Heavy Metal Contamination Alters the Co-Decomposition of Leaves of the Invasive Tree Rhus typhina L. and the Native Tree Koelreuteria paniculata Laxm
Abstract
:1. Introduction
2. Results
2.1. Differences in the Decomposition Variables
2.2. Differences in Soil pH
2.3. Differences in Soil Enzymatic Activities
2.4. Differences in Soil Bacterial Alpha Diversity
2.5. Differences in Soil Bacterial Community Structure
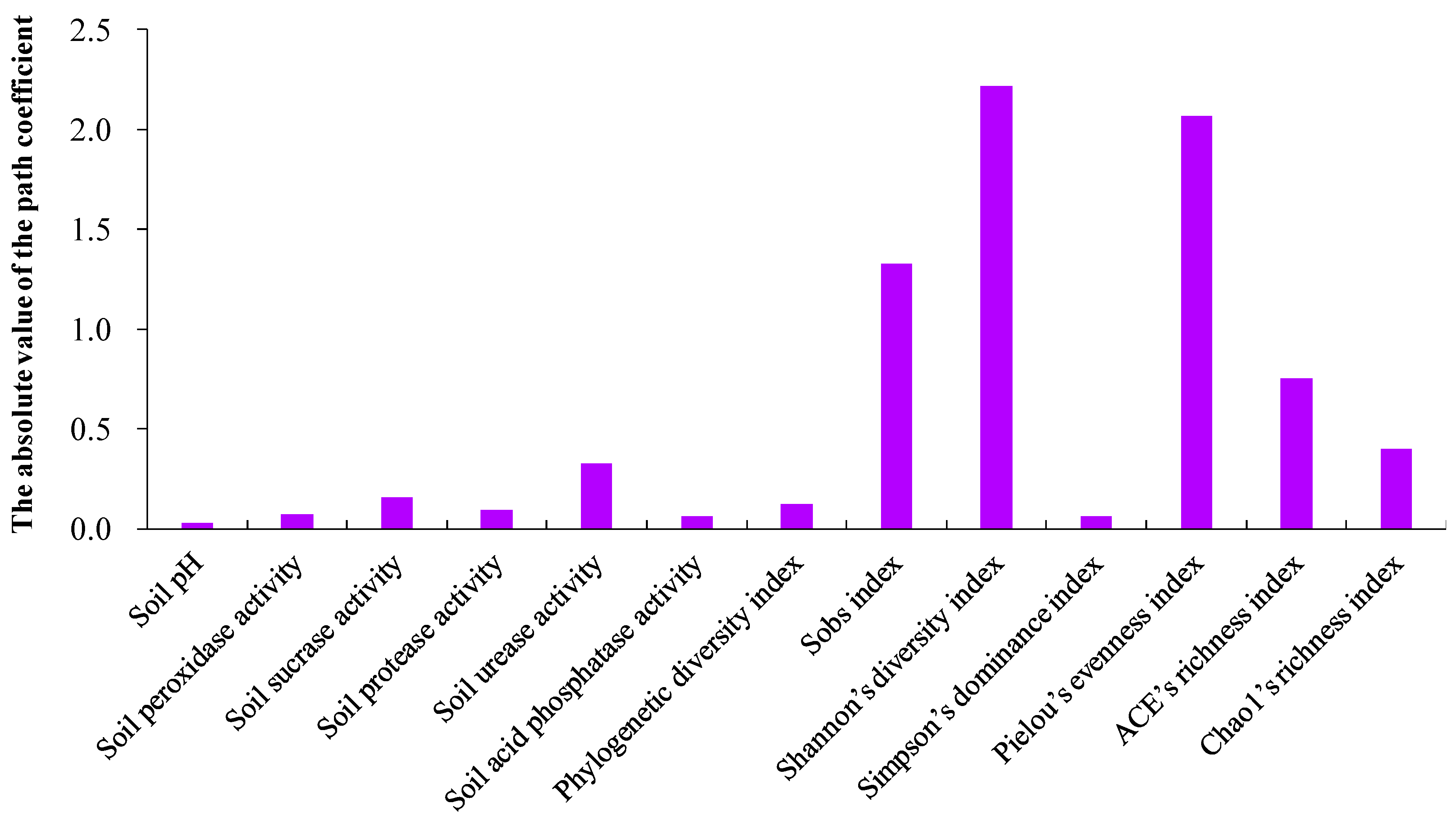
2.6. Contribution Intensity of Soil pH and Enzymatic Activities, and Soil Bacterial Alpha Diversity on K
3. Materials and Methods
3.1. Experimental Design
3.2. Determination of Decomposition Variables
3.3. Determination of Soil pH and Enzymatic Activities
3.4. Determination of Soil Bacterial Communities
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henry, A.L.; Gonzalez, E.; Bourgeois, B.; Sher, A.A. Invasive tree cover covaries with environmental factors to explain the functional composition of riparian plant communities. Oecologia 2021, 196, 1139–1152. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cheng, H.Y.; Wang, S.; Wei, M.; Du, D.L. Plant community and the influence of plant taxonomic diversity on community stability and invasibility: A case study based on Solidago canadensis L. Sci. Total Environ. 2021, 768, 144518. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, S.J.; Wakelin, A.; Peltzer, D.A.; Dickie, I.A. Pine invasion drives loss of soil fungal diversity. Biol. Invasions 2022, 24, 401–414. [Google Scholar] [CrossRef]
- Pysek, P.; Jarosik, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vila, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Yan, X.L.; Liu, Q.R.; Shou, H.Y.; Zeng, X.F.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.Y.; Qi, S.Y.; Ma, J.S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667–676. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, J.; Xiao, H.G.; Zhou, J.W.; Du, D.L. Floristic characteristics of alien invasive seed plant species in China. An. Acad. Bras. Ciências 2016, 88, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.P.; Liu, L.; Chu, H.; Ma, S.J.; Zhao, D.; Liang, R.R. Effects of exotic plant Rhus typhina invasion on soil properties in different forest types. Acta Ecol. Sin. 2015, 35, 5324–5330. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Xu, H.; Fan, Z.W.; Hou, Y.P. Effects of Rhus typhina invasion into young Pinus thunbergii forests on soil chemical properties. Ecol. Environ. Sci. 2013, 22, 1119–1123. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.; Wu, B.D.; Jiang, K.; Zhou, J.W.; Wang, C.Y. Variability of leaf functional traits of invasive tree Rhus typhina L. in North China. J. Cent. South Univ. 2020, 27, 155–163. [Google Scholar] [CrossRef]
