Biochar Improves Soil-Tomato Plant, Tomato Production, and Economic Benefits under Reduced Nitrogen Application in Northwestern China
Abstract
:1. Introduction
2. Methods and Materials
2.1. Experimental Site and Materials
2.2. Experimental Setup
2.3. Measurements
2.3.1. Soil Enzyme Activity and Microbial Abundance
2.3.2. Tomato Leaf Gas Exchange Parameters
2.3.3. Tomato Growth and Biomass
2.3.4. Tomato Quality, Yield, and Partial-Factor Productivity of N Fertilizer
2.3.5. The Yield, Quality of Tomatoes, and Economic Benefit Model
2.4. Statistical Analysis
3. Results
3.1. PCA Analysis of Soil‒Plant Parameters as Affected by the Treatments
3.2. Soil Microbial Abundance and Enzyme Activity
3.3. Leaf Gas Exchange Parameters
3.4. Tomato Growth and Biomass
3.5. Tomato Quality, Yield of Tomato, and Partial-Factor Productivity for N
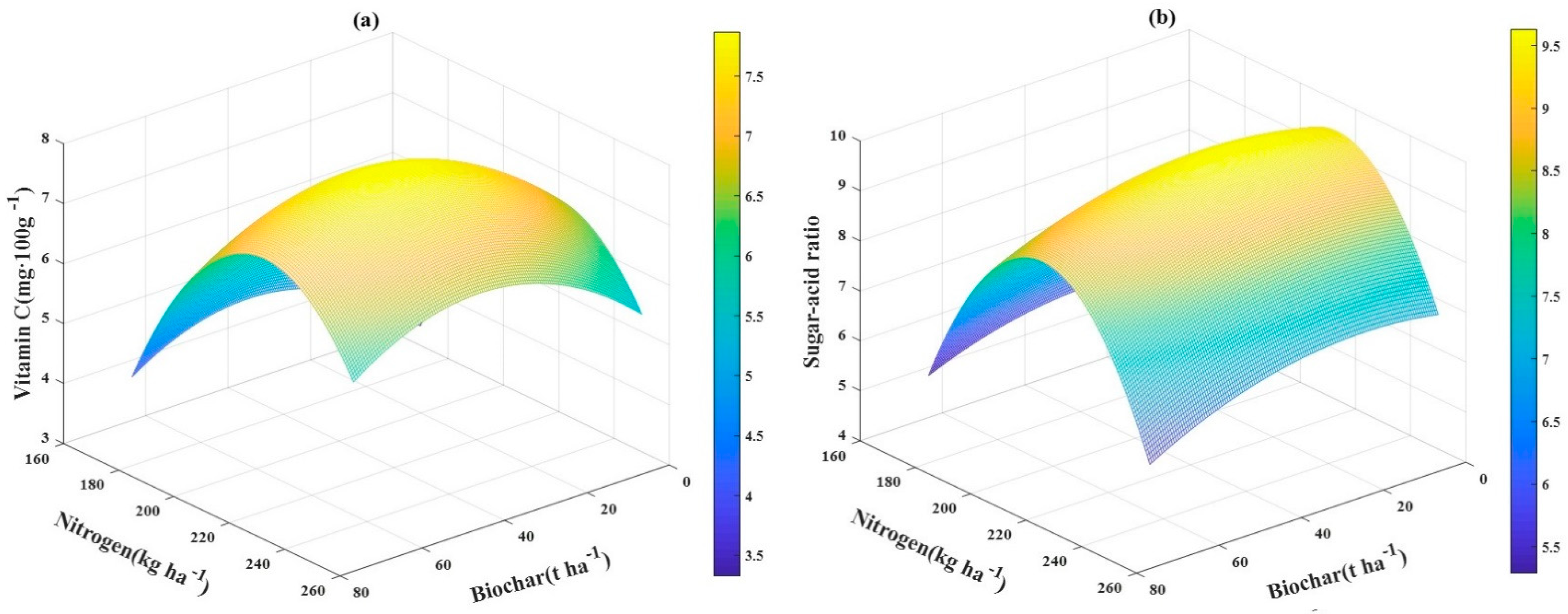
3.6. The Relationship between VC and Sugar–Acid Ratio and Application Rates of Biochar‒N Fertilizer
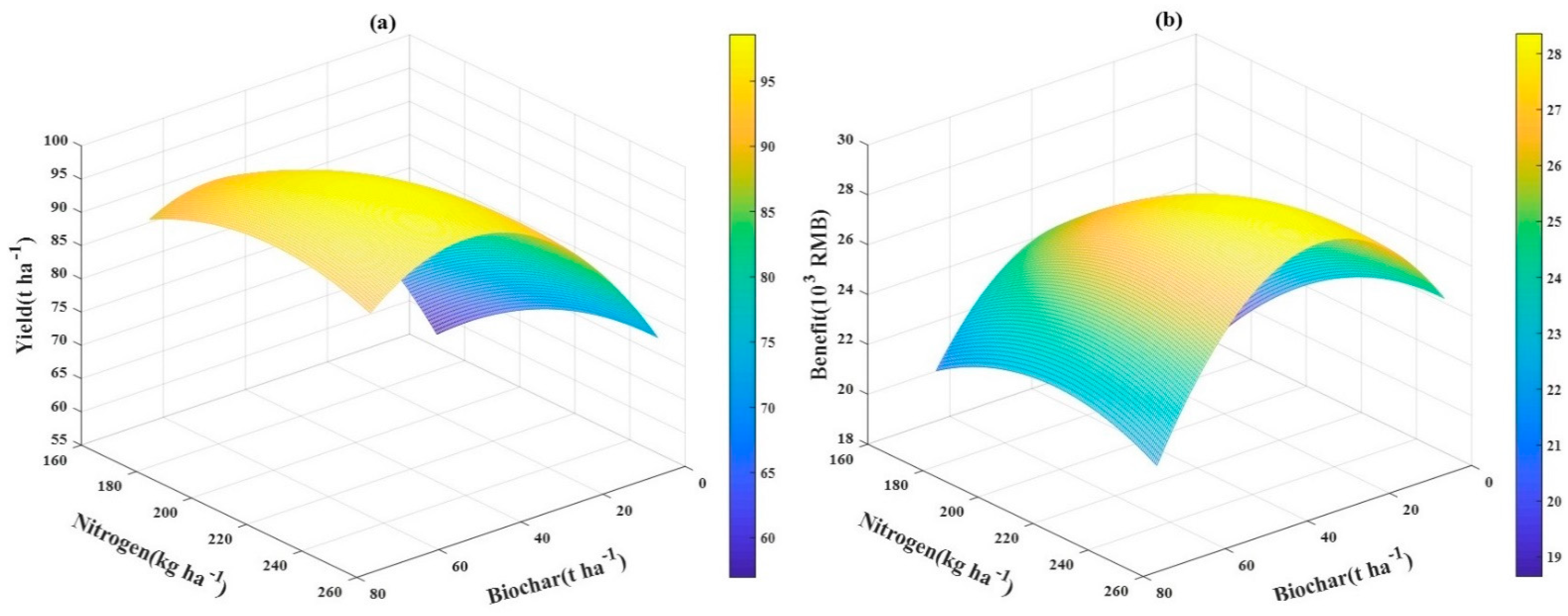
3.7. The Relationship between Tomato Yield and Economic Benefit and Application Rates of Biochar‒N Fertilizer
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatments | Yield (t/ha) | Input Cost | Income | Benefit | ||||
|---|---|---|---|---|---|---|---|---|
| Biochar | Fertilizer and Pesticide | Pipe System | Water and Electricity | Seedlings and Others | ||||
| N1C0 | 56.521 | 0.000 | 1.010 | 2.024 | 0.100 | 1.500 | 21.101 | 18.491 |
| N1C30 | 85.904 | 4.000 | 1.010 | 2.024 | 0.100 | 1.600 | 32.071 | 25.361 |
| N1C50 | 89.942 | 6.667 | 1.010 | 2.024 | 0.100 | 1.700 | 33.578 | 24.101 |
| N1C70 | 88.053 | 9.333 | 1.010 | 2.024 | 0.100 | 1.800 | 32.873 | 20.630 |
| N2C0 | 62.279 | 0.000 | 1.160 | 2.024 | 0.100 | 1.500 | 23.251 | 20.491 |
| N2C30 | 90.779 | 4.000 | 1.160 | 2.024 | 0.100 | 1.600 | 33.891 | 27.031 |
| N2C50 | 96.404 | 6.667 | 1.160 | 2.024 | 0.100 | 1.700 | 35.991 | 26.364 |
| N2C70 | 94.212 | 9.333 | 1.160 | 2.024 | 0.100 | 1.800 | 35.172 | 22.779 |
| N3C0 | 69.984 | 0.000 | 1.210 | 2.024 | 0.100 | 1.500 | 26.127 | 23.317 |
| N3C30 | 93.303 | 4.000 | 1.210 | 2.024 | 0.100 | 1.600 | 34.833 | 27.923 |
| N3C50 | 96.303 | 6.667 | 1.210 | 2.024 | 0.100 | 1.700 | 35.953 | 26.276 |
