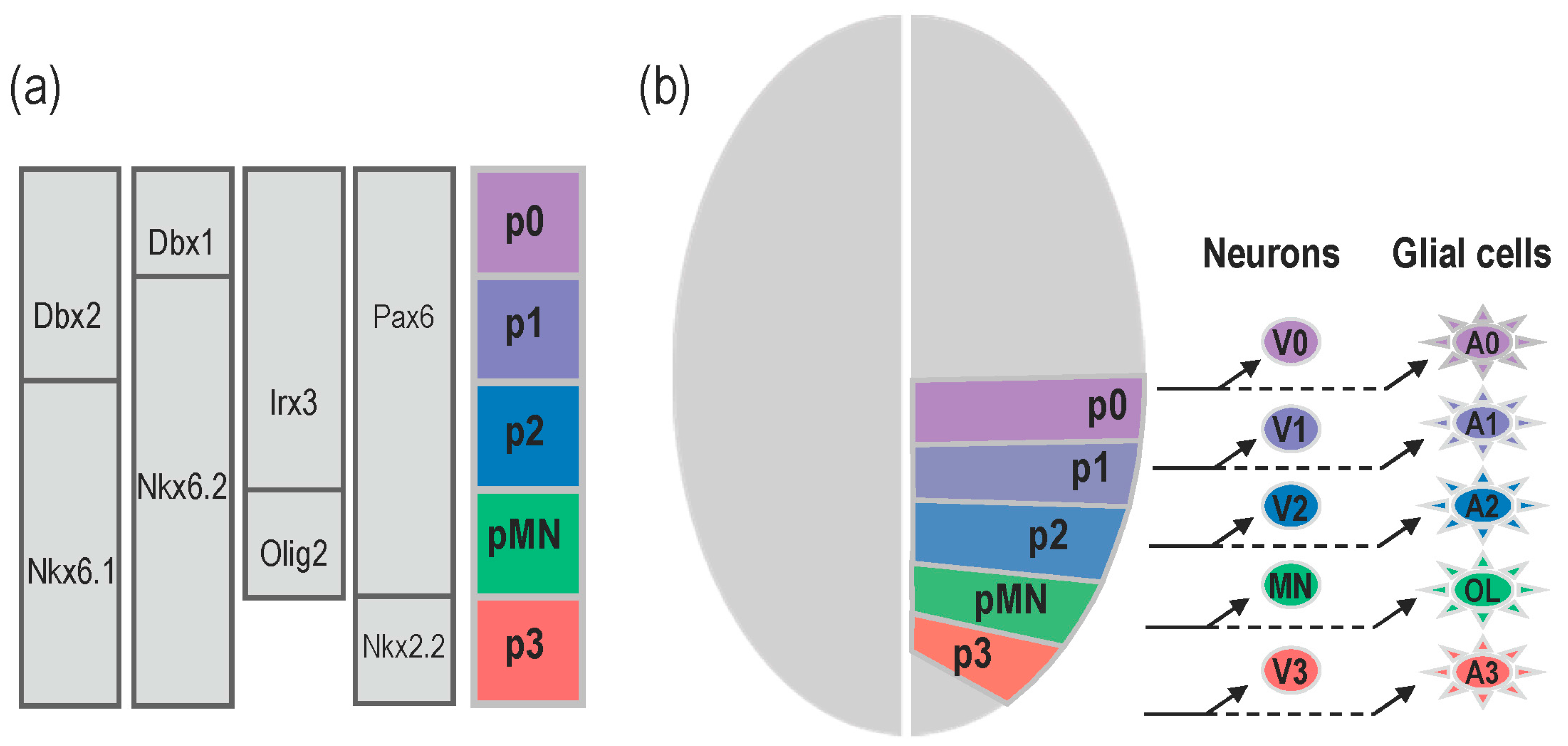
:1. Introduction
2. Specific Functions of the Notochord-Derived Shh
2.1. Shh Provided by the Notochord Triggers MFP Differentiation
2.2. Shh Provided by the Notochord Induces Neural Tube Patterning in Amniotes
2.3. Shh Provided by the Notochord Favours Progenitor Cell Proliferation in Amniotes
2.4. Role of the Notochord-Provided Shh in Zebrafish
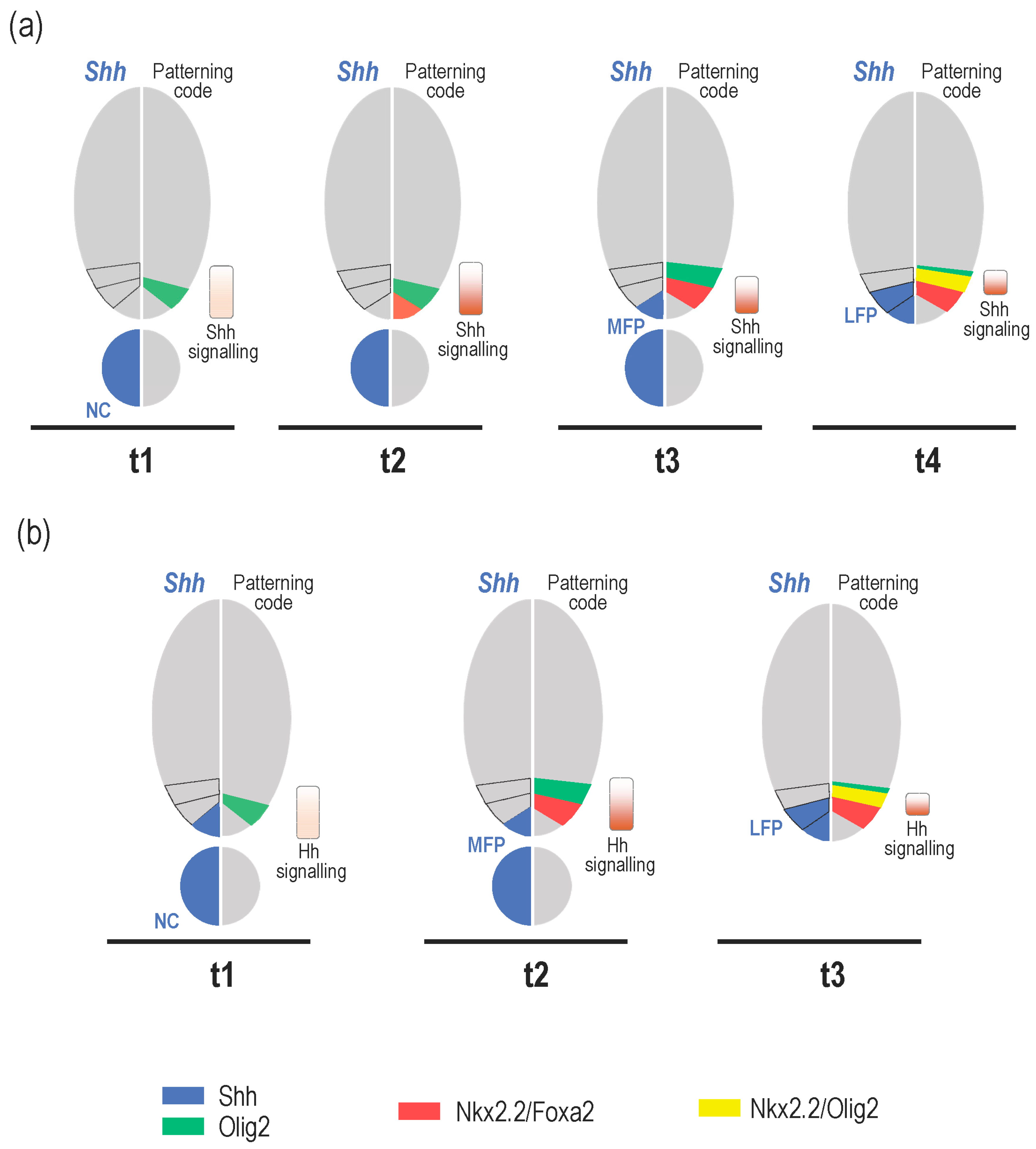
3. What Is the Relevance of Forming the MFP as a Secondary Signalling Centre?
3.1. Shh Provided by MFP Cells Is Required to Maintain Progenitor Domains in Amniotes
3.2. MFP-Provided Shh Is Required to Achieve the Neural Tube Patterning in Zebrafish
4. Role of Shh Provided by Lateral Floor Plate (LFP) Cells
4.1. Formation and Identity of LFP Cells
4.2. Is There a Specific Function for LFP-Derived Shh?
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jessell, T.M. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat. Rev. Genet. 2000, 1, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Dessaud, E.; McMahon, A.P.; Briscoe, J. Pattern formation in the vertebrate neural tube: A sonic hedgehog morphogen-regulated transcriptional network. Development 2008, 135, 2489–2503. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Zimmer, C. From cradle to grave: The multiple roles of fibroblast growth factors in neural development. Neuron 2011, 71, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Pierani, A.; Jessell, T.M.; Ericson, J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 2000, 101, 435–445. [Google Scholar] [CrossRef]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial-cell specification. Nature 2010, 468, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, Y.; Fujiwara, Y.; Orkin, S.H.; Rowitch, D.H. Specification of astrocytes by bhlh protein scl in a restricted region of the neural tube. Nature 2005, 438, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Hochstim, C.; Deneen, B.; Lukaszewicz, A.; Zhou, Q.; Anderson, D.J. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 2008, 133, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Li, H.; Fuentealba, L.C.; Molofsky, A.V.; Taveira-Marques, R.; Zhuang, H.; Tenney, A.; Murnen, A.T.; Fancy, S.P.; Merkle, F.; et al. Regional astrocyte allocation regulates cns synaptogenesis and repair. Science 2012, 337, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Anderson, D.J. The bhlh transcription factors olig2 and olig1 couple neuronal and glial subtype specification. Cell 2002, 109, 61–73. [Google Scholar] [CrossRef]
- Lu, Q.R.; Sun, T.; Zhu, Z.; Ma, N.; Garcia, M.; Stiles, C.D.; Rowitch, D.H. Common developmental requirement for olig function indicates a motor neuron/oligodendrocyte connection. Cell 2002, 109, 75–86. [Google Scholar] [CrossRef]
- Takebayashi, H.; Nabeshima, Y.; Yoshida, S.; Chisaka, O.; Ikenaka, K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 2002, 12, 1157–1163. [Google Scholar] [CrossRef]
- Park, H.C.; Mehta, A.; Richardson, J.S.; Appel, B. Olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. 2002, 248, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef]
- Roelink, H.; Augsburger, A.; Heemskerk, J.; Korzh, V.; Norlin, S.; Ruiz i Altaba, A.; Tanabe, Y.; Placzek, M.; Edlund, T.; Jessell, T.M. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 1994, 76, 761–775. [Google Scholar] [CrossRef]
- Martí, E.; Takada, R.; Bumcrot, D.A.; Sasaki, H.; McMahon, A.P. Distribution of sonic hedgehog peptides in the developing chick and mouse embryo. Development 1995, 121, 2537–2547. [Google Scholar] [PubMed]