- Xu, Z.W.; Guo, X.; Caplan, J.S.; Li, M.Y.; Guo, W.H. Novel plant-soil feedbacks drive adaption of invasive plants to soil legacies of native plants under nitrogen deposition. Plant Soil 2021, 467, 47–65. [Google Scholar] [CrossRef]
- Wang, G.M.; Jiang, G.M.; Yu, S.L.; Li, Y.H.; Liu, H. Invasion possibility and potential effects of Rhus typhina on beijing municipality. J. Integr. Plant Biol. 2008, 50, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Jiang, C.D.; Zhang, J.Z.; Zhang, H.J.; Shi, L. Ecophysiological evaluation of the potential invasiveness of Rhus typhina in its non-native habitats. Tree Physiol. 2009, 29, 1307–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Zhou, J.W.; Jiang, K.; Liu, J. Differences in leaf functional traits and allelopathic effects on seed germination and growth of Lactuca sativa between red and green leaves of Rhus typhina. S. Afr. J. Bot. 2017, 111, 17–22. [Google Scholar] [CrossRef]
- Yu, Y.L.; Cheng, H.Y.; Wang, C.Y.; Du, D.L. Heavy drought reduces the decomposition rate of the mixed litters of two composite invasive alien plants. J. Plant Ecol. 2023, 16, rtac047. [Google Scholar] [CrossRef]
- Zhong, S.S.; Xu, Z.L.; Yu, Y.L.; Cheng, H.Y.; Wang, S.; Wei, M.; Du, D.L.; Wang, C.Y. Acid deposition at higher acidity weakens the antagonistic responses during the co-decomposition of two Asteraceae invasive plants. Ecotoxicol. Environ. Saf. 2022, 243, 114012. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wei, M.; Wang, S.; Wu, B.D.; Du, D.L. Cadmium influences the litter decomposition of Solidago canadensis L. and soil N-fixing bacterial communities. Chemosphere 2020, 246, 125717. [Google Scholar] [CrossRef]
- Dekanova, V.; Svitkova, I.; Novikmec, M.; Svitok, M. Litter breakdown of invasive alien plant species in a pond environment: Rapid decomposition of Solidago canadensis may alter resource dynamics. Limnologica 2021, 90, 125911. [Google Scholar] [CrossRef]
- Aldorfová, A.; Münzbergová, Z. Conditions of plant cultivation affect the differences in intraspecific plant-soil feedback between invasive and native dominants. Flora 2019, 261, 151492. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Stanek, M.; Frac, M.; Oszust, K.; Woch, M.W.; Zubek, S. Invasive plant Reynoutria japonica produces large amounts of phenolic compounds and reduces the biomass but not activity of soil microbial communities. Sci. Total Environ. 2021, 767, 145439. [Google Scholar] [CrossRef]
