Biological Activities and Chemical Profiles of Kalanchoe fedtschenkoi Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC/MS Analysis
2.2. HPLC Analysis
2.3. TFC of Methanol Extract from K. fedtschenkoi
2.4. Antimicrobial Activity
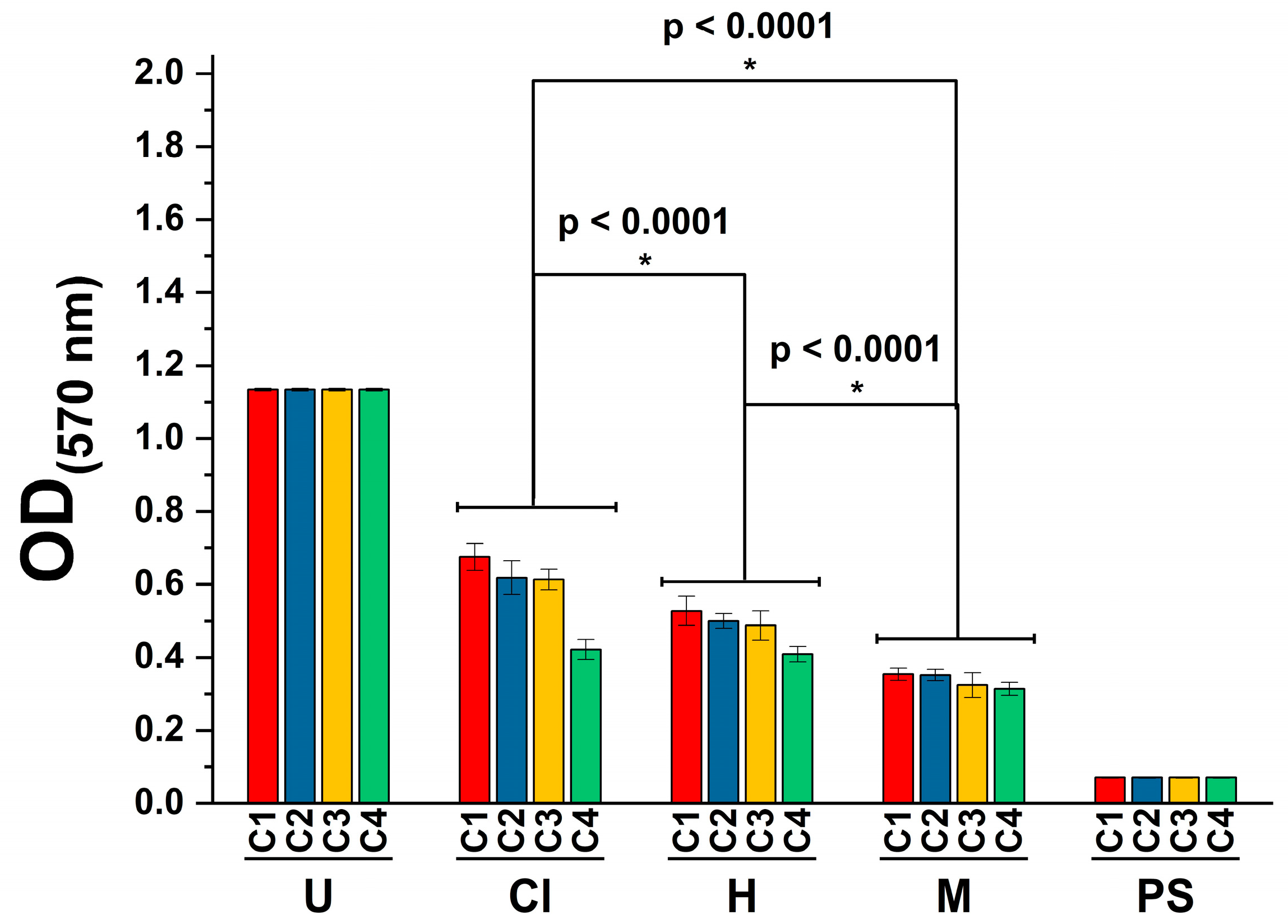
2.5. Cytotoxicity Activity
2.6. Anti-Inflammatory Activity
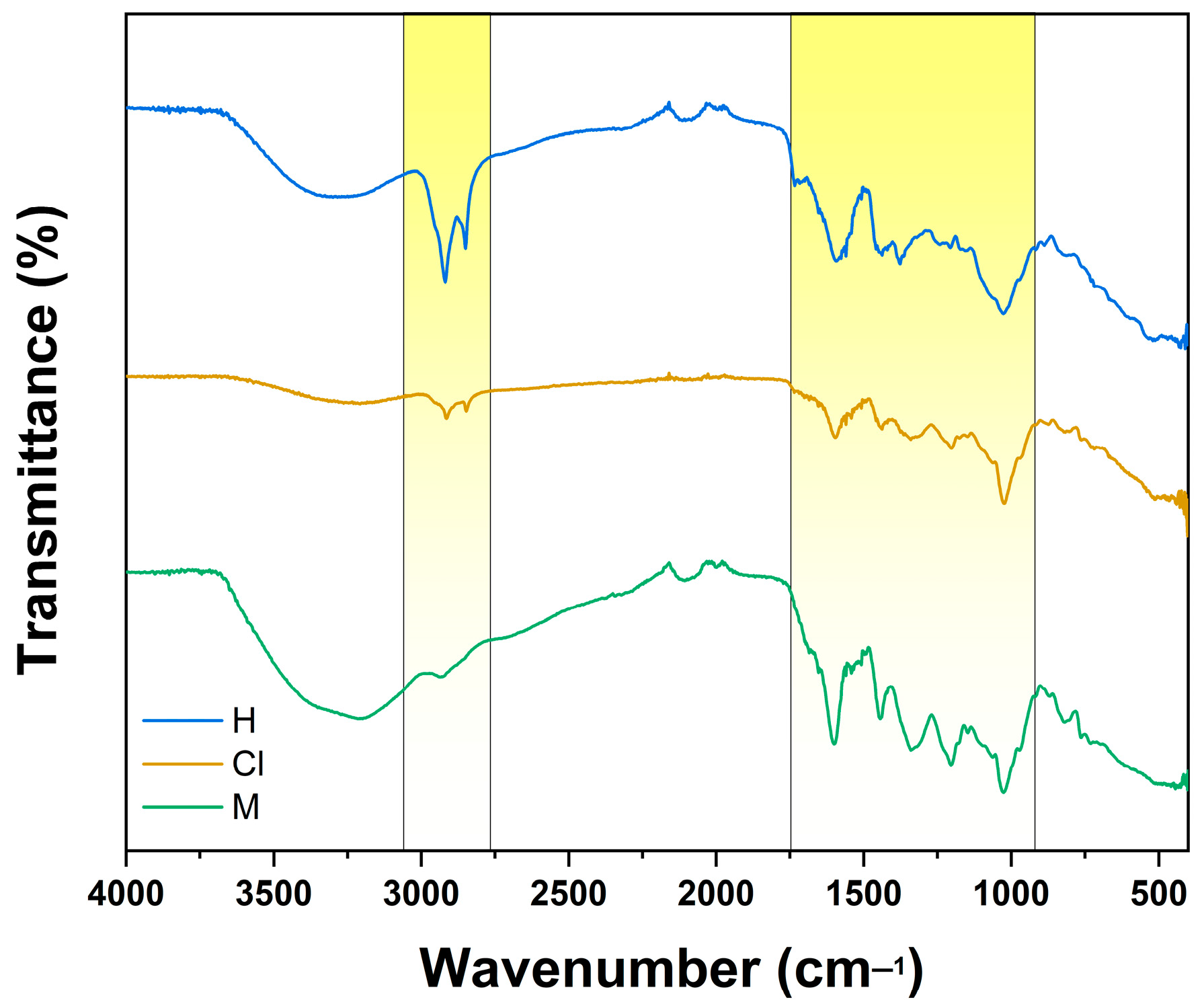
2.7. FTIR Analysis
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. GC/MS Analysis
3.3. HPLC Analysis
3.4. TFC Analysis
3.5. Strains and Culture Media
3.6. Microdilution Assay
3.7. Cytotoxicity Assay
3.8. Anti-Inflammatory Assay
3.9. FTIR Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chali, B.U.; Hasho, A.; Koricha, N.B. Preference and Practice of Traditional Medicine and Associated Factors in Jimma Town, Southwest Ethiopia. Evid. Based Complement. Alternat. Med. 2021, 2021, 9962892. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Gori, L. Herbal Medicine Today: Clinical and Research Issues. Evid. Based Complement. Alternat. Med. 2007, 4, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied. Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- AMELI, J. Communicable Diseases and Outbreak Control. Turk. J. Emerg. Med. 2016, 15, 20–26. [Google Scholar]
- Edemekong, P.F.; Huang, B. Epidemiology of Prevention of Communicable Diseases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Liang, Z.; Fan, S.; Gao, X.; Jia, H.; Li, B.; Shi, L.; Zhai, A.; Wu, C. Epidemiology and Prediction of Multidrug-Resistant Bacteria Based on Hospital Level. J. Glob. Antimicrob. Resist. 2022, 29, 155–162. [Google Scholar] [CrossRef]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-Communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Calcaterra, V.; Zuccotti, G. Non-Communicable Diseases and Rare Diseases: A Current and Future Public Health Challenge within Pediatrics. Children 2022, 9, 1491. [Google Scholar] [CrossRef]
- Kabir, A.; Karim, M.N.; Islam, R.M.; Romero, L.; Billah, B. Health System Readiness for Non-Communicable Diseases at the Primary Care Level: A Systematic Review. BMJ Open 2022, 12, e060387. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Deng, M. Crassulaceae. In Identification and Control of Common Weeds: Volume 2; Xu, Z., Deng, M., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 475–486. ISBN 978-94-024-1157-7. [Google Scholar]