- Roelink, H.; Porter, J.A.; Chiang, C.; Tanabe, Y.; Chang, D.T.; Beachy, P.A.; Jessell, T.M. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell 1995, 81, 445–455. [Google Scholar] [CrossRef]
- Placzek, M.; Briscoe, J. The floor plate: Multiple cells, multiple signals. Nat. Rev. Neurosci. 2005, 6, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Gritli-Linde, A.; Lewis, P.; McMahon, A.P.; Linde, A. The whereabouts of a morphogen: Direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev. Biol. 2001, 236, 364–386. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, C.E.; Jeong, J.; Guo, C.; Allen, B.L.; McMahon, A.P. Notochord-derived shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development 2008, 135, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Kicheva, A.; Ribeiro, A.; Blassberg, R.; Page, K.M.; Barnes, C.P.; Briscoe, J. Ptch1 and gli regulate shh signalling dynamics via multiple mechanisms. Nat. Commun. 2015, 6, 6709. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Thérond, P.P. The mechanisms of hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Ericson, J. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 2001, 11, 43–49. [Google Scholar] [CrossRef]
- Ribes, V.; Briscoe, J. Establishing and interpreting graded sonic hedgehog signaling during vertebrate neural tube patterning: The role of negative feedback. Cold Spring Harb. Perspect. Biol. 2009, 1, a002014. [Google Scholar] [CrossRef] [PubMed]
- Dessaud, E.; Yang, L.L.; Hill, K.; Cox, B.; Ulloa, F.; Ribeiro, A.; Mynett, A.; Novitch, B.G.; Briscoe, J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 2007, 450, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Dessaud, E.; Ribes, V.; Balaskas, N.; Yang, L.L.; Pierani, A.; Kicheva, A.; Novitch, B.G.; Briscoe, J.; Sasai, N. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. PLoS Biol. 2010, 8, e1000382. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; McMahon, A.P. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and hhip1. Development 2005, 132, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Balaskas, N.; Ribeiro, A.; Panovska, J.; Dessaud, E.; Sasai, N.; Page, K.M.; Briscoe, J.; Ribes, V. Gene regulatory logic for reading the sonic hedgehog signaling gradient in the vertebrate neural tube. Cell 2012, 148, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Dias, J.M.; Marklund, U.; Uhde, C.W.; Kurdija, S.; Lei, Q.; Sussel, L.; Rubenstein, J.L.; Matise, M.P.; Arnold, H.H.; et al. A homeodomain feedback circuit underlies step-function interpretation of a shh morphogen gradient during ventral neural patterning. Development 2010, 137, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Ribes, V.; Balaskas, N.; Sasai, N.; Cruz, C.; Dessaud, E.; Cayuso, J.; Tozer, S.; Yang, L.L.; Novitch, B.; Marti, E.; et al. Distinct sonic hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes Dev. 2010, 24, 1186–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lei, Q.; Oosterveen, T.; Ericson, J.; Matise, M.P. Tcf/lef repressors differentially regulate shh-gli target gene activation thresholds to generate progenitor patterning in the developing CNS. Development 2011, 138, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Vokes, S.A.; Ji, H.; McCuine, S.; Tenzen, T.; Giles, S.; Zhong, S.; Longabaugh, W.J.; Davidson, E.H.; Wong, W.H.; McMahon, A.P. Genomic characterization of gli-activator targets in sonic hedgehog-mediated neural patterning. Development 2007, 134, 1977–1989. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.A.; Nishi, Y.; Ma, W.; Vedenko, A.; Shokri, L.; Zhang, X.; McFarlane, M.; Baizabal, J.M.; Junker, J.P.; van Oudenaarden, A.; et al. Neural-specific sox2 input and differential gli-binding affinity provide context and positional information in shh-directed neural patterning. Genes Dev. 2012, 26, 2802–2816. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Song, J.Y.; Izzi, L.; Althaus, I.W.; Kang, J.S.; Charron, F.; Krauss, R.S.; McMahon, A.P. Overlapping roles and collective requirement for the coreceptors gas1, cdo, and boc in shh pathway function. Dev. Cell 2011, 20, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Rowitch, D.H. Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci. 2004, 5, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 2015, 8, a020453. [Google Scholar] [CrossRef] [PubMed]
- Ravanelli, A.M.; Appel, B. Motor neurons and oligodendrocytes arise from distinct cell lineages by progenitor recruitment. Genes Dev. 2015, 29, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Orentas, D.M.; Hayes, J.E.; Dyer, K.L.; Miller, R.H. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development 1999, 126, 2419–2429. [Google Scholar] [PubMed]
- Soula, C.; Danesin, C.; Kan, P.; Grob, M.; Poncet, C.; Cochard, P. Distinct sites of origin of oligodendrocytes and somatic motoneurons in the chick spinal cord: Oligodendrocytes arise from nkx2.2-expressing progenitors by a shh-dependent mechanism. Development 2001, 128, 1369–1379. [Google Scholar] [PubMed]
- Agius, E.; Soukkarieh, C.; Danesin, C.; Kan, P.; Takebayashi, H.; Soula, C.; Cochard, P. Converse control of oligodendrocyte and astrocyte lineage development by sonic hedgehog in the chick spinal cord. Dev. Biol. 2004, 270, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Shin, J.; Appel, B. Spatial and temporal regulation of ventral spinal cord precursor specification by hedgehog signaling. Development 2004, 131, 5959–5969. [Google Scholar] [CrossRef] [PubMed]
- Danesin, C.; Agius, E.; Escalas, N.; Ai, X.; Emerson, C.; Cochard, P.; Soula, C. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased sonic hedgehog (shh) activity: Involvement of sulfatase 1 in modulating shh signaling in the ventral spinal cord. J. Neurosci. 2006, 26, 5037–5048. [Google Scholar] [CrossRef] [PubMed]
- Touahri, Y.; Escalas, N.; Benazeraf, B.; Cochard, P.; Danesin, C.; Soula, C. Sulfatase 1 promotes the motor neuron-to-oligodendrocyte fate switch by activating shh signaling in olig2 progenitors of the embryonic ventral spinal cord. J. Neurosci. 2012, 32, 18018–18034. [Google Scholar] [CrossRef] [PubMed]
- Al Oustah, A.; Danesin, C.; Khouri-Farah, N.; Farreny, M.A.; Escalas, N.; Cochard, P.; Glise, B.; Soula, C. Dynamics of sonic hedgehog signaling in the ventral spinal cord are controlled by intrinsic changes in source cells requiring sulfatase 1. Development 2014, 141, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Choi, G.; Anderson, D.J. The bhlh transcription factor olig2 promotes oligodendrocyte differentiation in collaboration with nkx2.2. Neuron 2001, 31, 791–807. [Google Scholar] [CrossRef]
- Qi, Y.; Cai, J.; Wu, Y.; Wu, R.; Lee, J.; Fu, H.; Rao, M.; Sussel, L.; Rubenstein, J.; Qiu, M. Control of oligodendrocyte differentiation by the nkx2.2 homeodomain transcription factor. Development 2001, 128, 2723–2733. [Google Scholar] [PubMed]
