The Role of Apoptotic Signaling in Axon Guidance
Abstract
1. Introduction
Drosophila Netrin-B Is a Neurotrophic Factor That Blocks Cell Death
2. The Apoptotic Machinery and Guidance Receptors
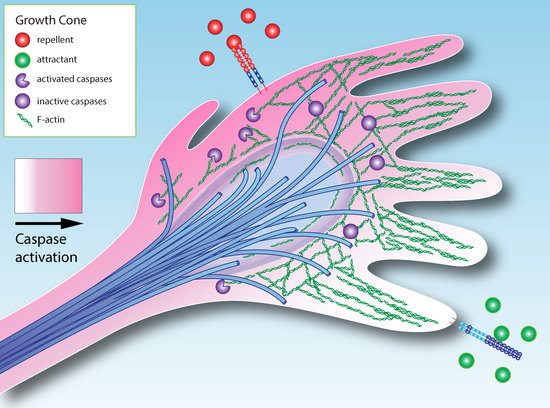
3. How Could Caspase Signaling Operate in Growth Cone Guidance?
4. Extracellular Modulation of Caspase Activity in the Growth Cone
5. Caspase Signaling at the Fly CNS Midline
6. Apoptotic Signaling in Axon Branching
7. Critical Experiments for the Activated Caspase Model
8. Conclusions
- Localized activation of caspases in the growth cone may modulate axon guidance.
- Axon attractants can promote cell survival, while repellents can promote cell death.
- Neurotrophic factor effects on axon guidance could be through caspase signaling.
- Based on an analogy with systems in which caspase signaling has non-apoptotic roles, we propose that the duration and intensity of caspase activation can modulate growth cone activity, while longer and stronger caspase activity can induce death.
- Crossing the CNS midline is associated with lower caspase activity.
- Correct wiring of the nervous system could result from the elimination of incorrectly navigating neurons due to increased activity of the cell death machinery.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lowery, L.A.; Van Vactor, D. The trip of the tip: Understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 2009, 10, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin, A.L.; Tessier-Lavigne, M. Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harbor Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.W.; Gupton, S.L.; Gertler, F.B. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harbor Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Gomez, T.M.; Letourneau, P.C. Actin dynamics in growth cone motility and navigation. J. Neurochem. 2014, 129, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Chacon, M.R.; Fazzari, P. FAK: Dynamic integration of guidance signals at the growth cone. Cell Adhes. Migr. 2011, 5, 52–55. [Google Scholar] [CrossRef]
- Dudanova, I.; Klein, R. Integration of guidance cues: Parallel signaling and crosstalk. Trends Neurosci. 2013, 36, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.G.; Kong, J.H.; Phan, K.D.; Kao, T.J.; Panaitof, S.C.; Cardin, J.; Eltzschig, H.; Kania, A.; Novitch, B.G.; Butler, S.J. Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 2017, 94, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Dominici, C.; Moreno-Bravo, J.A.; Puiggros, S.R.; Rappeneau, Q.; Rama, N.; Vieugue, P.; Bernet, A.; Mehlen, P.; Chedotal, A. Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 2017, 545, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wadsworth, W.G. SAX-3 (Robo) and UNC-40 (DCC) regulate a directional bias for axon guidance in response to multiple extracellular cues. PLoS ONE 2014, 9, e110031. [Google Scholar] [CrossRef] [PubMed]
- Stoeckli, E.T. Understanding axon guidance: Are we nearly there yet? Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Goodhill, G.J. Can Molecular Gradients Wire the Brain? Trends Neurosci. 2016, 39, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.L.W.; Correia, J.P.; Kennedy, T.E. Netrins: Versatile extracellular cues with diverse functions. Development 2011, 138, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Sabatelli, L.M.; Seeger, M.A. Guidance cues at the Drosophila CNS midline: Identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 1996, 17, 217–228. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Doyle, J.L.; Serafini, T.; Kennedy, T.E.; Tessier-Lavigne, M.; Goodman, C.S.; Dickson, B.J. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 1996, 17, 203–215. [Google Scholar] [CrossRef]