- Czortek, P.; Krolak, E.; Borkowska, L.; Bielecka, A. Impacts of soil properties and functional diversity on the performance of invasive plant species Solidago canadensis L. on post-agricultural wastelands. Sci. Total Environ. 2020, 729, 139077. [Google Scholar] [CrossRef]
- Yang, Q.; Carrillo, J.; Jin, H.y.; Shang, L.; Hovick, S.M.; Nijjer, S.; Gabler, C.A.; Li, B.; Siemann, E. Plant-soil biota interactions of an invasive species in its native and introduced ranges: Implications for invasion success. Soil Biol. Biochem. 2013, 65, 78–85. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zukswert, J.M. Invasive plant species and litter decomposition: Time to challenge assumptions. New Phytol. 2016, 209, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Arif, M.; Ding, D.D.; Li, J.J.; He, X.R.; Li, C.X. Invasive plants and species richness impact litter decomposition in Riparian Zones. Front. Plant Sci. 2022, 13, 955656. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.X.; Wan, D.J.; Tian, H.X.; He, W.X.; Wang, Z.Q.; Liu, Q. Pollution assessment of heavy metals in soils and plants around a molybdenum mine in Central China. Pol. J. Environ. Stud. 2019, 28, 123–133. [Google Scholar] [CrossRef]
- Chen, H.Y.; Teng, Y.G.; Lu, S.J.; Wang, Y.Y.; Wang, J.S. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Cheng, M.M.; Wu, L.H.; Huang, Y.J.; Luo, Y.M.; Christie, P. Total concentrations of heavy metals and occurrence of antibiotics in sewage sludges from cities throughout China. J. Soil. Sediment 2014, 14, 1123–1135. [Google Scholar] [CrossRef]
- Wang, H.T.; Wang, Q.J.; Cui, Z.Q.; Sun, Z.; Sun, C. Analysis of correlation among heavy metals elements and background value of heavy metal elements in soil of Nantong suburb. J. Anhui Agric. Sci. 2011, 39, 14062–14064. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jiang, K.; Zhou, J.W.; Liu, J.; Wu, B.D. Responses of soil N-fixing bacterial communities to redroot pigweed (Amaranthus retroflexus L.) invasion under Cu and Cd heavy metal soil pollution. Agric. Ecosyst. Environ. 2018, 267, 15–22. [Google Scholar] [CrossRef]
- Wu, Y.M.; Leng, Z.R.; Li, J.; Jia, H.; Yan, C.L.; Hong, H.L.; Wang, Q.; Lu, Y.Y.; Du, D.L. Increased fluctuation of sulfur alleviates cadmium toxicity and exacerbates the expansion of Spartina alterniflora in coastal wetlands. Environ. Pollut. 2022, 292, 118399. [Google Scholar] [CrossRef]
- Mesa-Marin, J.; Redondo-Gomez, S.; Rodriguez-Llorente, I.D.; Pajuelo, E.; Mateos-Naranjo, E. Microbial strategies in non-target invasive Spartina densiflora for heavy metal clean up in polluted saltmarshes. Estuar. Coast. Shelf Sci. 2020, 238, 106730. [Google Scholar] [CrossRef]