- Fu, H.; Qi, Y.; Tan, M.; Cai, J.; Takebayashi, H.; Nakafuku, M.; Richardson, W.; Qiu, M. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of olig2 and nkx2.2 in the control of oligodendrocyte differentiation. Development 2002, 129, 681–693. [Google Scholar] [PubMed]
- Dodd, J.; Jessell, T.M.; Placzek, M. The when and where of floor plate induction. Science 1998, 282, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Placzek, M.; Dodd, J.; Jessell, T.M. Discussion point. The case for floor plate induction by the notochord. Curr. Opin. Neurobiol. 2000, 10, 15–22. [Google Scholar] [CrossRef]
- Van Straaten, H.W.; Hekking, J.W.; Wiertz-Hoessels, E.J.; Thors, F.; Drukker, J. Effect of the notochord on the differentiation of a floor plate area in the neural tube of the chick embryo. Anat. Embryol. (Berl) 1988, 177, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Schoenwolf, G.C. Notochordal induction of cell wedging in the chick neural plate and its role in neural tube formation. J. Exp. Zool. 1989, 250, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Placzek, M.; Tanaka, H.; Dodd, J.; Jessell, T.M. Control of cell pattern in the developing nervous system: Polarizing activity of the floor plate and notochord. Cell 1991, 64, 635–647. [Google Scholar] [CrossRef]
- Placzek, M.; Tessier-Lavigne, M.; Yamada, T.; Jessell, T.; Dodd, J. Mesodermal control of neural cell identity: Floor plate induction by the notochord. Science 1990, 250, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Placzek, M.; Jessell, T.M.; Dodd, J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development 1993, 117, 205–218. [Google Scholar] [PubMed]
- Bovolenta, P.; Dodd, J. Perturbation of neuronal differentiation and axon guidance in the spinal cord of mouse embryos lacking a floor plate: Analysis of danforth's short-tail mutation. Development 1991, 113, 625–639. [Google Scholar] [PubMed]
- Clarke, J.D.; Holder, N.; Soffe, S.R.; Storm-Mathisen, J. Neuroanatomical and functional analysis of neural tube formation in notochordless xenopus embryos; laterality of the ventral spinal cord is lost. Development 1991, 112, 499–516. [Google Scholar] [PubMed]
- Ruiz i Altaba, A.; Placzek, M.; Baldassare, M.; Dodd, J.; Jessell, T.M. Early stages of notochord and floor plate development in the chick embryo defined by normal and induced expression of hnf-3 beta. Dev. Biol. 1995, 170, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.; Morton, S.; Kawakami, A.; Roelink, H.; Jessell, T.M. Two critical periods of sonic hedgehog signaling required for the specification of motor neuron identity. Cell 1996, 87, 661–673. [Google Scholar] [CrossRef]
- Strähle, U.; Lam, C.S.; Ertzer, R.; Rastegar, S. Vertebrate floor-plate specification: Variations on common themes. Trends Genet. 2004, 20, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.B.; Lapointe, F.; Le Douarin, N.M.; Teillet, M.A. Dual origin of the floor plate in the avian embryo. Development 2002, 129, 4785–4796. [Google Scholar] [PubMed]
- Cho, G.; Lim, Y.; Cho, I.T.; Simonet, J.C.; Golden, J.A. Arx together with foxa2, regulates shh floor plate expression. Dev. Biol. 2014, 393, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Concordet, J.P.; Ingham, P.W. A functionally conserved homolog of the drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 1993, 75, 1431–1444. [Google Scholar] [CrossRef]