- Thiede, J.; Eggli, U. Crassulaceae. In Flowering Plants Eudicots: Berberidopsidales, Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae; Kubitzki, K., Ed.; The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2007; pp. 83–118. ISBN 978-3-540-32219-1. [Google Scholar]
- Hassan, M.H.A.; Elwekeel, A.; Moawad, A.; Afifi, N.; Amin, E.; Amir, D.E. Phytochemical Constituents and Biological Activity of Selected Genera of Family Crassulaceae: A Review. S. Afr. J. Bot. 2021, 141, 383–404. [Google Scholar] [CrossRef]
- Vargas, A.; Herrera, I.; Nualart, N.; Guézou, A.; Gómez-Bellver, C.; Freire, E.; Jaramillo Díaz, P.; López-Pujol, J. The Genus Kalanchoe (Crassulaceae) in Ecuador: From Gardens to the Wild. Plants 2022, 11, 1746. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Nowak, P.; Wachowicz, B.; Piechocka, J.; Głowacki, R.; Moniuszko-Szajwaj, B.; Stochmal, A. Antioxidant Efficacy of Kalanchoe Daigremontiana Bufadienolide-Rich Fraction in Blood Plasma In Vitro. Pharm. Biol. 2016, 54, 3182–3188. [Google Scholar] [CrossRef]
- Phatak, R.S.; Hendre, A.S. In-Vitro Antiurolithiatic Activity of Kalanchoe Pinnata Extract. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 275–279. [Google Scholar]
- dos Santos Nascimento, L.B.; Casanova, L.M.; Costa, S.S. Bioactive Compounds from Kalanchoe Genus Potentially Useful for the Development of New Drugs. Life 2023, 13, 646. [Google Scholar] [CrossRef]
- Costa, S.S.; Muzitano, M.F.; Camargo, L.M.M.; Coutinho, M.A.S. Therapeutic Potential of Kalanchoe Species: Flavonoids and Other Secondary Metabolites. Nat. Prod. Commun. 2008, 3, 2151–2164. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, S.; Rani, K.; Sehgal, N.; Prakash, O. Compound Profiling in Methanol Extract of Kalanchoe Blossfeldiana (Flaming Katy) Leaves Through GC-MS Analysis and Evaluation of Its Bioactive Properties. Glob. J. Advnced Biol. Sci. 2015, 1, 38–49. [Google Scholar]
- Schuler, V.; Suter, K.; Fürer, K.; Eberli, D.; Horst, M.; Betschart, C.; Brenneisen, R.; Hamburger, M.; Mennet, M.; Schnelle, M.; et al. Bryophyllum Pinnatum Inhibits Detrusor Contractility in Porcine Bladder Strips—A Pharmacological Study towards a New Treatment Option of Overactive Bladder. Phytomedicine 2012, 19, 947–951. [Google Scholar] [CrossRef]
- El Abdellaoui, S.; Destandau, E.; Toribio, A.; Elfakir, C.; Lafosse, M.; Renimel, I.; André, P.; Cancellieri, P.; Landemarre, L. Bioactive Molecules in Kalanchoe Pinnata Leaves: Extraction, Purification, and Identification. Anal. Bioanal. Chem. 2010, 398, 1329–1338. [Google Scholar] [CrossRef]
