Anti-Austerity Activity of Thai Medicinal Plants: Chemical Constituents and Anti-Pancreatic Cancer Activities of Kaempferia parviflora
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anti-Austerity Activity of Thai Medicinal Plants
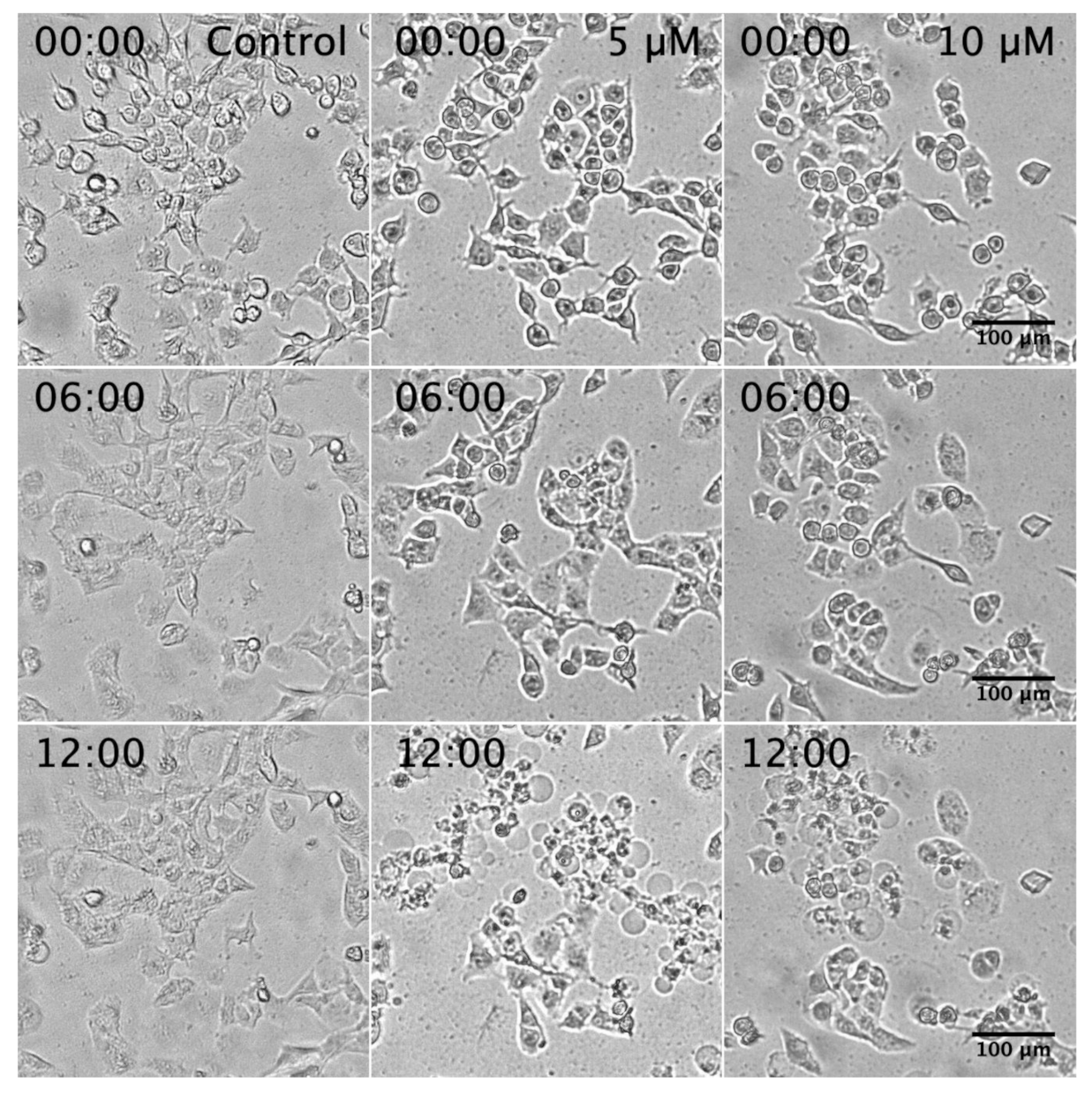
2.2. Assessment of PANC-1 Cell Death Induced by K. parviflora Extract
2.3. Effect of K. parviflora Extract on PANC-1 Colony Formation
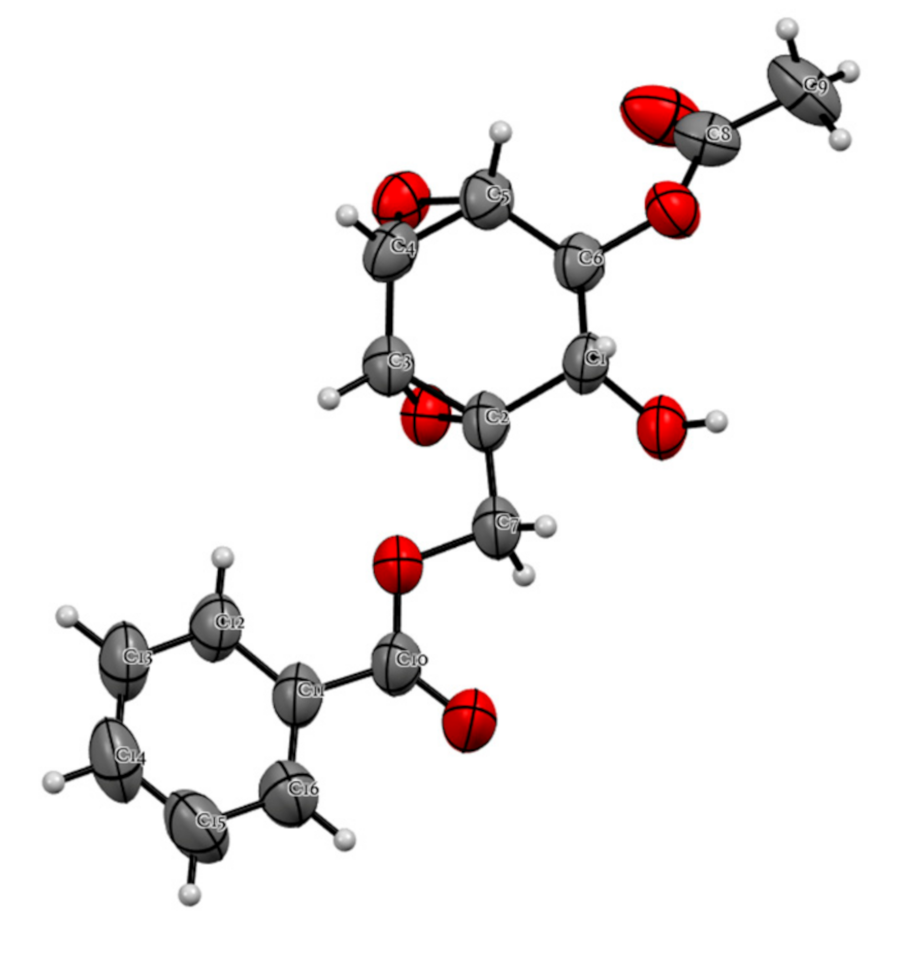
2.4. Chemical Constituents of K. parviflora and Their Anti-Austerity Activity against the PANC-1 Human Pancreatic Cancer Cells
2.5. Investigation of the Mechanism of Cell Death Induced by 5-Hydroxy-7-Methoxyflavone (3) in NDM
2.6. Effect of 5-Hydroxy-7-methoxyflavone (3) on PANC-1 Colony Formation in DMEM
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Phytochemical Investigation of K. parviflora
3.4. Chemicals and Antibodies
3.5. Cell Line and Cell Culture
3.6. Preferential Cytotoxicity against PANC-1 Pancreatic Cancer Cells
3.7. Morphological Analysis
3.8. Colony Formation Inhibition Assay
3.9. Western Blot Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foundation for Promotion of Cancer Research (FPCR). Cancer Statistics in Japan 2019. 2020. Available online: http://ganjoho.jp/en/professional/statistics/brochure/index.html (accessed on 15 October 2020).