- England, S.; Batista, M.F.; Mich, J.K.; Chen, J.K.; Lewis, K.E. Roles of hedgehog pathway components and retinoic acid signalling in specifying zebrafish ventral spinal cord neurons. Development 2011, 138, 5121–5134. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Litingtung, Y.; Lee, E.; Young, K.E.; Corden, J.L.; Westphal, H.; Beachy, P.A. Cyclopia and defective axial patterning in mice lacking sonic hedgehog gene function. Nature 1996, 383, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Pierani, A.; Brenner-Morton, S.; Chiang, C.; Jessell, T.M. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell 1999, 97, 903–915. [Google Scholar] [CrossRef]
- Wijgerde, M.; McMahon, J.A.; Rule, M.; McMahon, A.P. A direct requirement for hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002, 16, 2849–2864. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.; Briscoe, J.; Rashbass, P.; van Heyningen, V.; Jessell, T.M. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb. Symp. Quant. Biol. 1997, 62, 451–466. [Google Scholar] [PubMed]
- Sasaki, H.; Hogan, B.L. Hnf-3 beta as a regulator of floor plate development. Cell 1994, 76, 103–115. [Google Scholar] [CrossRef]
- Sasaki, H.; Hui, C.; Nakafuku, M.; Kondoh, H. A binding site for gli proteins is essential for hnf-3beta floor plate enhancer activity in transgenics and can respond to shh in vitro. Development 1997, 124, 1313–1322. [Google Scholar] [PubMed]
- Jeong, Y.; Epstein, D.J. Distinct regulators of shh transcription in the floor plate and notochord indicate separate origins for these tissues in the mouse node. Development 2003, 130, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Jeong, Y.; Misra, K.; Li, S.; Zelman, A.K.; Epstein, D.J.; Matise, M.P. Wnt signaling inhibitors regulate the transcriptional response to morphogenetic shh-gli signaling in the neural tube. Dev. Cell 2006, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Oosterveen, T.; Kurdija, S.; Alekseenko, Z.; Uhde, C.W.; Bergsland, M.; Sandberg, M.; Andersson, E.; Dias, J.M.; Muhr, J.; Ericson, J. Mechanistic differences in the transcriptional interpretation of local and long-range shh morphogen signaling. Dev. Cell 2012, 23, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Tenzen, T.; Allen, B.L.; Cole, F.; Kang, J.S.; Krauss, R.S.; McMahon, A.P. The cell surface membrane proteins cdo and boc are components and targets of the hedgehog signaling pathway and feedback network in mice. Dev. Cell 2006, 10, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Tenzen, T.; McMahon, A.P. The hedgehog-binding proteins gas1 and cdo cooperate to positively regulate shh signaling during mouse development. Genes Dev. 2007, 21, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.C.; Fan, C.M. Gas1 extends the range of hedgehog action by facilitating its signaling. Genes Dev. 2007, 21, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Sasai, N.; Kutejova, E.; Briscoe, J. Integration of signals along orthogonal axes of the vertebrate neural tube controls progenitor competence and increases cell diversity. PLoS Biol. 2014, 12, e1001907. [Google Scholar] [CrossRef] [PubMed]
- Matise, M.P.; Epstein, D.J.; Park, H.L.; Platt, K.A.; Joyner, A.L. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 1998, 125, 2759–2770. [Google Scholar] [PubMed]
- Bai, C.B.; Joyner, A.L. Gli1 can rescue the in vivo function of gli2. Development 2001, 128, 5161–5172. [Google Scholar] [PubMed]
- Bai, C.B.; Auerbach, W.; Lee, J.S.; Stephen, D.; Joyner, A.L. Gli2, but not gli1, is required for initial shh signaling and ectopic activation of the shh pathway. Development 2002, 129, 4753–4761. [Google Scholar] [PubMed]