- Newquist, G.; Drennan, J.M.; Lamanuzzi, M.; Walker, K.; Clemens, J.C.; Kidd, T. Blocking apoptotic signaling rescues axon guidance in Netrin mutants. Cell Rep. 2013, 3, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.S.; Holt, C.E. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron 2003, 37, 939–952. [Google Scholar] [CrossRef]
- Ohsawa, S.; Hamada, S.; Asou, H.; Kuida, K.; Uchiyama, Y.; Yoshida, H.; Miura, M. Caspase-9 activation revealed by semaphorin 7A cleavage is independent of apoptosis in the aged olfactory bulb. J. Neurosci. 2009, 29, 11385–11392. [Google Scholar] [CrossRef] [PubMed]
- Rotschafer, S.E.; Allen-Sharpley, M.R.; Cramer, K.S. Axonal Cleaved Caspase-3 Regulates Axon Targeting and Morphogenesis in the Developing Auditory Brainstem. Front. Neural Circuits 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Forsthoefel, D.J.; Liebl, E.C.; Kolodziej, P.A.; Seeger, M.A. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development 2005, 132, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.P.; Bashaw, G.J. Distinct functional domains of the Abelson tyrosine kinase control axon guidance responses to Netrin and Slit to regulate the assembly of neural circuits. Development 2013, 140, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Dorsten, J.N.; Varughese, B.E.; Karmo, S.; Seeger, M.A.; VanBerkum, M.F. In the absence of frazzled over-expression of Abelson tyrosine kinase disrupts commissure formation and causes axons to leave the embryonic CNS. PLoS ONE 2010, 5, e9822. [Google Scholar] [CrossRef] [PubMed]
- Muda, M.; Worby, C.A.; Simonson-Leff, N.; Clemens, J.C.; Dixon, J.E. Use of double-stranded RNA-mediated interference to determine the substrates of protein tyrosine kinases and phosphatases. Biochem. J. 2002, 366, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, D.; Clemens, J.C.; Shu, H.; Worby, C.A.; Xiao, J.; Muda, M.; Dixon, J.E.; Zipursky, S.L. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell 2000, 101, 671–684. [Google Scholar] [CrossRef]
- Sterne, G.R.; Kim, J.H.; Ye, B. Dysregulated Dscam levels act through Abelson tyrosine kinase to enlarge presynaptic arbors. eLife 2015, 4, e05196. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.A.; Simonson-Leff, N.; Clemens, J.C.; Huddler, D., Jr.; Muda, M.; Dixon, J.E. Drosophila Ack targets its substrate, the sorting nexin DSH3PX1, to a protein complex involved in axonal guidance. J. Biol. Chem. 2002, 277, 9422–9428. [Google Scholar] [CrossRef] [PubMed]
- Schoenherr, J.A.; Drennan, J.M.; Martinez, J.S.; Chikka, M.R.; Hall, M.C.; Chang, H.C.; Clemens, J.C. Drosophila activated Cdc42 kinase has an anti-apoptotic function. PLoS Genet. 2012, 8, e1002725. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Levi-Montalcini, R. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J. Exp. Zool. 1949, 111, 457–501. [Google Scholar] [CrossRef] [PubMed]
- Michalak, S.M.; Whitman, M.C.; Park, J.G.; Tischfield, M.A.; Nguyen, E.H.; Engle, E.C. Ocular Motor Nerve Development in the Presence and Absence of Extraocular Muscle. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2388–2396. [Google Scholar] [CrossRef]
- Foldi, I.; Anthoney, N.; Harrison, N.; Gangloff, M.; Verstak, B.; Nallasivan, M.P.; AlAhmed, S.; Zhu, B.; Phizacklea, M.; Losada-Perez, M.; et al. Three-tier regulation of cell number plasticity by neurotrophins and Tolls in Drosophila. J. Cell Biol. 2017, 216, 1421–1438. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, G.; Foldi, I.; Aurikko, J.; Wentzell, J.S.; Lim, M.A.; Fenton, J.C.; Gay, N.J.; Hidalgo, A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci. 2013, 16, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.B.; Deppmann, C.D. The evolutionary origins of antagonistic neurotrophin signaling. J. Cell Biol. 2017, 216, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.L.G.; Salvesen, G.S. A primer on caspase mechanisms. Semin. Cell Dev. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Clavier, A.; Rincheval-Arnold, A.; Colin, J.; Mignotte, B.; Guenal, I. Apoptosis in Drosophila: Which role for mitochondria? Apoptosis 2016, 21, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.I.; Kuranaga, E. Caspase-dependent non-apoptotic processes in development. Cell Death Differ. 2017, 24, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Hay, B.A.; Wassarman, D.A.; Rubin, G.M. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 1995, 83, 1253–1262. [Google Scholar] [CrossRef]
