Topsoil and Vegetation Dynamics 14 Years after Eucalyptus grandis Removal in Eastern Cape Province of South Africa
Abstract
:1. Introduction
2. Results
2.1. Effects of Alien Plant Clearing on Soil Properties
2.2. Effects of Alien Plant Clearing in Vegetation
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Experimental Design and Data Collection
4.3. Data Analysis
5. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species Name | Cleared | Natural |
|---|---|---|
| Acacia mearnsii | 25 | 0 |
| Anthospermum aethiopicum | 5 | 0 |
| Anthospermum spathulatum | 30 | 15 |
| Aspalathus subtingens | 0 | 35 |
| Asparagus suaveolens | 50 | 80 |
| Athanasia dentata | 5 | 15 |
| Chasmanthe aethiopica | 0 | 5 |
| Clutia daphnoides | 0 | 5 |
| Conyza scabrida | 0 | 5 |
| Crassula pellucida | 50 | 60 |
| Dovyalis rhamnoides | 5 | 5 |
| Eucalyptus grandis | 30 | 0 |
| Halleria lucida | 5 | 15 |
| Helichrysum cymosum | 20 | 70 |
| Helichrysum petiolare | 65 | 45 |
| Indigofera sp. | 5 | 10 |
| Maytenus acuminata | 0 | 5 |
| Metalasia muricata | 5 | 20 |
| Passerina rigida | 0 | 15 |
| Podocarpus latifolius | 0 | 5 |
| Protea neriifolia | 0 | 10 |
| Psychotria capensis | 0 | 5 |
| Pteronia incana | 0 | 25 |
| Searsia crenata | 25 | 15 |
| Rubus cuneifolius | 5 | 0 |
| Solanum linnaeanum | 10 | 0 |
| Zanthoxylum capense | 0 | 5 |
| Asplenium rutifolium | 5 | 20 |
| Centaurea benedicta | 15 | 0 |
| Centella asiatica | 70 | 65 |
| Conyza bonariensis | 95 | 20 |
| Erigeron canadensis | 5 | 0 |
| Erigeron sp. | 10 | 10 |
| Euphorbia epicyparissias | 0 | 15 |
| Pellaea mucronate | 0 | 25 |
| Pteridium aquilinum | 0 | 10 |
| Senecio macrocephalus | 40 | 60 |
| Thesium gnidiodes | 45 | 40 |
| Wahlenbergia procumbens | 5 | 0 |
| Agrostis lachnantha | 30 | 55 |
| Alloteropsis semialata | 25 | 0 |
| Aristida transvaalensis | 5 | 0 |
| Carex sp. | 20 | 20 |
| Cynodon dactylon | 5 | 0 |
| Cyperus albostriatus | 20 | 0 |
| Digitaria sanguinalis | 25 | 65 |
| Ehrharta erecta | 35 | 0 |
| Isolepis cernua | 5 | 0 |
| Pennisetum clandestinum | 20 | 50 |
| Sporobolus africanus | 20 | 5 |
References
- van Wilgen, B.W.; Measey, J.; Richardson, D.M.; Wilson, R.U.; Zengeya, T.A. Biological invasions in South Africa: An overview. In Biological Invasions in South Africa; van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–32. [Google Scholar]
- O’Connor, T.; van Wilgen, B.W. The impact of invasive alien plants on rangelands in South Africa. In Biological Invasions in South Africa; van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 457–486. [Google Scholar]