- Mansour, A.A.; Nissim-Eliraz, E.; Zisman, S.; Golan-Lev, T.; Schatz, O.; Klar, A.; Ben-Arie, N. Foxa2 regulates the expression of nato3 in the floor plate by a novel evolutionarily conserved promoter. Mol. Cell. Neurosci. 2011, 46, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.A.; Khazanov-Zisman, S.; Netser, Y.; Klar, A.; Ben-Arie, N. Nato3 plays an integral role in dorsoventral patterning of the spinal cord by segregating floor plate/p3 fates via nkx2.2 suppression and foxa2 maintenance. Development 2014, 141, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; McGlynn, S.; Matise, M.P. Floor plate-derived sonic hedgehog regulates glial and ependymal cell fates in the developing spinal cord. Development 2013, 140, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Motoyama, J.; Gasca, S.; Mo, R.; Sasaki, H.; Rossant, J.; Hui, C.C. Diminished sonic hedgehog signaling and lack of floor plate differentiation in gli2 mutant mice. Development 1998, 125, 2533–2543. [Google Scholar] [PubMed]
- Kicheva, A.; Bollenbach, T.; Ribeiro, A.; Valle, H.P.; Lovell-Badge, R.; Episkopou, V.; Briscoe, J. Coordination of progenitor specification and growth in mouse and chick spinal cord. Science 2014, 345, 1254927. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.B.; Lapointe, F.; Le Douarin, N.M.; Teillet, M.A. Anti-apoptotic role of sonic hedgehog protein at the early stages of nervous system organogenesis. Development 2001, 128, 4011–4020. [Google Scholar] [PubMed]
- Van Straaten, H.W.; Hekking, J.W. Development of floor plate, neurons and axonal outgrowth pattern in the early spinal cord of the notochord-deficient chick embryo. Anat. Embryol. (Berl) 1991, 184, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Van Straaten, H.W.; Hekking, J.W.; Beursgens, J.P.; Terwindt-Rouwenhorst, E.; Drukker, J. Effect of the notochord on proliferation and differentiation in the neural tube of the chick embryo. Development 1989, 107, 793–803. [Google Scholar] [CrossRef]
- Rowitch, D.H.; S-Jacques, B.; Lee, S.M.; Flax, J.D.; Snyder, E.Y.; McMahon, A.P. Sonic hedgehog regulates proliferation and inhibits differentiation of cns precursor cells. J. Neurosci. 1999, 19, 8954–8965. [Google Scholar] [PubMed]
- Goodrich, L.V.; Milenković, L.; Higgins, K.M.; Scott, M.P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 1997, 277, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Hynes, M.; Ye, W.; Wang, K.; Stone, D.; Murone, M.; Sauvage, F.; Rosenthal, A. The seven-transmembrane receptor smoothened cell-autonomously induces multiple ventral cell types. Nat. Neurosci. 2000, 3, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.E.; Ho, R.K.; Walker, C.; Kimmel, C.B. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 1993, 75, 99–111. [Google Scholar] [CrossRef]
- Schulte-Merker, S.; van Eeden, F.J.; Halpern, M.E.; Kimmel, C.B.; Nüsslein-Volhard, C. No tail (ntl) is the zebrafish homologue of the mouse t (brachyury) gene. Development 1994, 120, 1009–1015. [Google Scholar] [PubMed]
- Brand, M.; Heisenberg, C.P.; Warga, R.M.; Pelegri, F.; Karlstrom, R.O.; Beuchle, D.; Picker, A.; Jiang, Y.J.; Furutani-Seiki, M.; van Eeden, F.J.; et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development 1996, 123, 129–142. [Google Scholar] [PubMed]
- Schauerte, H.E.; van Eeden, F.J.; Fricke, C.; Odenthal, J.; Strahle, U.; Haffter, P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development 1998, 125, 2983–2993. [Google Scholar] [PubMed]