| N3C70 | 92.567 | 9.333 | 1.210 | 2.024 | 0.100 | 1.800 | 34.558 | 22.115 |
| N4C0 | 72.721 | 0.000 | 1.260 | 2.024 | 0.100 | 1.500 | 27.149 | 24.289 |
| N4C30 | 93.268 | 4.000 | 1.260 | 2.024 | 0.100 | 1.600 | 34.820 | 27.860 |
| N4C50 | 96.231 | 6.667 | 1.260 | 2.024 | 0.100 | 1.700 | 35.926 | 26.199 |
| N4C70 | 91.904 | 9.333 | 1.260 | 2.024 | 0.100 | 1.800 | 34.311 | 21.818 |
References
- Tian, X.; Li, Z.; Wang, L.; Wang, Y.; Li, B.; Duan, M.; Liu, B. Effects of Biochar Combined with Nitrogen Fertilizer Reduction on Rapeseed Yield and Soil Aggregate Stability in Upland of Purple Soils. Int. J. Environ. Res. Public Health 2019, 17, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.; Chen, X.; Zhang, F. Current nitrogen management status and measures to improve the intensive wheat–maize system in China. Ambio 2010, 39, 376–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Chen, C.; Chen, X.; Jiang, F.; Hopkins, I.; Zhang, X.; Han, Z.; Billy, G.; Benavides, J. Soil acidity, available phosphorus content, and optimal biochar and nitrogen fertilizer application rates: A five-year field trial in upland red soil, China. Field Crop. Res. 2019, 232, 77–87. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Lan, Y.; Liu, Z.Q.; Liu, X.L.; Miao, W.; He, T.Y.; Yang, X.; Meng, J.; Chen, W.F. Effect of biochar on the greenhouse gas emissions from farmland and its physicochemical properties. Matec Web Conf. 2016, 63, 03010. [Google Scholar] [CrossRef] [Green Version]
- Majeed, A.J.; Dikici, H.; Demir, Ö.F. Effect of Biochar and Nitrogen Applications on Growth of Corn (Zea mays L.) Plants. Turk. J. Agric. Food Sci. Technol. 2018, 6, 346. [Google Scholar] [CrossRef] [Green Version]
- Awad, Y.M.; Ok, Y.S.; Abrigata, J.; Beiyuan, J.; Beckers, F.; Tsang, D.C.W.; Rinklebe, J. Pine sawdust biomass and biochars at different pyrolysis temperatures change soil redox processes. Sci. Total Environ. 2018, 625, 147–154. [Google Scholar] [CrossRef]