- Xu, Z.L.; Zhong, S.S.; Yu, Y.L.; Wang, Y.Y.; Cheng, H.Y.; Du, D.L.; Wang, C.Y. Rhus typhina L. triggered greater allelopathic effects than Koelreuteria paniculata Laxm under ammonium fertilization. Sci. Hortic. 2023, 309, 111703. [Google Scholar] [CrossRef]
- Jiang, K.; Wu, B.D.; Wang, C.Y.; Ran, Q. Ecotoxicological effects of metals with different concentrations and types on the morphological and physiological performance of wheat. Ecotoxicol. Environ. Saf. 2019, 167, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wu, B.D.; Jiang, K.; Zhou, J.W. Effects of different types of heavy metal pollution on functional traits of invasive redroot pigweed and native red amaranth. Int. J. Environ. Res. 2018, 12, 419–427. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.; Wu, B.D.; Cheng, H.Y.; Wang, C.Y. Heavy metal pollution improves allelopathic effects of Canada goldenrod on lettuce germination. Plant Biol. 2020, 22, 832–838. [Google Scholar] [CrossRef]
- Lu, Y.J.; Wang, Y.F.; Wu, B.D.; Wang, S.; Wei, M.; Du, D.L.; Wang, C.Y. Allelopathy of three Compositae invasive alien species on indigenous Lactuca sativa L. enhanced under Cu and Pb pollution. Sci. Hortic. 2020, 267, 109323. [Google Scholar] [CrossRef]
- Shang, E.; Xu, E.Q.; Zhang, H.Q.; Huang, C.H. Spatial-temporal trends and pollution source analysis for heavy metal contamination of cultivated soils in five major grain producing regions of China. Environ. Sci. 2018, 39, 4670–4683. [Google Scholar]
- Chen, N.C.; Zheng, Y.J.; He, X.F.; Li, X.F.; Zhang, X.X. Analysis of the Report on the national general survey of soil contamination. J. Agro-Environ. Sci. 2017, 36, 1689–1692. [Google Scholar]
- Wang, C.Y.; Wu, B.D.; Jiang, K.; Wei, M.; Wang, S. Effects of different concentrations and types of Cu and Pb on soil N-fixing bacterial communities in the wheat rhizosphere. Appl. Soil Ecol. 2019, 144, 51–59. [Google Scholar] [CrossRef]
- Statistics, Z.B.o. Zhenjiang Statistical Yearbook 2022; China Statistics Press: Beijing, China, 2022.
- Zhang, W.L.; Xu, A.G.; Zhang, R.L.; Ji, H.J. Review of soil classification and revision of China soil classification system. Sci. Agric. Sin. 2014, 47, 3214–3230. [Google Scholar]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Hoorens, B.; Aerts, R.; Stroetenga, M. Does initial litter chemistry explain litter mixture effects on decomposition. Oecologia 2003, 137, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.L.; Scullion, J.; Worgan, H.; Gwynn-Jones, D. Litter of the invasive shrub Rhododendron ponticum (Ericaceae) modifies the decomposition rate of native UK woodland litter. Ecol. Indic. 2019, 107, 105597. [Google Scholar] [CrossRef]