- Zakharchenko, N.S.; Belous, A.S.; Biryukova, Y.K.; Medvedeva, O.A.; Belyakova, A.V.; Masgutova, G.A.; Trubnikova, E.V.; Buryanov, Y.I.; Lebedeva, A.A. Immunomodulating and Revascularizing Activity of Kalanchoe Pinnata Synergize with Fungicide Activity of Biogenic Peptide Cecropin P1. J. Immunol. Res. 2017, 2017, 3940743. [Google Scholar] [CrossRef]
- Richwagen, N.; Lyles, J.T.; Dale, B.L.F.; Quave, C.L. Antibacterial Activity of Kalanchoe Mortagei and K. Fedtschenkoi Against ESKAPE Pathogens. Front. Pharmacol. 2019, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.M.; dos Santos Nascimento, L.B.; Casanova, L.M.; Castricini, S.D.; de Souza, J.E.E.; Yien, R.M.K.; Costa, S.S.; Tavares, E.S. Kalanchoe fedtschenkoi R. Hamet & H. Perrier, a Non-Conventional Food Plant in Brazil: HPLC-DAD-ESI-MS/MS Profile and Leaf Histochemical Location of Flavonoids. J. Appl. Bot. Food Qual. 2022, 95, 154–166. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; McCubrey, J.A.; Ramarosandratana, A.V.; Sajeva, M.; Zito, P.; Notarbartolo, M. Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell. Pharmaceuticals 2019, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.; Silva, S.A.G.D.; Souza, M.L.M.; Lima, L.M.T.R.R.; Rossi-Bergmann, B.; de Moraes, V.L.G.; Costa, S.S. Isolation and Chemical Analysis of a Fatty Acid Fraction of Kalanchoe Pinnata with a Potent Lymphocyte Suppressive Activity. Planta Med. 2000, 66, 134–137. [Google Scholar] [CrossRef]
- Indriyanti, N.; Garmana, A.; Setiawan, F.; Setiawan, F. Repairing Effects of Aqueous Extract of Kalanchoe Pinnata (Lmk) Pers. on Lupus Nephritis Mice. Pharmacogn. J. 2018, 10, 548–552. [Google Scholar] [CrossRef]
- Pereira, K.M.F.; Grecco, S.S.; Figueiredo, C.R.; Hosomi, J.K.; Nakamura, M.U.; Lago, J.H.G. Chemical Composition and Cytotoxicity of Kalanchoe Pinnata Leaves Extracts Prepared Using Accelerated System Extraction (ASE). Nat. Prod. Commun. 2018, 13, 163–166. [Google Scholar] [CrossRef]
- Saleh, M.M.; Ghoneim, M.M.; Kottb, S.; El-Hela, A.A. Biologically Active Secondary Metabolites from Kalanchoe Tomentosa. J. Biomed. Pharm. Res. 2014, 3, 136–140. [Google Scholar]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Sztormowska-Achranowicz, K.; Kowalczyk, M.; Soluch, A.; Ochocka, J.R. An In Vitro Anticancer, Antioxidant, and Phytochemical Study on Water Extract of Kalanchoe Daigremontiana Raym.-Hamet and H. Perrier. Molecules 2022, 27, 2280. [Google Scholar] [CrossRef]