- Ye, J.; Joseph, S.D.; Ji, M.; Nielsen, S.; Mitchell, D.R.G.; Donne, S.; Horvat, J.; Wang, J.; Munroe, P.; Thomas, T. Chemolithotrophic processes in the bacterial communities on the surface of mineral-enriched biochars. ISME J. 2017, 11, 1087–1101. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, P.; Chen, D.; Shi, G.; Cheng, K.; Bian, R.; Liu, X.; Zhang, X.; Zheng, J.; Crowley, D.E. Short-term biochar manipulation of microbial nitrogen transformation in wheat rhizosphere of a metal contaminated Inceptisol from North China plain. Sci. Total Environ. 2018, 640, 1287–1296. [Google Scholar] [CrossRef]
- Keabetswe, L.; Shao, G.C.; Cui, J.; Lu, J.; Stimela, T. A combination of biochar and regulated deficit irrigation improves tomato fruit quality: A comprehensive quality analysis. Folia Hortic. 2019, 31, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Rick, C.M. The tomato. Sci. Am. 1978, 239, 76–89. [Google Scholar] [CrossRef]
- Brunetti, G.; Traversa, A.; De Mastro, F.; Cocozza, C. Short term effects of synergistic inorganic and organic fertilization on soil properties and yield and quality of plum tomato. Sci. Hortic. 2019, 252, 342–347. [Google Scholar] [CrossRef]
- Feng, Z.; Zhu, L. Impact of biochar on soil N2O emissions under different biochar-carbon/fertilizer-nitrogen ratios at a constant moisture condition on a silt loam soil. Sci. Total Environ. 2017, 584–585, 776–782. [Google Scholar] [CrossRef]
- García-Gaines, R.A.; Frankenstein, S. USCS and the USDA Soil Classification System: Development of a Mapping Scheme; United States Army Corps of Engineers: Washington, DC, USA, 2015. [Google Scholar]
- Du, Y.; Niu, W.; Zhang, Q.; Cui, B.; Gu, X.; Guo, L.; Liang, B. Effects of Nitrogen on Soil Microbial Abundance, Enzyme Activity, and Nitrogen Use Efficiency in Greenhouse Celery under Aerated Irrigation. Soil Sci. Soc. Am. J. 2018, 82, 606. [Google Scholar] [CrossRef] [Green Version]
- Fisher, K.A.; Yarwood, S.A.; James, B.R. Soil urease activity and bacterial ureC gene copy numbers: Effect of pH. Geoderma 2017, 285, 1–8. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Kaushal, J.; Mehandia, S.; Singh, G.; Raina, A.; Arya, S.K. Catalase enzyme: Application in bioremediation and food industry. Biocatal. Agric. Biotechnol. 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Wang, J.; Liu, L.; Zhang, M.; Xu, J. Effects of Artificial Soil Aeration Volume and Frequency on Soil Enzyme Activity and Microbial Abundance when Cultivating Greenhouse Tomato. Soil Sci. Soc. Am. J. 2016, 80, 1208–1221. [Google Scholar] [CrossRef]