- Zhang, J.E. Experimental Methods and Techniques Commonly Used in Ecology; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Perucci, P.; Casucci, C.; Dumontet, S. An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol. Biochem. 2000, 32, 1927–1933. [Google Scholar] [CrossRef]
- Guan, S.Y. Soil Enzyme and Its Research Methods; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Wear, E.K.; Wilbanks, E.G.; Nelson, C.E.; Carlson, C.A. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ. Microbiol. 2018, 20, 2709–2726. [Google Scholar] [CrossRef] [Green Version]
- Nwuche, C.O.; Ugoji, E.O. Effects of heavy metal pollution on the soil microbial activity. Int. J. Environ. Sci. Technol. 2008, 5, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Gu, P.X.; Liu, X.Y.; Huang, X.; Wang, J.Y.; Zhang, S.Y.; Ji, J.H. Effect of crop straw biochars on the remediation of Cd-contaminated farmland soil by hyperaccumulator Bidens pilosa L. Ecotoxicol. Environ. Saf. 2021, 219, 112332. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.M.; Wu, H.P.; Hua, S.S.; Liu, J.Y.; Yuan, Y.J.; Xiao, H.B.; Deng, L.J.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557–558, 785–790. [Google Scholar] [CrossRef]
- Liao, C.Z.; Luo, Y.Q.; Jiang, L.F.; Zhou, X.H.; Wu, X.W.; Fang, C.M.; Chen, J.K.; Li, B. Invasion of Spartina alterniflora enhanced ecosystem carbon and nitrogen stocks in the Yangtze Estuary, China. Ecosystems 2007, 10, 1351–1361. [Google Scholar] [CrossRef]
- Liao, C.Z.; Peng, R.H.; Luo, Y.Q.; Zhou, X.H.; Wu, X.W.; Fang, C.M.; Chen, J.K.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef]
- Maan, I.; Kaur, A.; Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Arora, N.K. Variations in leaf litter decomposition explain invasion success of Broussonetia papyrifera over confamilial non-invasive Morus alba in urban habitats. Urban For. Urban Green. 2022, 67, 127408. [Google Scholar] [CrossRef]
- Mitchell, J.D.; Lockaby, B.G.; Brantley, E.F. Influence of Chinese Privet (Ligustrum sinense) on decomposition and nutrient availability in riparian forests. Invasive Plant Sci. Manag. 2011, 4, 437–447. [Google Scholar] [CrossRef]
- Schuster, M.J.; Dukes, J.S. Non-additive effects of invasive tree litter shift seasonal N release: A potential invasion feedback. Oikos 2014, 123, 1101–1111. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Fromin, N.; Barantal, S. Functional diversity of terrestrial microbial decomposers and their substrates. Comptes Rendus Biol. 2011, 334, 393–402. [Google Scholar] [CrossRef]