- Bhatti, M.; Kamboj, A.; Saluja, A.K. Phytochemical Screening and In-Vitro Evaluation of Antioxidant Activities of Various Extracts of Leaves and Stems of Kalanchoe Crenata. J. Pharm. Nutr. Sci. 2012, 2, 104–114. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Félix-Silva, J.; da Cunha, L.M.; dos Santos Gomes, J.A.; da Silva Siqueira, E.M.; Gimenes, L.P.; Lopes, N.P.; Soares, L.A.L.; de Freitas Fernandes-Pedrosa, M.; Zucolotto, S.M. Inhibitory Effects of Hydroethanolic Leaf Extracts of Kalanchoe Brasiliensis and Kalanchoe Pinnata (Crassulaceae) against Local Effects Induced by Bothrops Jararaca Snake Venom. PLoS ONE 2016, 11, e0168658. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Asztemborska, M.; Krauze-Baranowska, M.; Godlewska, S.; Gucwa, M.; Moniuszko-Szajwaj, B.; Stochmal, A.; Ochocka, J.R. Identification of Flavonoids and Bufadienolides and Cytotoxic Effects of Kalanchoe Daigremontiana Extracts on Human Cancer Cell Lines. Planta Med. 2020, 86, 239–246. [Google Scholar] [CrossRef]
- Aisyah, L.S.; Yun, Y.F.; Julaeha, E.; Herlina, T.; Zainuddin, A.; Hermawan, W.; Supratman, U.; Hayashi, H. Flavonoids from the Fresh Leaves of Kalanchoe Tomentosa (Crassulaceae). Open Chem. J. 2015, 2, 36–39. [Google Scholar] [CrossRef]
- Asiedu-Gyekye, I.J.; Antwi, D.A.; Bugyei, K.A.; Awortwe, C. Comparative Study of Two Kalanchoe Species: Total Flavonoid and Phenolic Contents and Antioxidant Properties. Afr. J. Pure. Appl. Chem. 2012, 6, 65–73. [Google Scholar] [CrossRef]
- Liang, J.; Huang, X.; Ma, G. Antimicrobial Activities and Mechanisms of Extract and Components of Herbs in East Asia. RSC Adv. 2022, 12, 29197–29213. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Hałasa, R.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Ochocka, R. Biological Activities of Leaf Extracts from Selected Kalanchoe Species and Their Relationship with Bufadienolides Content. Pharm. Biol. 2020, 58, 732–740. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, e8825387. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The Antibacterial Activity and Mechanism of Action of Luteolin Against Trueperella Pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.N.; de Dieu Tamokou, J.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and Antioxidant Activity of Kaempferol Rhamnoside Derivatives from Bryophyllum Pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- El-Shamy, A.M.; Fathy, F.I.; Abdel-Rahman, E.H.; Sabry, M.M. Phytochemical, Biological and Botanical Studies of Klanchoe Blossfeldiana Poelln. Int. J. Pharm. Photon. 