- Le Maitre, D.C.; Blignaut, J.N.; Clulow, A.; Dzikiti, S.; Everson, C.G.; Örgens, A.H.M.; Gush, M.B. Impacts of plant invasions on terrestrial water flows in South Africa. In Biological Invasions in South Africa; van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 429–456. [Google Scholar]
- Ngorima, A.; Shackleton, C.M. Livelihood benefits and costs from an invasive alien tree (Acacia dealbata) to rural communities in the Eastern Cape, South Africa. J. Environ. Manag. 2019, 229, 158–165. [Google Scholar] [CrossRef] [PubMed]
- van Wilgen, B.W.; Scott, D.F. Managing fires on the Cape Peninsula: Dealing with the inevitable. J. Mediterr. Ecol. 2001, 2, 197–208. [Google Scholar]
- Gaertner, M.; Larson, B.M.H.; Irlich, U.M.; Holmes, P.M.; Stafford, L.; van Wilgen, B.W.; Richardson, D.M. Managing invasive species in cities: A framework from Cape Town, South Africa. Landsc. Urban Plan. 2016, 151, 1–9. [Google Scholar] [CrossRef]
- Holmes, P.M.; Esler, K.J.; Gaertner, M.; Geerts, S.; Hall, S.A.; Nsikani, M.M.; Richardson, D.M.; Ruwanza, S. Biological invasions and ecological restoration in South Africa. In Biological Invasions in South Africa; van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 665–700. [Google Scholar]
- van Wilgen, B.W.; Le Maitre, D.C.; Cowling, R.M. Ecosystem services, efficiency, sustainability and equity: South Africa’s Working for Water programme. Trends Ecol. Evol. 1998, 13, 379. [Google Scholar] [CrossRef]
- van Wilgen, B.W.; Forsyth, G.G.; Le Maitre, D.C.; Wannenburg, A.; Kotze, J.D.F.; ven den Berg, E.; Henderson, L. An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa. Biol. Conserv. 2012, 148, 28–38. [Google Scholar] [CrossRef]
- van Wilgen, B.W.; Wilson, J.R.; Wannenburgh, A.; Foxcroft, L.C. The extent and effectiveness of alien plant control projects in South Africa. In Biological Invasions in South Africa; van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 597–628. [Google Scholar]
- Ruwanza, S.; Gaertner, M.; Esler, K.J.; Richardson, D.M. Medium-term vegetation recovery after removal of invasive Eucalyptus camaldulensis stands along a South African river. S. Afr. J. Bot. 2018, 119, 63–68. [Google Scholar] [CrossRef]
- Reinecke, M.K.; Pigot, A.; King, J.M. Spontaneous succession of riparian fynbos: Is unassisted recovery a viable restoration strategy? S. Afr. J. Bot. 2008, 74, 412–420. [Google Scholar] [CrossRef]
- Ruwanza, S.; Gaertner, M.; Esler, K.J.; Richardson, D.M. The effectiveness of active and passive restoration on recovery of indigenous vegetation in riparian zones in the Western Cape, South Africa: A preliminary assessment. S. Afr. J. Bot. 2013, 88, 132–141. [Google Scholar] [CrossRef]