- Brunel, C.; Gros, R.; Ziarelli, F.; Farnet Da Silva, A.M. Additive or non-additive effect of mixing oak in pine stands on soil properties depends on the tree species in Mediterranean forests. Sci. Total Environ. 2017, 590–591, 676–685. [Google Scholar] [CrossRef]
- Yu, H.X.; Le Roux, J.J.; Jiang, Z.Y.; Sun, F.; Peng, C.L.; Li, W.H. Soil nitrogen dynamics and competition during plant invasion: Insights from Mikania micrantha invasions in China. New Phytol. 2021, 229, 3440–3452. [Google Scholar] [CrossRef]
- Torres, N.; Herrera, I.; Fajardo, L.; Bustamante, R.O. Meta-analysis of the impact of plant invasions on soil microbial communities. BMC Ecol. Evol. 2021, 21, 172. [Google Scholar] [CrossRef]
- Allison, S.D.; Nielsen, C.; Hughes, R.F. Elevated enzyme activities in soils under the invasive nitrogen-fixing tree Falcataria moluccana. Soil Biol. Biochem. 2006, 38, 1537–1544. [Google Scholar] [CrossRef]
- Yu, Y.L.; Cheng, H.Y.; Wang, S.; Wei, M.; Wang, C.Y.; Du, D.L. Drought may be beneficial to the competitive advantage of Amaranthus spinosus. J. Plant Ecol. 2022, 15, 494–508. [Google Scholar] [CrossRef]
- Murugan, R.; Beggi, F.; Prabakaran, N.; Maqsood, S.; Joergensen, R.G. Changes in plant community and soil ecological indicators in response to Prosopis juliflora and Acacia mearnsii invasion and removal in two biodiversity hotspots in Southern India. Soil Ecol. Lett. 2020, 2, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Stefanowicz, A.M.; Stanek, M.; Nobis, M.; Zubek, S. Species-specific effects of plant invasions on activity, biomass, and composition of soil microbial communities. Biol. Fertil. Soils 2016, 52, 841–852. [Google Scholar] [CrossRef] [Green Version]


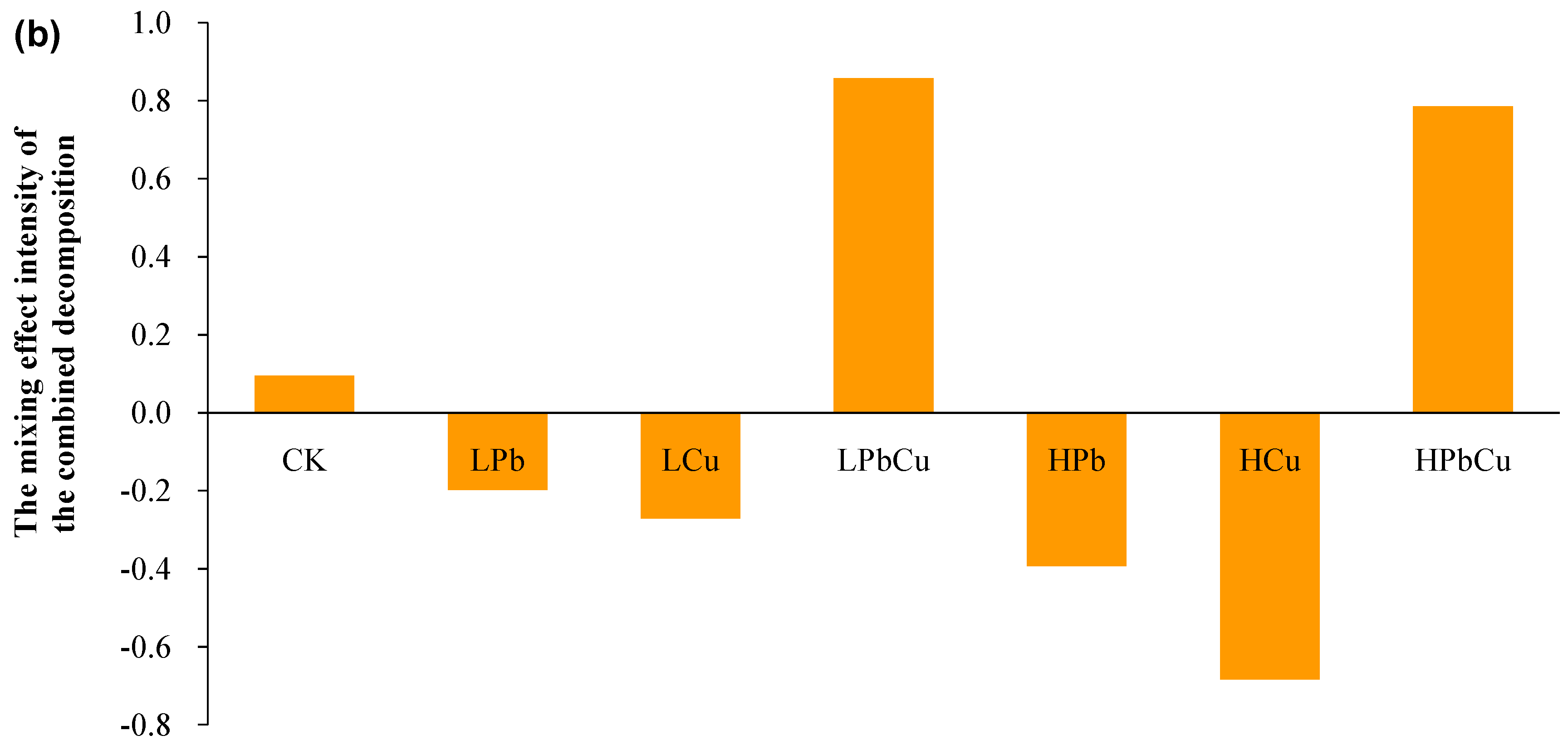

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhong, S.; Yu, Y.; Li, Y.; Li, C.; Xu, Z.; Liu, J.; Wang, C.; Du, D. Heavy Metal Contamination Alters the Co-Decomposition of Leaves of the Invasive Tree Rhus typhina L. and the Native Tree Koelreuteria paniculata Laxm. Plants 2023, 12, 2523. https://doi.org/10.3390/plants12132523
Xu Z, Zhong S, Yu Y, Li Y, Li C, Xu Z, Liu J, Wang C, Du D. Heavy Metal Contamination Alters the Co-Decomposition of Leaves of the Invasive Tree Rhus typhina L. and the Native Tree Koelreuteria paniculata Laxm. Plants. 2023; 12(13):2523. https://doi.org/10.3390/plants12132523
Chicago/Turabian StyleXu, Zhelun, Shanshan Zhong, Youli Yu, Yue Li, Chuang Li, Zhongyi Xu, Jun Liu, Congyan Wang, and Daolin Du. 2023. "Heavy Metal Contamination Alters the Co-Decomposition of Leaves of the Invasive Tree Rhus typhina L. and the Native Tree Koelreuteria paniculata Laxm" Plants 12, no. 13: 2523. https://doi.org/10.3390/plants12132523