- Huang, J. The Effect of Biochar Application on Soil Microbial Biomass and Soil Enzymes. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2012. [Google Scholar]
- Thwe, A.A.; Vercambre, G.; Gautier, H.; Gay, F.; Phattaralerphong, J.; Kasemsap, P. Response of photosynthesis and chlorophyll fluorescence to acute ozone stress in tomato (Solanum lycopersicum Mill.). Photosynthetica 2014, 52, 105–116. [Google Scholar] [CrossRef]
- Zou, X.; Niu, W.; Liu, J.; Li, Y.; Liang, B.; Guo, L.; Guan, Y. Effects of Residual Mulch Film on the Growth and Fruit Quality of Tomato (Lycopersicon esculentum Mill.). Water Air Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Du, Y.-D.; Zhang, Q.; Cui, B.-J.; Sun, J.; Wang, Z.; Ma, L.-H.; Niu, W.-Q. Aerated irrigation improves tomato yield and nitrogen use efficiency while reducing nitrogen application rate. Agric. Water Manag. 2020, 235, 106152. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Lü, W.; Gu, J.; Zou, X.; Wang, J.; Liu, L.; Zhang, M.; Xu, J. Aerated irrigation improving photosynthesis characteristics and dry matter accumulation of greenhouse tomato. Trans. Chin. Soc. Agric. Eng. 2016, 32, 125–132. [Google Scholar]
- Bhat, R. Impact of ultraviolet radiation treatments on the quality of freshly prepared tomato (Solanum lycopersicum) juice. Food Chem. 2016, 213, 635–640. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, M.P. Nutritional characterisation of commercial traditional pasteurised tomato juices: Carotenoids, vitamin C and radical-scavenging capacity. Food Chem. 2006, 98, 749–756. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Improving Flavour of Tomatoes. In Controlled Environment Horticulture: Improving Quality of Vegetables and Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2019; pp. 199–205. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Dayal, D.; Mandal, K.G.; Wanjari, R.H.; Hati, K.M. Optimization of fertilizer schedules in fallow and groundnut-based cropping systems and an assessment of system sustainability. Field Crop. Res. 2003, 80, 83–98. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Anawar, H.M. Application of Biochars for Soil Constraints: Challenges and Solutions. Pedosphere 2015, 25, 631–638. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S.; et al. Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Guo, L.; Bornø, M.L.; Niu, W.; Liu, F. Biochar amendment improves shoot biomass of tomato seedlings and sustains water relations and leaf gas exchange rates under different irrigation and nitrogen regimes. Agric. Water Manag. 2020. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; She, D.; Liu, Z.; Elshaikh, N.A.; Shao, G.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Traversi, M.L.; Faraloni, C.; Valagussa, M.; Pozzi, A. Influence of biochar application on nutritional quality of tomato (Lycopersicon esculentum). Crop Pasture Sci. 2015, 66, 747. [Google Scholar] [CrossRef] [Green Version]
- Suthar, R.; Wang, C.; Nunes, M.; Chen, J.; Sargent, S.; Bucklin, R.; Gao, B. Bamboo Biochar Pyrolyzed at Low Temperature Improves Tomato Plant Growth and Fruit Quality. Agriculture 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Fan, C.H.; Zhang, H.; Chen, Z.Z.; Sun, L.Y.; Xiong, Z.Q. Combined effects of nitrogen fertilization and biochar on the net global warming potential, greenhouse gas intensity and net ecosystem economic budget in intensive vegetable agriculture in southeastern China. Atmos. Environ. 2015, 100, 10–19. [Google Scholar] [CrossRef]
- Mete, F.; Mia, S.; Dijkstra, F.; Abuyusuf, M.; Hossain, A. Synergistic Effects of Biochar and NPK Fertilizer on Soybean Yield in an Alkaline Soil. Pedosphere 2015, 25, 713–719. [Google Scholar] [CrossRef]
- Cheng, N.; Peng, Y.; Kong, Y.; Li, J. Combined effects of biochar addition and nitrogen fertilizer reduction on the rhizosphere metabolomics of maize (Zea mays L.) seedlings. Plant Soil 2018, 433, 19–35. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]

| Factors | Urease | Phosphatase | Catalase | Bacteria | Fungi | Actinomycetes |
|---|---|---|---|---|---|---|
| N | *** | *** | *** | *** | *** | *** |
| C | *** | *** | *** | *** | *** | *** |
| N × C | *** | ** | ** | *** | *** | * |
| Variables | Nitrogen | Biochar | |||
|---|---|---|---|---|---|
| C0 | C30 | C50 | C70 | ||
| Urease (mg g−1 24 h−1) | N1 | 1.48 ± 0.05 Cb | 1.71 ± 0.09 Bc | 1.78 ± 0.04 ABb | 1.82 ± 0.03 Ab |