- Hay, B.A.; Wolff, T.; Rubin, G.M. Expression of baculovirus P35 prevents cell death in Drosophila. Development 1994, 120, 2121–2129. [Google Scholar] [PubMed]
- Quinn, L.; Coombe, M.; Mills, K.; Daish, T.; Colussi, P.; Kumar, S.; Richardson, H. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. EMBO J. 2003, 22, 3568–3579. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Jones, D.; Zhou, L.; Steller, H.; Chu, Y. Deterin, a new inhibitor of apoptosis from Drosophila melanogaster. J. Biol. Chem. 2000, 275, 22157–22165. [Google Scholar] [CrossRef] [PubMed]
- Vernooy, S.Y.; Chow, V.; Su, J.; Verbrugghe, K.; Yang, J.; Cole, S.; Olson, M.R.; Hay, B.A. Drosophila Bruce can potently suppress Rpr- and Grim-dependent but not Hid-dependent cell death. Curr. Biol. 2002, 12, 1164–1168. [Google Scholar] [CrossRef]
- Bergmann, A. The role of ubiquitylation for the control of cell death in Drosophila. Cell Death Differ. 2010, 17, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kuranaga, E.; Kanuka, H.; Tonoki, A.; Takemoto, K.; Tomioka, T.; Kobayashi, M.; Hayashi, S.; Miura, M. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell 2006, 126, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Muro, I.; Hay, B.A.; Clem, R.J. The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J. Biol. Chem. 2002, 277, 49644–49650. [Google Scholar] [CrossRef] [PubMed]
- Arama, E.; Agapite, J.; Steller, H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell 2003, 4, 687–697. [Google Scholar] [CrossRef]
- Huh, J.R.; Vernooy, S.Y.; Yu, H.; Yan, N.; Shi, Y.; Guo, M.; Hay, B.A. Multiple apoptotic caspase cascades are required in nonapoptotic roles for Drosophila spermatid individualization. PLoS Biol. 2004, 2, E15. [Google Scholar] [CrossRef] [PubMed]
- Kanuka, H.; Kuranaga, E.; Takemoto, K.; Hiratou, T.; Okano, H.; Miura, M. Drosophila caspase transduces Shaggy/GSK-3beta kinase activity in neural precursor development. EMBO J. 2005, 24, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Zhu, S.; Younger, S.; Jan, L.Y.; Jan, Y.N. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron 2006, 51, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Kondo, S.; Krzyzanowska, A.; Hiromi, Y.; Truman, J.W. Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 2006, 9, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Hinck, L.; Nishiyama, M.; Poo, M.M.; Tessier-Lavigne, M.; Stein, E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 1999, 97, 927–941. [Google Scholar] [CrossRef]
- Forcet, C.; Ye, X.; Granger, L.; Corset, V.; Shin, H.; Bredesen, D.E.; Mehlen, P. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc. Natl. Acad. Sci. USA 2001, 98, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Rabizadeh, S.; Snipas, S.J.; Assa-Munt, N.; Salvesen, G.S.; Bredesen, D.E. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 1998, 395, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Castets, M.; Broutier, L.; Molin, Y.; Brevet, M.; Chazot, G.; Gadot, N.; Paquet, A.; Mazelin, L.; Jarrosson-Wuilleme, L.; Scoazec, J.Y.; et al. DCC constrains tumour progression via its dependence receptor activity. Nature 2012, 482, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Lu, X.; McKenna, W.L.; Washington, R.; Boyette, A.; Strickland, P.; Dillon, A.; Kaprielian, Z.; Tessier-Lavigne, M.; Hinck, L. UNC5A promotes neuronal apoptosis during spinal cord development independent of netrin-1. Nat. Neurosci. 2006, 9, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Furne, C.; Rama, N.; Corset, V.; Chedotal, A.; Mehlen, P. Netrin-1 is a survival factor during commissural neuron navigation. Proc. Natl. Acad. Sci. USA 2008, 105, 14465–14470. [Google Scholar] [CrossRef] [PubMed]