- Odenthal, J.; van Eeden, F.J.; Haffter, P.; Ingham, P.W.; Nüsslein-Volhard, C. Two distinct cell populations in the floor plate of the zebrafish are induced by different pathways. Dev. Biol. 2000, 219, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Currie, P.D.; Ingham, P.W. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature 1996, 382, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R.; Abouheif, E.; Meyer, A. Evolution and orthology of hedgehog genes. Trends Genet. 1996, 12, 496–497. [Google Scholar] [CrossRef]
- Ekker, S.C.; Ungar, A.R.; Greenstein, P.; von Kessler, D.P.; Porter, J.A.; Moon, R.T.; Beachy, P.A. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr. Biol. 1995, 5, 944–955. [Google Scholar] [CrossRef]
- Etheridge, L.A.; Wu, T.; Liang, J.O.; Ekker, S.C.; Halpern, M.E. Floor plate develops upon depletion of tiggy-winkle and sonic hedgehog. Genesis 2001, 30, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.E.; Eisen, J.S. Hedgehog signaling is required for primary motoneuron induction in zebrafish. Development 2001, 128, 3485–3495. [Google Scholar] [PubMed]
- Chen, W.; Burgess, S.; Hopkins, N. Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development 2001, 128, 2385–2396. [Google Scholar] [PubMed]
- Karlstrom, R.O.; Talbot, W.S.; Schier, A.F. Comparative synteny cloning of zebrafish you-too: Mutations in the hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999, 13, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.M.; Amores, A.; Lewis, K.E.; Yan, Y.L.; Postlethwait, J.H.; Eisen, J.S.; Westerfield, M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development 2001, 128, 3497–3509. [Google Scholar] [PubMed]
- Hatta, K.; Kimmel, C.B.; Ho, R.K.; Walker, C. The cyclops mutation blocks specification of the floor plate of the zebrafish central nervous system. Nature 1991, 350, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Rebagliati, M.R.; Toyama, R.; Haffter, P.; Dawid, I.B. Cyclops encodes a nodal-related factor involved in midline signaling. Proc. Natl. Acad. Sci. USA 1998, 95, 9932–9937. [Google Scholar] [CrossRef] [PubMed]
- Feldman, B.; Gates, M.A.; Egan, E.S.; Dougan, S.T.; Rennebeck, G.; Sirotkin, H.I.; Schier, A.F.; Talbot, W.S. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 1998, 395, 181–185. [Google Scholar] [PubMed]
- Schier, A.F.; Neuhauss, S.C.; Helde, K.A.; Talbot, W.S.; Driever, W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development 1997, 124, 327–342. [Google Scholar] [PubMed]
- Strähle, U.; Jesuthasan, S.; Blader, P.; Garcia-Villalba, P.; Hatta, K.; Ingham, P.W. One-eyed pinhead is required for development of the ventral midline of the zebrafish (danio rerio) neural tube. Genes Funct. 1997, 1, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Talbot, W.S.; Schier, A.F. Positional cloning identifies zebrafish one-eyed pinhead as a permissive egf-related ligand required during gastrulation. Cell 1998, 92, 241–251. [Google Scholar] [CrossRef]
- Strähle, U.; Blader, P.; Henrique, D.; Ingham, P.W. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 1993, 7, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Kinzel, D.; Neuner, C.; Schartl, M.; Volff, J.N.; Winkler, C. Hedgehog and retinoid signalling confines nkx2.2b expression to the lateral floor plate of the zebrafish trunk. Mech. Dev. 2005, 122, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Muller, F.; Fischer, N.; Biellmann, D.; Neumann, C.; Blader, P.; Strahle, U. Cyclops-independent floor plate differentiation in zebrafish embryos. Dev. Dyn. 2003, 226, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Cayuso, J.; Ulloa, F.; Cox, B.; Briscoe, J.; Martí, E. The sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating gli activity. Development 2006, 133, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Guner, B.; Karlstrom, R.O. Cloning of zebrafish nkx6.2 and a comprehensive analysis of the conserved transcriptional response to hedgehog/gli signaling in the zebrafish neural tube. Gene Expr. Patterns 2007, 7, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, S.E.; Layden, M.J.; Von Ohlen, T.; Doe, C.Q.; Eisen, J.S. Zebrafish and fly nkx6 proteins have similar cns expression patterns and regulate motoneuron formation. Development 2004, 131, 5221–5232. [Google Scholar] [CrossRef] [PubMed]
- Gribble, S.L.; Nikolaus, O.B.; Dorsky, R.I. Regulation and function of dbx genes in the zebrafish spinal cord. Dev. Dyn. 2007, 236, 3472–3483. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.E.; Bates, J.; Eisen, J.S. Regulation of iro3 expression in the zebrafish spinal cord. Dev. Dyn. 2005, 232, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, W.C.; Kalus, I.; Padva, M.; Baldwin, R.J.; Merry, C.L.; Dierks, T. The heparanome--the enigma of encoding and decoding heparan sulfate sulfation. J. Biotechnol. 2007, 129, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Wojcinski, A.; Nakato, H.; Soula, C.; Glise, B. Dsulfatase-1 fine-tunes hedgehog patterning activity through a novel regulatory feedback loop. Dev. Biol. 2011, 358, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Gorsi, B.; Whelan, S.; Stringer, S.E. Dynamic expression patterns of 6-o endosulfatases during zebrafish development suggest a subfunctionalisation event for sulf2. Dev. Dyn. 2010, 239, 3312–3323. [Google Scholar] [CrossRef] [PubMed]
- Barth, K.A.; Wilson, S.W. Expression of zebrafish nk2.2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development 1995, 121, 1755–1768. [Google Scholar] [PubMed]
- Schäfer, M.; Kinzel, D.; Winkler, C. Discontinuous organization and specification of the lateral floor plate in zebrafish. Dev. Biol. 2007, 301, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Odenthal, J.; Nüsslein-Volhard, C. Fork head domain genes in zebrafish. Dev. Genes Evol. 1998, 208, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Strähle, U.; Blader, P.; Ingham, P.W. Expression of axial and sonic hedgehog in wildtype and midline defective zebrafish embryos. Int. J. Dev. Biol. 1996, 40, 929–940. [Google Scholar] [PubMed]
- Braquart-Varnier, C.; Danesin, C.; Clouscard-Martinato, C.; Agius, E.; Escalas, N.; Benazeraf, B.; Ai, X.; Emerson, C.; Cochard, P.; Soula, C. A subtractive approach to characterize genes with regionalized expression in the gliogenic ventral neuroepithelium: Identification of chick sulfatase 1 as a new oligodendrocyte lineage gene. Mol. Cell. Neurosci. 2004, 25, 612–628. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danesin, C.; Soula, C. Moving the Shh Source over Time: What Impact on Neural Cell Diversification in the Developing Spinal Cord? J. Dev. Biol. 2017, 5, 4. https://doi.org/10.3390/jdb5020004
Danesin C, Soula C. Moving the Shh Source over Time: What Impact on Neural Cell Diversification in the Developing Spinal Cord? Journal of Developmental Biology. 2017; 5(2):4. https://doi.org/10.3390/jdb5020004
Chicago/Turabian StyleDanesin, Cathy, and Cathy Soula. 2017. "Moving the Shh Source over Time: What Impact on Neural Cell Diversification in the Developing Spinal Cord?" Journal of Developmental Biology 5, no. 2: 4. https://doi.org/10.3390/jdb5020004