- Ndou, E.; Ruwanza, S. Soil and vegetation recovery following alien tree clearing in the Eastern Cape Province of South Africa. Afr. J. Ecol. 2016, 54, 460–470. [Google Scholar] [CrossRef]
- Fill, J.M.; Kritzinger-Klopper, S.; Van Wilgen, B.W. Short-term vegetation recovery after alien plant clearing along the Rondegat River, South Africa. Restor. Ecol. 2018, 26, 434–438. [Google Scholar] [CrossRef]
- Holmes, P.M.; Esler, K.J.; Richardson, D.M.; Witkowski, E.T.F. Guidelines for improved management of riparian zones invaded by alien plants in South Africa. S. Afr. J. Bot. 2008, 74, 538–552. [Google Scholar] [CrossRef]
- Blanchard, R.; Holmes, P.M. Riparian vegetation recovery after invasive alien tree clearance in the fynbos biome. S. Afr. J. Bot. 2008, 74, 421–431. [Google Scholar] [CrossRef]
- Hagen, D.; Evju, M. Using short-term monitoring data to achieve goals in a large-scale restoration. Ecol. Soc. 2013, 18, 29. [Google Scholar] [CrossRef]
- Gerhart, V.J.; Waugh, W.J.; Glenn, E.P.; Pepper, I.L. Ecological restoration. In Monitoring and Characterization of the Environment; Artiola, J.F., Pepper, I.L., Brusseau, M.L., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 357–375. [Google Scholar]
- Prach, K.; Marrs, R.; Pyšek, P.; van Diggelen, R. Manipulation of succession. In Linking Restoration and Ecological Succession; Walker, L.R., Walker, J., Hobbs, R.J., Eds.; Springer Science + Business Media: New York, NY, USA, 2007; pp. 121–149. [Google Scholar]
- Rodríguez-Echeverría, S.; Afonso, C.; Correia, M.; Lorenzo, P.; Roiloa, S.R. The effect of soil legacy on competition and invasion by Acacia dealbata Link. Plant Ecol. 2013, 214, 1139–1146. [Google Scholar] [CrossRef]
- Zhu, S.-C.; Zheng, H.-X.; Liu, W.-S.; Liu, C.; Guo, M.-N.; Huot, H.; Morel, J.L.; Qiu, R.-L.; Chao, Y.; Tang, Y.-T. Plant-soil feedbacks for the restoration of degraded mine lands: A Review. Front. Microbiol. 2022, 12, 751794. [Google Scholar] [CrossRef]
- Nsikani, M.M.; Geerts, S.; Ruwanza, S.; Richardson, D.M. Secondary invasion and weedy native species dominance after clearing invasive alien plants in South Africa: Status quo and prognosis. S. Afr. J. Bot. 2020, 132, 338–345. [Google Scholar] [CrossRef]
- Kerr, T.F.; Ruwanza, S. Does Eucalyptus grandis invasion and removal affect soils and vegetation in the Eastern Cape Province, South Africa? Austral Ecol. 2016, 41, 328–338. [Google Scholar] [CrossRef]
- Nsikani, M.M.; Novoa, A.; van Wilgen, B.W.; Keet, J.; Gaertner, M. Acacia saligna’s soil legacy effects persist up to 10 years after clearing: Implications for ecological restoration. Austral Ecol. 2017, 42, 880–889. [Google Scholar] [CrossRef]