- Bin, J.M.; Han, D.; Lai Wing Sun, K.; Croteau, L.P.; Dumontier, E.; Cloutier, J.F.; Kania, A.; Kennedy, T.E. Complete Loss of Netrin-1 Results in Embryonic Lethality and Severe Axon Guidance Defects without Increased Neural Cell Death. Cell Rep. 2015, 12, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus-Follini, A.; Bashaw, G.J. The Intracellular Domain of the Frazzled/DCC Receptor Is a Transcription Factor Required for Commissural Axon Guidance. Neuron 2015, 87, 751–763. [Google Scholar] [CrossRef] [PubMed]
- VanZomeren-Dohm, A.; Sarro, J.; Flannery, E.; Duman-Scheel, M. The Drosophila Netrin receptor frazzled/DCC functions as an invasive tumor suppressor. BMC Dev. Biol. 2011, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Eroshkin, A.; Gramatikova, S.; Gramatikoff, K.; Zhang, Y.; Smith, J.W.; Osterman, A.L.; Godzik, A. CutDB: A proteolytic event database. Nucleic Acids Res. 2007, 35, D546–D549. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Seaman, J.E.; Agard, N.; Hsu, G.W.; Julien, O.; Mahrus, S.; Nguyen, H.; Shimbo, K.; Yoshihara, H.A.; Zhuang, M.; et al. The DegraBase: A database of proteolysis in healthy and apoptotic human cells. Mol. Cell. Proteom. 2013, 12, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Luthi, A.U.; Martin, S.J. The CASBAH: A searchable database of caspase substrates. Cell Death Differ. 2007, 14, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Mashima, T.; Naito, M.; Noguchi, K.; Miller, D.K.; Nicholson, D.W.; Tsuruo, T. Actin cleavage by CPP-32/apopain during the development of apoptosis. Oncogene 1997, 14, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.; Sytnyk, V.; Schachner, M.; Leshchyns’ka, I. Clustering of the neural cell adhesion molecule (NCAM) at the neuronal cell surface induces caspase-8- and -3-dependent changes of the spectrin meshwork required for NCAM-mediated neurite outgrowth. J. Biol. Chem. 2010, 285, 42046–42057. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.N.; Baehrecke, E.H. Caspases function in autophagic programmed cell death in Drosophila. Development 2004, 131, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Akhter, A.; Caution, K.; Abu Khweek, A.; Tazi, M.; Abdulrahman, B.A.; Abdelaziz, D.H.; Voss, O.H.; Doseff, A.I.; Hassan, H.; Azad, A.K.; et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 2012, 37, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brieher, W.M.; Scimone, M.L.; Kang, S.J.; Zhu, H.; Yin, H.; von Andrian, U.H.; Mitchison, T.; Yuan, J. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat. Cell Biol. 2007, 9, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Jiao, S.; Jia, J.M.; Chen, Y.; Chen, C.Y.; Gucek, M.; Markey, S.P.; Li, Z. The novel caspase-3 substrate Gap43 is involved in AMPA receptor endocytosis and long-term depression. Mol. Cell. Proteom. 2013, 12, 3719–3731. [Google Scholar] [CrossRef] [PubMed]
- Geisbrecht, E.R.; Montell, D.J. A role for Drosophila IAP1-mediated caspase inhibition in Rac-dependent cell migration. Cell 2004, 118, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Koto, A.; Kuranaga, E.; Miura, M. Temporal regulation of Drosophila IAP1 determines caspase functions in sensory organ development. J. Cell Biol. 2009, 187, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Takeda, M.; Kuranaga, E.; Ueda, R.; Aigaki, T.; Miura, M.; Hayashi, S. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Curr. Biol. 2006, 16, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garijo, A.; Shlevkov, E.; Morata, G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development 2009, 136, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Rudrapatna, V.A.; Bangi, E.; Cagan, R.L. Caspase signalling in the absence of apoptosis drives Jnk-dependent invasion. EMBO Rep. 2013, 14, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Portela, M.; Richardson, H.E. Death takes a holiday—Non-apoptotic role for caspases in cell migration and invasion. EMBO Rep. 2013, 14, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.L.; Espinosa, J.S.; Xu, Y.; Davidson, N.; Kovacs, G.T.; Barres, B.A. Retinal ganglion cells do not extend axons by default: Promotion by neurotrophic signaling and electrical activity. Neuron 2002, 33, 689–702. [Google Scholar] [CrossRef]