2013, 104, 189–205. [Google Scholar]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas Aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. 2022, 7, 199. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus Aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Mayorga, O.A.S.; da Costa, Y.F.G.; da Silva, J.B.; Scio, E.; Ferreira, A.L.P.; de Sousa, O.V.; Alves, M.S. Kalanchoe brasiliensis Cambess., a Promising Natural Source of Antioxidant and Antibiotic Agents against Multidrug-Resistant Pathogens for the Treatment of Salmonella Gastroenteritis. Oxidative Med. Cell. Longev. 2019, 2019, e9245951. [Google Scholar] [CrossRef]
- McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. 8-Cytotoxicity of African Medicinal Plants Against Normal Animal and Human Cells. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–233. ISBN 978-0-12-800018-2. [Google Scholar]
- Canga, I.; Vita, P.; Oliveira, A.I.; Castro, M.Á.; Pinho, C. In Vitro Cytotoxic Activity of African Plants: A Review. Molecules 2022, 27, 4989. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Gomez-Flores, R.; Verde-Star, M.J.; Tamez-Guerra, P.; Romo-Sáenz, C.I.; Chávez-Montes, A.; Rodríguez-Garza, N.E.; Quintanilla-Licea, R. In Vitro Cytotoxic Activity of Methanol Extracts of Selected Medicinal Plants Traditionally Used in Mexico against Human Hepatocellular Carcinoma. Plants 2022, 11, 2862. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Rathor, L. Medicinal Plants: A Rich Source of Bioactive Molecules Used in Drug Development. In Evidence Based Validation of Traditional Medicines: A comprehensive Approach; Mandal, S.C., Chakraborty, R., Sen, S., Eds.; Springer: Singapore, 2021; pp. 195–209. ISBN 9789811581274. [Google Scholar]
- Abdel-Hameed, E.-S.S.; Bazaid, S.A.; Shohayeb, M.M.; El-Sayed, M.M.; El-Wakil, E.A. Phytochemical Studies and Evaluation of Antioxidant, Anticancer and Antimicrobial Properties of Conocarpus erectus L. Growing in Taif, Saudi Arabia. Eur. J. Med. Plants 2012, 2, 93–112. [Google Scholar] [CrossRef]
- Widiyastuti, Y.; Sholikhah, I.Y.M.; Haryanti, S. Cytotoxic Activities of Ethanolic and Dichloromethane Extract of Leaves, Stems, and Flowers of Jarong [Stachytarpheta jamaicensis (L.) Vahl.] on HeLa and T47D Cancer Cell Line; AIP Publishing LLC: Surakarta, Indonesia, 2019; p. 020101. [Google Scholar]