| N2 | 1.78 ± 0.03 Ca | 2.09 ± 0.06 Bb | 2.33 ± 0.07 Aa | 2.27 ± 0.06 Aa | |
| N3 | 1.81 ± 0.05 Ca | 2.42 ± 0.06 Aa | 2.33 ± 0.03 ABa | 2.28 ± 0.02 Ba | |
| N4 | 1.84 ± 0.03 Ca | 2.46 ± 0.04 ABa | 2.38 ± 0.06 Aa | 2.30 ± 0.07 Aa | |
| Phosphatase (μg g−1 1 h−1) | N1 | 8.67 ± 0.50 Cc | 11.63 ± 0.47 Bb | 12.17 ± 0.59 Ac | 12.60 ± 0.40 Ac |
| N2 | 10.52 ± 0.41 Cb | 12.96 ± 1.39 Bb | 16.36 ± 0.96 Aa | 15.84 ± 1.40 Aa | |
| N3 | 12.37 ± 0.49 Da | 15.07 ± 0.60 Aa | 14.50 ± 0.50 Ab | 14.13 ± 0.74 Cb | |
| N4 | 12.47 ± 0.63 Ba | 15.05 ± 1.11 Aa | 14.98 ± 0.95 Aab | 13.54 ± 0.54 Abc | |
| Catalase (mg L−1) | N1 | 0.62 ± 0.11 Bc | 0.90 ± 0.06 Ac | 0.96 ± 0.05 Ac | 0.93 ± 0.06 Ac |
| N2 | 0.72 ± 0.11 Dc | 1.25 ± 0.06 Cb | 2.01 ± 0.16 Aa | 1.71 ± 0.06 Ba | |
| N3 | 0.92 ± 0.08 Bb | 1.49 ± 0.09 Aa | 1.43 ± 0.06 Ab | 1.41 ± 0.07 Ab | |
| N4 | 1.12 ± 0.10 Ca | 1.62 ± 0.21 Aa | 1.39 ± 0.16 Bb | 1.30 ± 0.14 Bb | |
| Bacteria (108 g−1) | N1 | 2.24 ± 0.25 Ba | 2.30 ± 0.44 ABb | 2.66 ± 0.48 ABc | 2.82 ± 0.40 Ab |
| N2 | 2.81 ± 0.26 Da | 3.67 ± 0.25 Ca | 5.32 ± 0.36 Aa | 4.33 ± 0.28 Ba | |
| N3 | 2.92 ± 0.17 Ca | 4.11 ± 0.26 Ba | 4.84 ± 0.36 Aab | 3.81 ± 0.12 Ba | |
| N4 | 2.15 ± 0.42 Ca | 4.06 ± 0.36 Ba | 3.95 ± 0.31 Ab | 3.95 ± 0.25 Ba | |
| Fungi (105 g−1) | N1 | 1.22 ± 0.17 Bb | 1.26 ± 0.15 ABc | 1.48 ± 0.28 Ac | 1.37 ± 0.16 ABc |
| N2 | 1.71 ± 0.70 Ca | 3.24 ± 0.30 Bb | 3.17 ± 0.30 Aa | 2.70 ± 0.29 Ba | |
| N3 | 2.02 ± 0.19 Bb | 3.89 ± 0.23 Aa | 3.48 ± 0.16 Aa | 3.43 ± 0.35 Aa | |
| N4 | 2.71 ± 0.70 Cab | 3.33 ± 0.27 Aa | 3.05 ± 0.22 Ab | 2.92 ± 0.14 Bb | |
| Actinomycetes (106 g−1) | N1 | 1.69 ± 0.59 Bc | 1.97 ± 0.30 ABb | 2.00 ± 0.62 ABb | 2.19 ± 0.56 Ab |
| N2 | 2.50 ± 0.46 Ca | 3.46 ± 0.45 BCa | 4.70 ± 0.36 Aa | 4.12 ± 0.30 Ba | |
| N3 | 2.75 ± 0.09 Cb | 3.98 ± 0.20 ABa | 4.59 ± 0.17 Aa | 3.37 ± 0.26 Ba | |
| N4 | 2.30 ± 0.44 Ba | 3.87 ± 0.25 Aa | 4.16 ± 0.41 Aa | 3.77 ± 0.23 Aa | |
| Factors | Pn | gs | Tr | Plant Height | Stem Thickness | Biomass |
|---|---|---|---|---|---|---|
| N | *** | *** | *** | *** | *** | *** |
| C | *** | *** | *** | *** | ** | *** |
| N × C | *** | * | * | ** | * | *** |
| Variables | Nitrogen | Biochar | |||
|---|---|---|---|---|---|
| C0 | C30 | C50 | C70 | ||