- Lentz, S.I.; Knudson, C.M.; Korsmeyer, S.J.; Snider, W.D. Neurotrophins support the development of diverse sensory axon morphologies. J. Neurosci. 1999, 19, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Michod, D.; Walicki, J.; Murphy, B.M.; Kasibhatla, S.; Martin, S.J.; Widmann, C. Partial cleavage of RasGAP by caspases is required for cell survival in mild stress conditions. Mol. Cell. Biol. 2004, 24, 10425–10436. [Google Scholar] [CrossRef] [PubMed]
- Gilman, C.P.; Mattson, M.P. Do apoptotic mechanisms regulate synaptic plasticity and growth-cone motility? Neuromol. Med. 2002, 2, 197–214. [Google Scholar] [CrossRef]
- Campbell, D.S.; Okamoto, H. Local caspase activation interacts with Slit-Robo signaling to restrict axonal arborization. J. Cell Biol. 2013, 203, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Igaki, T.; Yamamoto-Goto, Y.; Tokushige, N.; Kanda, H.; Miura, M. Down-regulation of DIAP1 triggers a novel Drosophila cell death pathway mediated by Dark and DRONC. J. Biol. Chem. 2002, 277, 23103–23106. [Google Scholar] [CrossRef] [PubMed]
- Muro, I.; Means, J.C.; Clem, R.J. Cleavage of the apoptosis inhibitor DIAP1 by the apical caspase DRONC in both normal and apoptotic Drosophila cells. J. Biol. Chem. 2005, 280, 18683–18688. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Chen, P.; Oliver, H.; Abrams, J.M. Unrestrained caspase-dependent cell death caused by loss of Diap1 function requires the Drosophila Apaf-1 homolog, Dark. EMBO J. 2002, 21, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.C.; Ricci, J.E.; Droin, N.M.; Green, D.R. The role of ARK in stress-induced apoptosis in Drosophila cells. J. Cell Biol. 2002, 156, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Linhoff, M.W.; Potts, P.R.; Deshmukh, M. Decreased apoptosome activity with neuronal differentiation sets the threshold for strict IAP regulation of apoptosis. J. Cell Biol. 2004, 167, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Erturk, A.; Wang, Y.; Sheng, M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J. Neurosci. 2014, 34, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Hollville, E.; Deshmukh, M. Physiological functions of non-apoptotic caspase activity in the nervous system. Semin. Cell Dev. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Delloye-Bourgeois, C.; Chedotal, A. Novel roles for Slits and netrins: Axon guidance cues as anticancer targets? Nat. Rev. Cancer 2011, 11, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Gara, R.K.; Kumari, S.; Ganju, A.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Slit/Robo pathway: A promising therapeutic target for cancer. Drug Discov. Today 2015, 20, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Colin, J.; Garibal, J.; Clavier, A.; Szuplewski, S.; Risler, Y.; Milet, C.; Gaumer, S.; Guenal, I.; Mignotte, B. Screening of suppressors of bax-induced cell death identifies glycerophosphate oxidase-1 as a mediator of debcl-induced apoptosis in Drosophila. Genes Cancer 2015, 6, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Manhire-Heath, R.; Golenkina, S.; Saint, R.; Murray, M.J. Netrin-dependent downregulation of Frazzled/DCC is required for the dissociation of the peripodial epithelium in Drosophila. Nat. Commun. 2013, 4, 2790. [Google Scholar] [CrossRef] [PubMed]