- Subash-Babu, P.; Li, D.K.; Alshatwi, A.A. In Vitro Cytotoxic Potential of Friedelin in Human MCF-7 Breast Cancer Cell: Regulate Early Expression of Cdkn2a and PRb1, Neutralize Mdm2-P53 Amalgamation and Functional Stabilization of P53. Exp. Toxicol. Pathol. 2017, 69, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on Its Anti-Tumor Effect and Mechanism of Action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.C.; Parker, R.S. The Cytotoxicity of Vitamin E Is Both Vitamer- and Cell-Specific and Involves a Selectable Trait. J. Nutr. 2004, 134, 3335–3342. [Google Scholar] [CrossRef]
- Yadegarynia, S.; Pham, A.; Ng, A.; Nguyen, D.; Lialiutska, T.; Bortolazzo, A.; Sivryuk, V.; Bremer, M.; White, J.B. Profiling Flavonoid Cytotoxicity in Human Breast Cancer Cell Lines: Determination of Structure-Function Relationships. Nat. Prod. Commun. 2012, 7, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Fokou, F.W.; Karaosmanoğlu, O.; Beng, V.P.; Sivas, H. Cytotoxicity of the Methanol Extracts of Elephantopus Mollis, Kalanchoe Crenata and 4 Other Cameroonian Medicinal Plants towards Human Carcinoma Cells. BMC Complement. Altern. Med. 2017, 17, 280. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Zidorn, C.; Kasprzycka, M.; Szymczak, G.; Szewczyk, K. Phenolic Acid Content, Antioxidant and Cytotoxic Activities of Four Kalanchoë Species. Saudi J. Biol. Sci. 2018, 25, 622–630. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Abdullah, Z.; Knolle, P.A. Scaling of Immune Responses against Intracellular Bacterial Infection. EMBO J. 2014, 33, 2283–2294. [Google Scholar] [CrossRef]
- Stokes, B.A.; Yadav, S.; Shokal, U.; Smith, L.C.; Eleftherianos, I. Bacterial and Fungal Pattern Recognition Receptors in Homologous Innate Signaling Pathways of Insects and Mammals. Front. Microbiol. 2015, 6, 19. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation and Pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and Stigmasterol Suppress Tumor Angiogenesis and Inhibit Cholangiocarcinoma Growth in Mice via Downregulation of Tumor Necrosis Factor-α. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.W.L.; Guerra, G.C.B.; de Souza Araújo, D.F.; de Araújo Júnior, R.F.; de Araújo, A.A.; de Carvalho, T.G.; Fernandes, J.M.; Diez-Echave, P.; Hidalgo-García, L.; Rodriguez-Cabezas, M.E.; et al. Anti-Inflammatory and Chemopreventive Effects of Bryophyllum Pinnatum (Lamarck) Leaf Extract in Experimental Colitis Models in Rodents. Front. Pharmacol. 2020, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, E.R.D.; Félix-Silva, J.; Xavier-Santos, J.B.; Fernandes, J.M.; Guerra, G.C.B.; de Araújo, A.A.; de Souza Araújo, D.F.; de Santis Ferreira, L.; da Silva Júnior, A.A.; de Freitas Fernandes-Pedrosa, M.; et al. Local Anti-Inflammatory Activity: Topical Formulation Containing Kalanchoe Brasiliensis and Kalanchoe Pinnata Leaf Aqueous Extract. Biomed. Pharm. 2019, 113, 108721. [Google Scholar] [CrossRef]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/Quercetin Sol–Gel Hybrids as Antioxidant Dental Implant Materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef]