| Pn (mmol m−2 s−1) | N1 | 21.25 ± 1.07 Cc | 26.32 ± 0.31 Bd | 29.90 ± 0.59 Ad | 28.90 ± 0.26 Ac |
| N2 | 23.25 ± 1.07 Db | 29.96 ± 0.72 Cb | 32.77 ± 0.36 Bc | 35.20 ± 1.15 Aa | |
| N3 | 24.08 ± 0.29 Dab | 31.58 ± 0.34 Ca | 35.86 ± 0.43 Aa | 34.12 ± 0.45 Ba | |
| N4 | 25.08 ± 0.77 Ca | 27.07 ± 0.59 Bc | 33.61 ± 0.96 Ab | 34.29 ± 0.88 Aa | |
| gs (mol m−2 s−1) | N1 | 0.36 ± 0.03 Dc | 0.43 ± 0.01 Cb | 0.56 ± 0.03 Ab | 0.50 ± 0.01 Bb |
| N2 | 0.39 ± 0.04 Cbc | 0.49 ± 0.04 Ba | 0.62 ± 0.05 Aa | 0.57 ± 0.02 Aa | |
| N3 | 0.41 ± 0.02 Cb | 0.50 ± 0.01 Ba | 0.60 ± 0.03 Aab | 0.59 ± 0.02 Aa | |
| N4 | 0.46 ± 0.02 Ca | 0.50 ± 0.04 BCa | 0.61 ± 0.02 Aab | 0.52 ± 0.04 Bb | |
| Tr (mmol m−2 s−1) | N1 | 9.18 ± 0.14 Cb | 11.28 ± 0.33 Bc | 11.86 ± 0.38 ABc | 12.23 ± 0.38 Ac |
| N2 | 9.81 ± 0.49 Dab | 11.63 ± 0.41 Cc | 12.73 ± 0.59 Bb | 13.71 ± 0.72 Ab | |
| N3 | 10.09 ± 0.08 Ca | 13.73 ± 0.95 Ba | 14.11 ± 0.39 Ba | 15.10 ± 0.39 Aa | |
| N4 | 10.46 ± 0.34 Ca | 12.50 ± 0.41 Bb | 13.25 ± 0.32 Ab | 14.07 ± 0.23 Ab | |
| Plant height (cm) | N1 | 107.00 ± 1.50 Cd | 122.17 ± 0.29 Bc | 125.17 ± 0.29 Ad | 126.33 ± 0.29 Ac |
| N2 | 112.331.61 Cc | 124.00 ± 0.50 Bb | 131.87 ± 0.33 Ab | 130.67 ± 1.26 Ab | |
| N3 | 114.55 ± 1.00 Cb | 129.67 ± 0.29 Ba | 133.33 ± 0.76 Aa | 133.50 ± 0.87 Aa | |
| N4 | 115.33 ± 1.04 Ca | 128.67 ± 1.04 Aa | 130.47 ± 0.96 Bc | 129.33 ± 1.04 ABb | |
| Stem thickness (cm) | N1 | 12.15 ± 0.27 Bb | 12.39 ± 0.18 Bc | 12.66 ± 0.28 ABb | 13.05 ± 0.18 Ab |
| N2 | 13.38 ± 0.44 Ba | 13.68 ± 0.45 Ab | 14.25 ± 0.38 Aa | 14.03 ± 0.53 Aa | |
| N3 | 13.45 ± 0.17 Aa | 13.83 ± 0.19 Ab | 13.92 ± 0.25 Aa | 13.56 ± 0.14 Aa | |
| N4 | 13.82 ± 0.28 Ba | 14.54 ± 0.20 Aa | 14.14 ± 0.53 ABa | 13.88 ± 0.16 Ba | |
| Biomass (g) | N1 | 30.32 ± 0.37 Dd | 40.36 ± 0.13 Ab | 36.68 ± 0.30 Bc | 31.94 ± 0.38 Cd |
| N2 | 31.10 ± 0.46 Dc | 40.67 ± 0.28 Bb | 43.43 ± 0.07 Aa | 39.71 ± 0.34 Ca | |
| N3 | 32.36 ± 0.49 Db | 40.85 ± 0.85 Ba | 42.34 ± 0.40 Ab | 37.41 ± 0.54 Cc | |
| N4 | 33.80 ± 0.19 Da | 40.08 ± 0.30 Bb | 42.92 ± 0.39 Aab | 38.35 ± 0.63 Cb | |
| Factors | Yield | VC | Sugar‒Acid Ratio | PFP of N |
|---|---|---|---|---|
| N | *** | *** | *** | *** |
| C | *** | *** | *** | *** |
| N × C | *** | *** | ns | *** |