- Vaughen, J.; Igaki, T. Slit-Robo Repulsive Signaling Extrudes Tumorigenic Cells from Epithelia. Dev. Cell 2016, 39, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Manor, O.; Schachner, M.; Yaron, A.; Tessier-Lavigne, M.; Behar, O. The Semaphorin receptor PlexinA3 mediates neuronal apoptosis during dorsal root ganglia development. J. Neurosci. 2008, 28, 12427–12432. [Google Scholar] [CrossRef] [PubMed]
- Wehner, A.B.; Abdesselem, H.; Dickendesher, T.L.; Imai, F.; Yoshida, Y.; Giger, R.J.; Pierchala, B.A. Semaphorin 3A is a retrograde cell death signal in developing sympathetic neurons. Development 2016, 143, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.; Kang, Y.S.; Park, S. EphA receptors form a complex with caspase-8 to induce apoptotic cell death. Mol. Cells 2015, 38, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Liu, J.; Luo, H.; Gou, K.; Cui, S. Prostaglandin F2alpha upregulates Slit/Robo expression in mouse corpus luteum during luteolysis. J. Endocrinol. 2013, 218, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.E.; Fegan, K.S.; Ren, X.; Hillier, S.G.; Duncan, W.C. Glucocorticoid regulation of SLIT/ROBO tumour suppressor genes in the ovarian surface epithelium and ovarian cancer cells. PLoS ONE 2011, 6, e27792. [Google Scholar] [CrossRef] [PubMed]
- Winberg, M.L.; Mitchell, K.J.; Goodman, C.S. Genetic analysis of the mechanisms controlling target selection: Complementary and combinatorial functions of netrins, semaphorins, and IgCAMs. Cell 1998, 93, 581–591. [Google Scholar] [CrossRef]
- Jiao, S.; Li, Z. Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron 2011, 70, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Serradj, N.; Ueno, M.; Liang, M.; Li, J.; Baccei, M.L.; Martin, J.H.; Yoshida, Y. Skilled Movements Require Non-apoptotic Bax/Bak Pathway-Mediated Corticospinal Circuit Reorganization. Neuron 2017, 94, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Truman, J.W. Cellular mechanisms of dendrite pruning in Drosophila: Insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development 2005, 132, 3631–3642. [Google Scholar] [CrossRef] [PubMed]
- Rogulja-Ortmann, A.; Luer, K.; Seibert, J.; Rickert, C.; Technau, G.M. Programmed cell death in the embryonic central nervous system of Drosophila melanogaster. Development 2007, 134, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Williams, D.W. More alive than dead: Non-apoptotic roles for caspases in neuronal development, plasticity and disease. Cell Death Differ. 2017, 24, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Bergmann, A. The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila. Cell Death Differ. 2010, 17, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Kidd, T.; Brose, K.; Mitchell, K.J.; Fetter, R.D.; Tessier-Lavigne, M.; Goodman, C.S.; Tear, G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998, 92, 205–215. [Google Scholar] [CrossRef]
- Brankatschk, M.; Dickson, B.J. Netrins guide Drosophila commissural axons at short range. Nat. Neurosci. 2006, 9, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Pennack, J.A.; McQuilton, P.; Forero, M.G.; Mizuguchi, K.; Sutcliffe, B.; Gu, C.J.; Fenton, J.C.; Hidalgo, A. Drosophila neurotrophins reveal a common mechanism for nervous system formation. PLoS Biol. 2008, 6, e284. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tessier-Lavigne, M. En passant neurotrophic action of an intermediate axonal target in the developing mammalian CNS. Nature 1999, 401, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Watanabe, K.; Ono, K.; Tomita, K.; Tamamaki, N.; Ikenaka, K.; Takebayashi, H. Role of motoneuron-derived neurotrophin 3 in survival and axonal projection of sensory neurons during neural circuit formation. Development 2012, 139, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, R.; Zweifel, L.S.; Glebova, N.O.; Lonze, B.E.; Valdez, G.; Ye, H.; Ginty, D.D. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 2004, 118, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, B.; Forero, M.G.; Zhu, B.; Robinson, I.M.; Hidalgo, A. Neuron-type specific functions of DNT1, DNT2 and Spz at the Drosophila neuromuscular junction. PLoS ONE 2013, 8, e75902. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A.; Tugentman, M.; Shilo, B.Z.; Steller, H. Regulation of cell number by MAPK-dependent control of apoptosis: A mechanism for trophic survival signaling. Dev. Cell 2002, 2, 159–170. [Google Scholar] [CrossRef]