- Khan, N.; Jamila, N.; Ejaz, R.; Nishan, U.; Kim, K.S. Volatile Oil, Phytochemical, and Biological Activities Evaluation of Trachyspermum Ammi Seeds by Chromatographic and Spectroscopic Methods. Anal. Lett. 2020, 53, 984–1001. [Google Scholar] [CrossRef]
- Din, S.; Hamid, S.; Yaseen, A.; Yatoo, A.M.; Ali, S.; Shamim, K.; Mahdi, W.A.; Alshehri, S.; Rehman, M.U.; Shah, W.A. Isolation and Characterization of Flavonoid Naringenin and Evaluation of Cytotoxic and Biological Efficacy of Water Lilly (Nymphaea mexicana Zucc.). Plants 2022, 11, 3588. [Google Scholar] [CrossRef]
- Bhatt, N.; Mehata, M.S. A Sustainable Approach to Develop Gold Nanoparticles with Kalanchoe Fedtschenkoi and Their Interaction with Protein and Dye: Sensing and Catalytic Probe. Plasmonics 2023. [Google Scholar] [CrossRef]
- Cuevas-Cianca, S.I.; Leal, A.C.L.; Hernández, L.R.; Arreola, E.S.; Bach, H. Antimicrobial, Toxicity, and Anti-Inflammatory Activities of Buddleja Perfoliata Kunth. Phytomed. Plus 2022, 2, 100357. [Google Scholar] [CrossRef]
- Rahim, N.A.; Roslan, M.N.F.; Muhamad, M.; Seeni, A. Antioxidant Activity, Total Phenolic and Flavonoid Content and LC–MS Profiling of Leaves Extracts of Alstonia Angustiloba. Separations 2022, 9, 234. [Google Scholar] [CrossRef]
- Bermúdez-Jiménez, C.; Romney, M.G.; Roa-Flores, S.A.; Martínez-Castañón, G.; Bach, H. Hydrogel-Embedded Gold Nanorods Activated by Plasmonic Photothermy with Potent Antimicrobial Activity. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102093. [Google Scholar] [CrossRef] [PubMed]
- Cruz Paredes, C.; Bolívar Balbás, P.; Gómez-Velasco, A.; Juárez, Z.N.; Sánchez Arreola, E.; Hernández, L.R.; Bach, H. Antimicrobial, Antiparasitic, Anti-Inflammatory, and Cytotoxic Activities of Lopezia Racemosa. Sci. World J. 2013, 2013, e237438. [Google Scholar] [CrossRef]
- Juárez, Z.N.; Bach, H.; Sánchez-Arreola, E.; Bach, H.; Hernández, L.R. Protective Antifungal Activity of Essential Oils Extracted from Buddleja Perfoliata and Pelargonium Graveolens against Fungi Isolated from Stored Grains. J. Appl. Microbiol. 2016, 120, 1264–1270. [Google Scholar] [CrossRef]

| Extract | Match | R match | Rt (min) | % | Name |