| Variables | Nitrogen | Biochar | |||
|---|---|---|---|---|---|
| C0 | C30 | C50 | C70 | ||
| Yield (t ha−1) | N1 | 56.52 ± 0.33 Dd | 85.91 ± 0.31 Cc | 89.94 ± 0.29 Ab | 88.06 ± 0.42 Bd |
| N2 | 62.28 ± 0.18 Dc | 90.78 ± 0.15 Cb | 96.40 ± 0.16 Aa | 94.21 ± 0.24 Ba | |
| N3 | 69.98 ± 0.22 Db | 93.30 ± 0.22 Ca | 96.30 ± 0.11 Aa | 92.57 ± 0.20 Bb | |
| N4 | 72.72 ± 0.09 Da | 93.27 ± 0.31 Ba | 96.23 ± 0.30 Aa | 91.90 ± 0.16 Cc | |
| VC (mg·100 g−1) | N1 | 3.77 ± 0.08 Dd | 4.28 ± 0.07 Bd | 4.57 ± 0.08 Ad | 4.26 ± 0.07 Cc |
| N2 | 5.14 ± 0.14 Dc | 6.57 ± 0.09 Bc | 7.23 ± 0.08 Ab | 5.70 ± 0.17 Cb | |
| N3 | 6.14 ± 0.07 Da | 7.96 ± 0.10 Aa | 7.75 ± 0.08 Ba | 7.20 ± 0.19 Ca | |
| N4 | 5.42 ± 0.09 Db | 6.63 ± 0.08 Ab | 6.10 ± 0.08 Bc | 5.85 ± 0.08 Cb | |
| Sugar‒acid ratio | N1 | 5.16 ± 0.12 | 5.72 ± 0.37 | 5.56 ± 0.37 | 4.88 ± 0.08 |
| N2 | 9.27 ± 0.82 | 9.59 ± 0.56 | 9.54 ± 0.39 | 8.48 ± 0.58 | |
| N3 | 8.77 ± 0.33 | 8.39 ± 0.77 | 8.86 ± 0.53 | 8.03 ± 0.27 | |
| N4 | 6.90 ± 0.29 | 6.66 ± 0.17 | 6.70 ± 0.70 | 5.72 ± 0.52 | |
| PFP of N (kg kg−1) | N1 | 332.48 ± 1.94 Da | 505.32± 1.81 Ca | 529.07 ± 1.70 Aa | 517.96 ± 2.45 Ba |
| N2 | 327.79± 0.92 Db | 477.78± 0.79 Cb | 507.39 ± 0.82 Ab | 495.85 ± 1.29 Bb | |
| N3 | 333.26 ± 1.05 Da | 444.30 ± 1.04 Bc | 458.59 ± 0.52 Ac | 440.80 ± 0.93 Cc | |
| N4 | 290.89 ± 0.36 Dc | 373.07± 1.24 Bd | 384.92 ± 1.21 Ad | 367.61 ± 0.62 Cd | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Yu, H.; Kharbach, M.; Zhang, W.; Wang, J.; Niu, W. Biochar Improves Soil-Tomato Plant, Tomato Production, and Economic Benefits under Reduced Nitrogen Application in Northwestern China. Plants 2021, 10, 759. https://doi.org/10.3390/plants10040759
Guo L, Yu H, Kharbach M, Zhang W, Wang J, Niu W. Biochar Improves Soil-Tomato Plant, Tomato Production, and Economic Benefits under Reduced Nitrogen Application in Northwestern China. Plants. 2021; 10(4):759. https://doi.org/10.3390/plants10040759
Chicago/Turabian StyleGuo, Lili, Huiwen Yu, Mourad Kharbach, Wenqian Zhang, Jingwei Wang, and Wenquan Niu. 2021. "Biochar Improves Soil-Tomato Plant, Tomato Production, and Economic Benefits under Reduced Nitrogen Application in Northwestern China" Plants 10, no. 4: 759. https://doi.org/10.3390/plants10040759