- Goldberg, J.L. How does an axon grow? Genes Dev. 2003, 17, 941–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; Brose, K.; Arnott, D.; Kidd, T.; Goodman, C.S.; Henzel, W.; Tessier-Lavigne, M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 1999, 96, 771–784. [Google Scholar] [CrossRef]
- Nguyen Ba-Charvet, K.T.; Brose, K.; Ma, L.; Wang, K.H.; Marillat, V.; Sotelo, C.; Tessier-Lavigne, M.; Chedotal, A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J. Neurosci. 2001, 21, 4281–4289. [Google Scholar] [CrossRef] [PubMed]
- Dascenco, D.; Erfurth, M.-L.; Izadifar, A.; Song, M.; Sachse, S.; Bortnick, R.; Urwyler, O.; Petrovic, M.; Ayaz, D.; He, H.; et al. Slit and Receptor Tyrosine Phosphatase 69D Confer Spatial Specificity to Axon Branching via Dscam1. Cell 2015. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.; Nurcombe, V.; Reid, K.; Bartlett, P.; Little, M. N-terminal Slit2 promotes survival and neurite extension in cultured peripheral neurons. Neuroreport 2002, 13, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zheng, B. Axon plasticity in the mammalian central nervous system after injury. Trends Neurosci. 2014, 37, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Yoshida, W.; Toriyama, M.; Shimada, T.; Manning, C.F.; Saito, M.; Kohno, K.; Trimmer, J.S.; Watanabe, R.; Inagaki, N. Gradient-reading and mechano-effector machinery for netrin-1-induced axon guidance. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, D.; Sekine, S.; Barsi-Rhyne, B.; Hu, J.; Chen, B.; Gilbert, L.A.; Ishikawa, H.; Leonetti, M.D.; Marshall, W.F.; Weissman, J.S.; et al. Versatile protein tagging in cells with split fluorescent protein. Nat. Commun. 2016, 7, 11046. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, D.; McGorty, R.; Kamiyama, R.; Kim, M.D.; Chiba, A.; Huang, B. Specification of Dendritogenesis Site in Drosophila aCC Motoneuron by Membrane Enrichment of Pak1 through Dscam1. Dev. Cell 2015, 35, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Geden, M.J.; Deshmukh, M. Axon degeneration: Context defines distinct pathways. Curr. Opin. Neurobiol. 2016, 39, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Bardet, P.L.; Kolahgar, G.; Mynett, A.; Miguel-Aliaga, I.; Briscoe, J.; Meier, P.; Vincent, J.P. A fluorescent reporter of caspase activity for live imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 13901–13905. [Google Scholar] [CrossRef] [PubMed]
- Melzer, J.; Broemer, M. Nerve-racking—Apoptotic and non-apoptotic roles of caspases in the nervous system of Drosophila. Eur. J. Neurosci. 2016, 44, 1683–1690. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellermeyer, R.; Heydman, L.M.; Mastick, G.S.; Kidd, T. The Role of Apoptotic Signaling in Axon Guidance. J. Dev. Biol. 2018, 6, 24. https://doi.org/10.3390/jdb6040024
Kellermeyer R, Heydman LM, Mastick GS, Kidd T. The Role of Apoptotic Signaling in Axon Guidance. Journal of Developmental Biology. 2018; 6(4):24. https://doi.org/10.3390/jdb6040024
Chicago/Turabian StyleKellermeyer, Riley, Leah M. Heydman, Grant S. Mastick, and Thomas Kidd. 2018. "The Role of Apoptotic Signaling in Axon Guidance" Journal of Developmental Biology 6, no. 4: 24. https://doi.org/10.3390/jdb6040024
APA StyleKellermeyer, R., Heydman, L. M., Mastick, G. S., & Kidd, T. (2018). The Role of Apoptotic Signaling in Axon Guidance. Journal of Developmental Biology, 6(4), 24. https://doi.org/10.3390/jdb6040024