|---|---|---|---|---|---|
| 749 | 776 | 45.11 | 0.59 | n-hexadecanoic acid | |
| 888 | 902 | 54.86 | 1.74 | Phytol | |
| 735 | 750 | 58.22 | 0.58 | Stearic acid | |
| 885 | 916 | 94.96 | 1.23 | Squalene | |
| Hexane | 878 | 883 | 103.47 | 3.83 | -Tocopherol |
| 770 | 809 | 109.55 | 0.84 | -Tocopherol | |
| 883 | 887 | 116.01 | 4.35 | -Tocopherol | |
| 767 | 771 | 125.10 | 0.88 | Stigmasterol | |
| 919 | 925 | 128.74 | 15.34 | Heptacosane | |
| 813 | 884 | 131.90 | 1.44 | Simiarenol | |
| 854 | 856 | 138.60 | 1.90 | Friedelin | |
| 759 | 804 | 148.48 | 0.62 | Octadecanal | |
| 704 | 723 | 151.54 | 0.305 | 2-Hexadecanol | |
| 756 | 785 | 37.82 | 4.34 | Phytol | |
| 726 | 756 | 45.09 | 1.86 | Hexadecanoic acid | |
| 757 | 772 | 58.28 | 2.97 | Stearic acid | |
| Chloroform | 678 | 750 | 95.54 | 0.44 | 1-Hexadecanol |
| 680 | 683 | 97.30 | 0.24 | 1-Pentatriacontanol | |
| 853 | 862 | 103.79 | 4.85 | -Tocopherol | |
| 747 | 850 | 109.43 | 5.48 | 1-Docosene | |
| 812 | 850 | 110.75 | 3.83 | -Tocopherol | |
| 856 | 869 | 115.85 | 8.13 | -Tocopherol | |
| 724 | 730 | 122.14 | 4.48 | -Stigmasterol | |
| 895 | 913 | 128.09 | 24.22 | Heptacosane | |
| 750 | 836 | 131.18 | 2.34 | -Simiarenol | |
| 748 | 799 | 136.77 | 0.25 | Octadecanal | |
| 742 | 861 | 148.59 | 0.62 | Hexadecanal |
| Extract | Bacteria | Fungi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRSA | SA | AB | PA | EC | LM | ABc | PAc | CA | CN | |
| Hexane | R | R | R | R | R | R | R | R | R | R |
| Chloroform | R | R | R | 150 | R | R | R | R | R | R |
| Methanol | 150 | R | R | R | R | R | R | R | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-Méndez, J.L.; Bach, H.; Lorenzo-Leal, A.C.; Navarro-López, D.E.; López-Mena, E.R.; Hernández, L.R.; Sánchez-Arreola, E. Biological Activities and Chemical Profiles of Kalanchoe fedtschenkoi Extracts. Plants 2023, 12, 1943. https://doi.org/10.3390/plants12101943
Mejía-Méndez JL, Bach H, Lorenzo-Leal AC, Navarro-López DE, López-Mena ER, Hernández LR, Sánchez-Arreola E. Biological Activities and Chemical Profiles of Kalanchoe fedtschenkoi Extracts. Plants. 2023; 12(10):1943. https://doi.org/10.3390/plants12101943
Chicago/Turabian StyleMejía-Méndez, Jorge L., Horacio Bach, Ana C. Lorenzo-Leal, Diego E. Navarro-López, Edgar R. López-Mena, Luis Ricardo Hernández, and Eugenio Sánchez-Arreola. 2023. "Biological Activities and Chemical Profiles of Kalanchoe fedtschenkoi Extracts" Plants 12, no. 10: 1943. https://doi.org/10.3390/plants12101943