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Bauden, M.; et al. Pancreatic cancer: Yesterday, today and tomorrow. Future Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutsem, E.V.; Vervenne, W.L.; Bennouna, J.; Humblet, Y.; Gill, S.; Laethem, J.L.V.; Verslype, C.; Scheithauer, W.; Shang, A.; Cosaert, J.; et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2009, 27, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Izuishi, K.; Kato, K.; Ogura, T.; Esumi, H. Remarkable tolerance of tumor cells to nutrient deprivation: Possible new biochemical target for cancer therapy. Cancer Res. 2000, 60, 6201–6207. [Google Scholar] [PubMed]
- Awale, S.; Linn, T.Z.; Li, F.; Tezuka, Y.; Myint, A.; Tomida, A.; Yamori, T.; Esumi, H.; Kadota, S. Identification of chrysoplenetin from vitex negundo as a potential cytotoxic agent against PANC-1 and a Panel of 39 human cancer cell lines (JFCR-39). Phytother. Res. 2011, 25, 1770–1775. [Google Scholar] [CrossRef]
- Awale, S.; Lu, J.; Kalauni, S.K.; Kurashima, Y.; Tezuka, Y.; Kadota, S.; Esumi, H. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res. 2006, 66, 1751–1757. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.; Athikomkulchai, S.; Miyatake, R.; Saiki, I.; Esumi, H.; Awale, S. (+)-Grandifloracin, an anti-austerity agent, induces autophagic PANC-1 pancreatic cancer cell death. Drug Des. Dev. Ther. 2014, 8, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.X.; Do, T.N.V.; Le, T.H.; Nguyen, M.T.T.; Nguyen, N.T.; Esumi, H.; Awale, S. Chemical constituents of Mangifera indica and their anti-austerity activity against the PANC-1 human pancreatic cancer cell line. J. Nat. Prod. 2016, 79, 2053–2059. [Google Scholar] [CrossRef]
- Awale, S.; Dibwe, D.F.; Balachandran, C.; Fayez, S.; Feineis, D.; Lombe, B.K.; Bringmann, G. Ancistrolikokine E3, a 5,8’-coupled naphthylisoquinoline alkaloid, eliminates the tolerance of cancer cells to nutrition starvation by inhibition of the Akt/mTOR/Autophagy signaling pathway. J. Nat. Prod. 2018, 81, 2282–2291. [Google Scholar] [CrossRef]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef]
- Rujjanawate, C.; Kanjanapothi, D.; Amornlerdpison, D.; Pojanagaroon, S. Anti-gastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol. 2005, 102, 120–122. [Google Scholar] [CrossRef]
- Potikanond, S.; Sookkhee, S.; Takuathung, M.N.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia parviflora extract exhibits anti-cancer activity against Hela cervical cancer cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramee, S.; Sookkhee, S.; Sakonwasun, C.; Takuathung, M.N.; Mungkornasawakul, P.; Nimlamool, W.; Potikanond, S. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complementary Altern. Med. 2018, 18, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanasombat, S.; Teckchuen, N. Antimicrobial, antioxidant and anticancer activities of Thai local vegetables. J. Med. Plant Res. 2009, 3, 443–449. [Google Scholar] [CrossRef]
- Sun, S.; Phrutivorapongkul, A.; Dibwe, D.F.; Balachandran, C.; Awale, S. Chemical constituents of Thai Citrus hystrix and their anti-austerity activity against the PANC-1 human pancreatic cancer cell line. J. Nat. Prod. 2018, 81, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Pancharoen, O.; Tuntiwachwuttikul, P.; Taylor, W.C. Cyclohexane diepoxides from Kaempferia rotunda. Phytochemistry 1996, 43, 305–308. [Google Scholar] [CrossRef]
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 1143, 227–233. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Lee, C.; Chong, Y. Synthesis of a complete series of O-Methyl analogues of naringenin and apigenin. Bull. Korean Chem. Soc. 2007, 28, 2527–2530. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedula, V.S.P.; Prakash, I. Isolation of stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2002, 1, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Chhetri, B.K.; Lavoie, S.; Sweeney-Jones, A.M.; Kubanek, J. Recent trends in the structural revision of natural products. Nat. Prod. Rep. 2018, 35, 514–531. [Google Scholar] [CrossRef]
- Mcllwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeb, A.; Haque, S.U.; Olowokure, O. Pulmonary metastases in pancreatic cancer, is there a survival influence? J. Gastrointest. Oncol. 2015, 6, E48–E51. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Bagga, M.; Kaur, A.; Kaur, A.; Westermarck, J.; Abankwa, D. ColonyArea: An imageJ plugin to automatically quantify colony formation in clonogenic assays. PLoS ONE 2014, 9, e92444. [Google Scholar] [CrossRef] [PubMed]






| Scientific Name | Part Used | Family | Extraction Solvent | Preferential Cytotoxicity a |
|---|---|---|---|---|
| Phlogacanthuspulcherrimus | leaves | Acanthaceae | 70% EtOH | >100 |
| Clinacanthus nutans | leaves | Acanthaceae | 70% EtOH | >100 |
| Polyscias fruticosa | shoot | Araliaceae | 70% EtOH | 12.0 ± 1.5 |
| Eupatorium stoechadosmum | leaves | Asteraceae | 70% EtOH | >100 |
| Gymnema inodorum | leaves | Asclepiadaceae | 70% EtOH | 38.0 ± 1.7 |
| Dolichandrone serrulata | flowers | Bignoniaceae | 70% EtOH | >100 |
| Plukenetia volubilis | endocarps | Euphorbiaceae | 70% EtOH | 71.5 ± 1.5 |
| Plectranthus amboinicus | leaves | Lamiaceae | 70% EtOH | 88.9 ± 1.8 |
| Hibiscus sabdariffa | calyx | Malvaceae | 70% EtOH | >100 |
| Derris scandens | flowers | Papilionaceae | 95% EtOH | 0.8 ± 0.2 |
| Antidesma thwaitesianum | leaves | Phyllanthaceae | 70% EtOH | >100 |
| Antidesma thwaitesianum | fruits | Phyllanthaceae | 95% EtOH | 90.8 ± 1.7 |
| Piper sarmentosum | leaves | Piperaceae | 70% EtOH | 91.9 ± 1.8 |
| Boesenbergia pandurata | rhizomes | Zingiberaceae | 100% CH2Cl2 | 0.5 ± 0.1 |
| Polygonum odoratum | leaves & twigs | Polgonaceae | 70% EtOH | 31.3 ± 1.4 |
| Breynia vitis-idaea | leaves | Euphorbiaceae | 70% EtOH | 87.9 ± 1.3 |
| Morinda citrifolia | fruits | Rubiaceae | 70% EtOH | >100 |
| Murraya paniculata | leaves | Rutaceae | 70% EtOH | 44.9 ± 1.8 |
| Clausena anisata | leaves | Rutaceae | 70% EtOH | >100 |
| Citrus hystrix | leaves | Rutaceae | 70% EtOH | 56.4 ± 1.6 |
| Citrus hystrix | fruit peels | Rutaceae | 70% EtOH | 8.9 ± 1.4 |
| Zanthoxylum myriacanthum | pericarps | Rutaceae | 70% EtOH | 21.7 ± 2.1 |
| Zanthoxylum myriacanthum | leaves | Rutaceae | 70% EtOH | 81.3 ± 1.8 |
| Houttuynia cordata | leaves | Saururaceae | 70% EtOH | >100 |
| Kaempferia parviflora | rhizomes | Zingiberaceae | 100% CH2Cl2 | 3.3 ± 0.5 |
| Position | Compound 1 | |
|---|---|---|
| δC, Type | δH (J in Hz) | |
| 1 | 56.0, C | |
| 2 | 66.0, CH | 4.13, dd (10.4, 4.8) |
| 3 | 69.8, CH | 5.17, dd (4.8, 2.8) |
| 4 | 51.0, CH | 3.31, t (2.8) |
| 5 | 48.0, CH | 3.55, t (2.8) |
| 6 | 53.8, CH | 3.70, d (2.8) |
| 7a | 64.8, CH2 | 4.54, d (12.0) |
| 7b | 64.8, CH2 | 4.42, d (12.0) |
| 1’ | 129.4, C | |
| 2’,6’ | 129.9, CH | 8.05, m |
| 3’,5’ | 128.7, CH | 7.47, m |
| 4’ | 133.7, CH | 7.59, m |
| C=O | 166.1, C | |
| C=O | 170.0, C | |
| 2-OH | 2.69, d (10.4) | |
| Me | 20.6, CH3 | 1.89, s |
| Compound | PC50, μM a | Compound | PC50, μM a |
|---|---|---|---|
| Extract | 3.3 ± 0.5 μg/mL | ||
| 1 | >100 | 9 | 50.3 ± 1.5 |
| 2 | >100 | 10 | 85.3 ± 1.8 |
| 3 | 0.8 ± 0.1 | 11 | >100 |
| 4 | >100 | 12 | >100 |
| 5 | 16.0 ± 1.3 | 13 | 63.7 ± 1.5 |
| 6 | 73.2 ± 2.2 | 14 | >100 |
| 7 | >100 | Arctigenin Should b | 0.8 ± 0.2 |
| 8 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Kim, M.J.; Dibwe, D.F.; Omar, A.M.; Athikomkulchai, S.; Phrutivorapongkul, A.; Okada, T.; Tsuge, K.; Toyooka, N.; Awale, S. Anti-Austerity Activity of Thai Medicinal Plants: Chemical Constituents and Anti-Pancreatic Cancer Activities of Kaempferia parviflora. Plants 2021, 10, 229. https://doi.org/10.3390/plants10020229
Sun S, Kim MJ, Dibwe DF, Omar AM, Athikomkulchai S, Phrutivorapongkul A, Okada T, Tsuge K, Toyooka N, Awale S. Anti-Austerity Activity of Thai Medicinal Plants: Chemical Constituents and Anti-Pancreatic Cancer Activities of Kaempferia parviflora. Plants. 2021; 10(2):229. https://doi.org/10.3390/plants10020229
Chicago/Turabian StyleSun, Sijia, Min Jo Kim, Dya Fita Dibwe, Ashraf M. Omar, Sirivan Athikomkulchai, Ampai Phrutivorapongkul, Takuya Okada, Kiyoshi Tsuge, Naoki Toyooka, and Suresh Awale. 2021. "Anti-Austerity Activity of Thai Medicinal Plants: Chemical Constituents and Anti-Pancreatic Cancer Activities of Kaempferia parviflora" Plants 10, no. 2: 229. https://doi.org/10.3390/plants10020229