- Tererai, F.; Gaertner, M.; Jacobs, S.M.; Richardson, D.M. Eucalyptus camaldulensis invasion in riparian zones reveals few significant effects on soil physico-chemical properties. River Res. Appl. 2015, 31, 590–601. [Google Scholar] [CrossRef]
- Grove, S.; Parker, I.M.; Haubensak, K.A. Persistence of a soil legacy following removal of a nitrogen-fixing invader. Biol. Invasions 2015, 17, 2621–2631. [Google Scholar] [CrossRef]
- Memiaghe, H.R. Old Field Restoration: Vegetation Response to Soil Changes and Restoration Efforts in Western Cape Lowlands. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2008. [Google Scholar]
- Scott, A.J.; Morgan, J.W. Recovery of soil and vegetation in semi-arid Australian old fields. J. Arid Environ. 2012, 76, 61–71. [Google Scholar] [CrossRef]
- Feng, H.; Liu, Y. Combined effects of precipitation and air temperature on soil moisture in different land covers in a humid basin. J. Hydrol. 2015, 531, 1129–1140. [Google Scholar] [CrossRef]
- Stavi, I.; Barkai, D.; Knoll, Y.M.; Zaady, E. Livestock grazing impact on soil wettability and erosion risk in post-fire agricultural lands. Sci. Total Environ. 2016, 573, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Hallett, P.D.; Bachmann, J.; Czachor, H.; Urbanek, E.; Zhang, B. Hydrophobicity of soil. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Galatowitsch, S.; Richardson, D.M. Riparian scrub recovery after clearing of invasive alien trees in headwater streams of the Western Cape, South Africa. Biol. Conserv. 2005, 122, 509–521. [Google Scholar] [CrossRef]
- Zahawi, R.A.; Augspurger, C.K. Tropical forest restoration: Tree islands as recruitment foci in degraded lands of Honduras. Ecol. Appl. 2006, 16, 464–478. [Google Scholar] [CrossRef]
- Ren, H.; Yang, L.; Liu, N. Nurse plant theory and its application in ecological restoration in lower subtropics of China. Prog. Nat. Sci. 2008, 18, 137–142. [Google Scholar] [CrossRef]
- Fourie, S. Composition of the soil seed bank in alien-invaded grassy fynbos: Potential for recovery after clearing. S. Afr. J. Bot. 2008, 74, 445–453. [Google Scholar] [CrossRef]
- Vosse, S.; Esler, K.J.; Richardson, D.M.; Holmes, P.M. Can riparian seed banks initiate restoration after alien plant invasion? Evidence from the Western Cape, South Africa. S. Afr. J. Bot. 2008, 74, 432–444. [Google Scholar] [CrossRef]
- Bennett, J.A.; Klironomos, J. Mechanisms of plant–soil feedback: Interactions among biotic and abiotic drivers. New Phytol. 2019, 222, 91–96. [Google Scholar] [CrossRef]
- Shiponeni, N.N.; Milton, S.J. Seed dispersal in the dung of large herbivores: Implications for restoration of Renosterveld shrubland old fields. Biodiver. Conserv. 2006, 15, 3161–3175. [Google Scholar] [CrossRef]
- Baltzinger, C.; Karimi, S.; Shukla, U. Plants on the move: Hitch-hiking with ungulates distributes diaspores across landscapes. Front. Ecol. Evol. 2019, 7, 38. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland; Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- Black, C.A. Methods of Soil Analysis: Part I Physical and Mineralogical Properties; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Doerr, S.H.; Thomas, A.D. The role of soil moisture in controlling water repellency: New evidence from forest soils in Portugal. J. Hydrol. 2000, 231–232, 134–147. [Google Scholar] [CrossRef]
- Bisdom, E.B.A.; Dekker, L.W.; Schoute, J.F.T. Water repellency of sieve fractions from sandy soils and relationships with organic material and soil structure. Geoderma 1993, 56, 105–118. [Google Scholar] [CrossRef]
- Leung, Y.-F.; Meyer, K. Soil compaction as indicated by penetration resistance: A comparison of two types of penetrometers. In Protecting Our Diverse Heritage: The Role of Parks, Protected Areas, and Cultural Sites, Proceedings of the George Wright Society/National Park Service Joint Conference, San Diego, CA, USA, 14–18 April 2003; George Wright Society: Hancock, MI, USA, 2003; pp. 370–375. [Google Scholar]
- Rhoades, J.D. Cation exchanger capacity. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 147–157. [Google Scholar]
- Bray, R.H.; Krutz, L.T. Determination of total, organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organic carbon fractions and soil quality changes in an Oxic Paleustalf under different pasture leys. Soil Sci. Soc. Am. J. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 159–162. [Google Scholar]
- Goldblatt, P.; Manning, J. Cape Plants. A Conspectus of the Cape Flora of South Africa; Strelitzia 9; National Botanical Institute: Pretoria, South Africa, 2000. [Google Scholar]
- Manning, J. Field Guide to Fynbos; Struik: Cape Town, South Africa, 2007. [Google Scholar]
- Manning, J.C.; Goldblatt, P. Plants of the Greater Cape Floristic Region 1: The Core Cape Flora; Strelitzia 29; South African National Biodiversity Institute: Pretoria, South Africa, 2012. [Google Scholar]
- South African National Biodiversity Institute (SANBI). PlantZAfrica.com. 2017. Available online: http://pza.sanbi.org/ (accessed on 10 October 2022).
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 14.0. 2019. Available online: http://tibco.com (accessed on 10 November 2022).
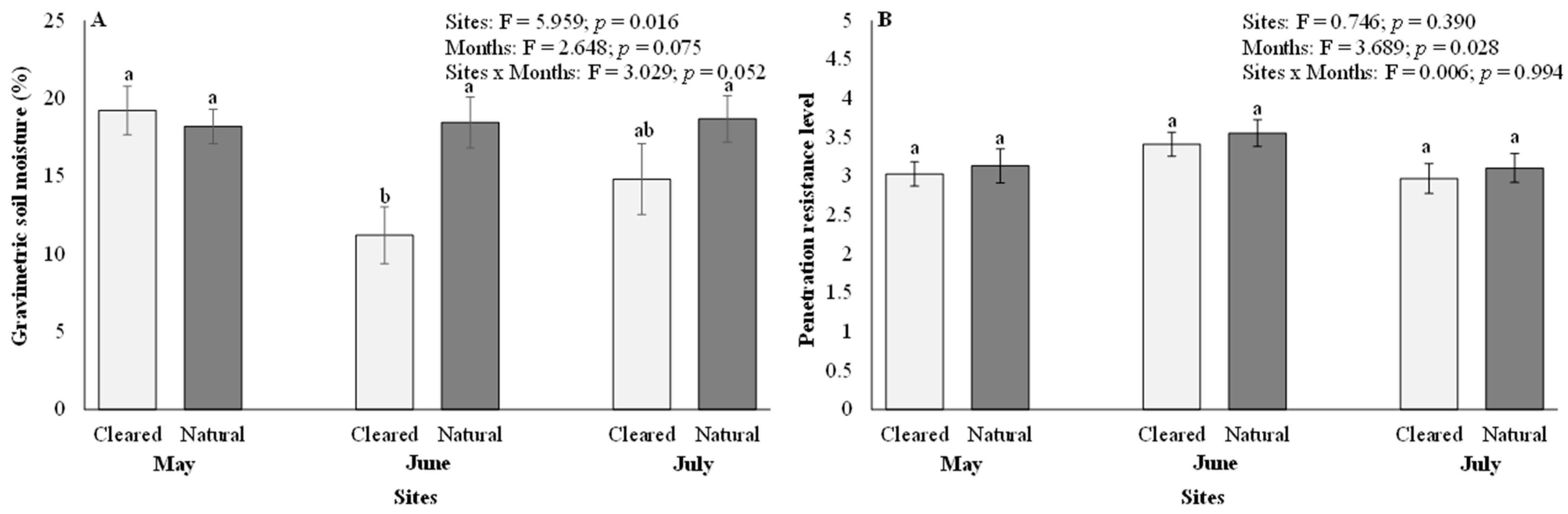
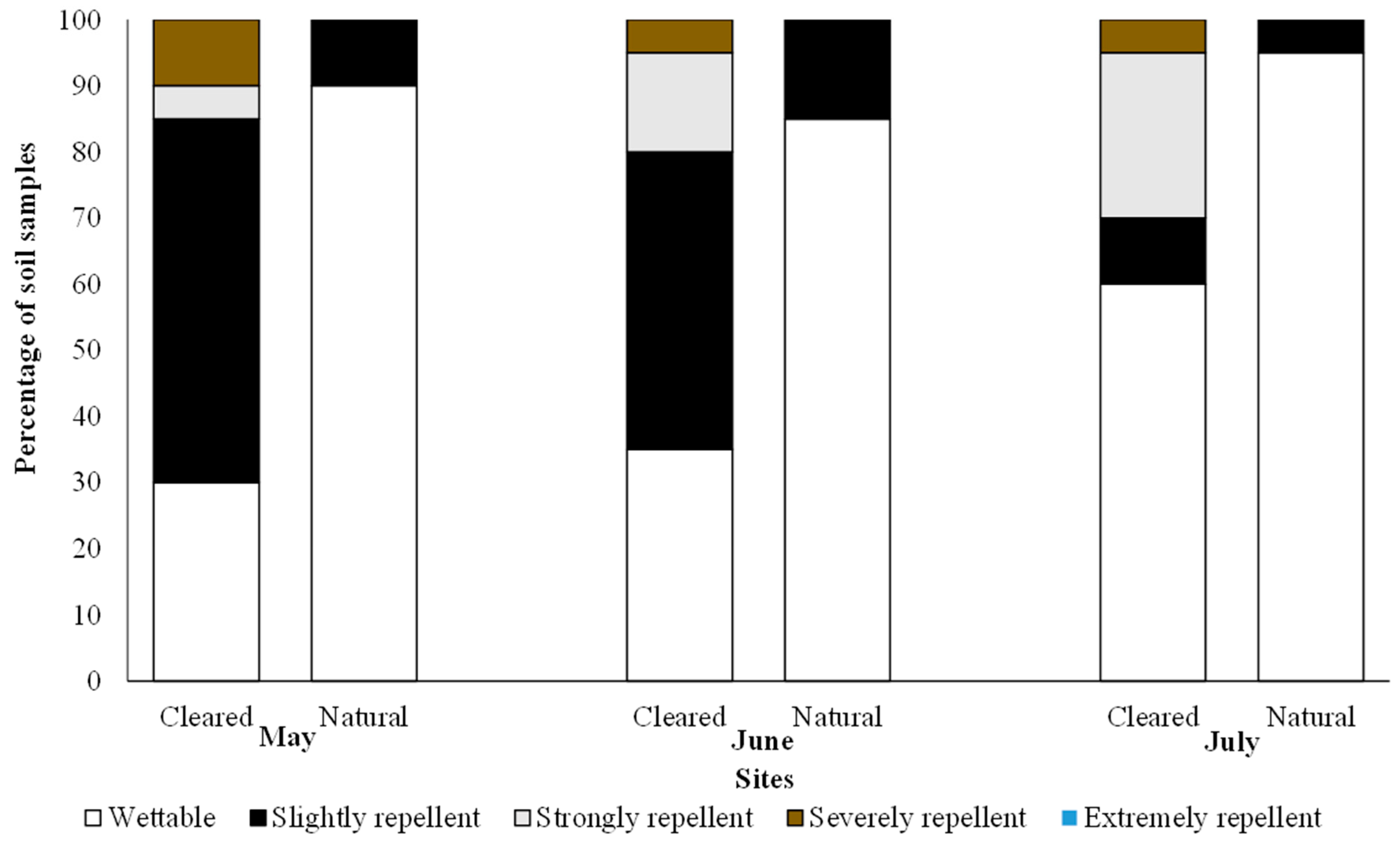
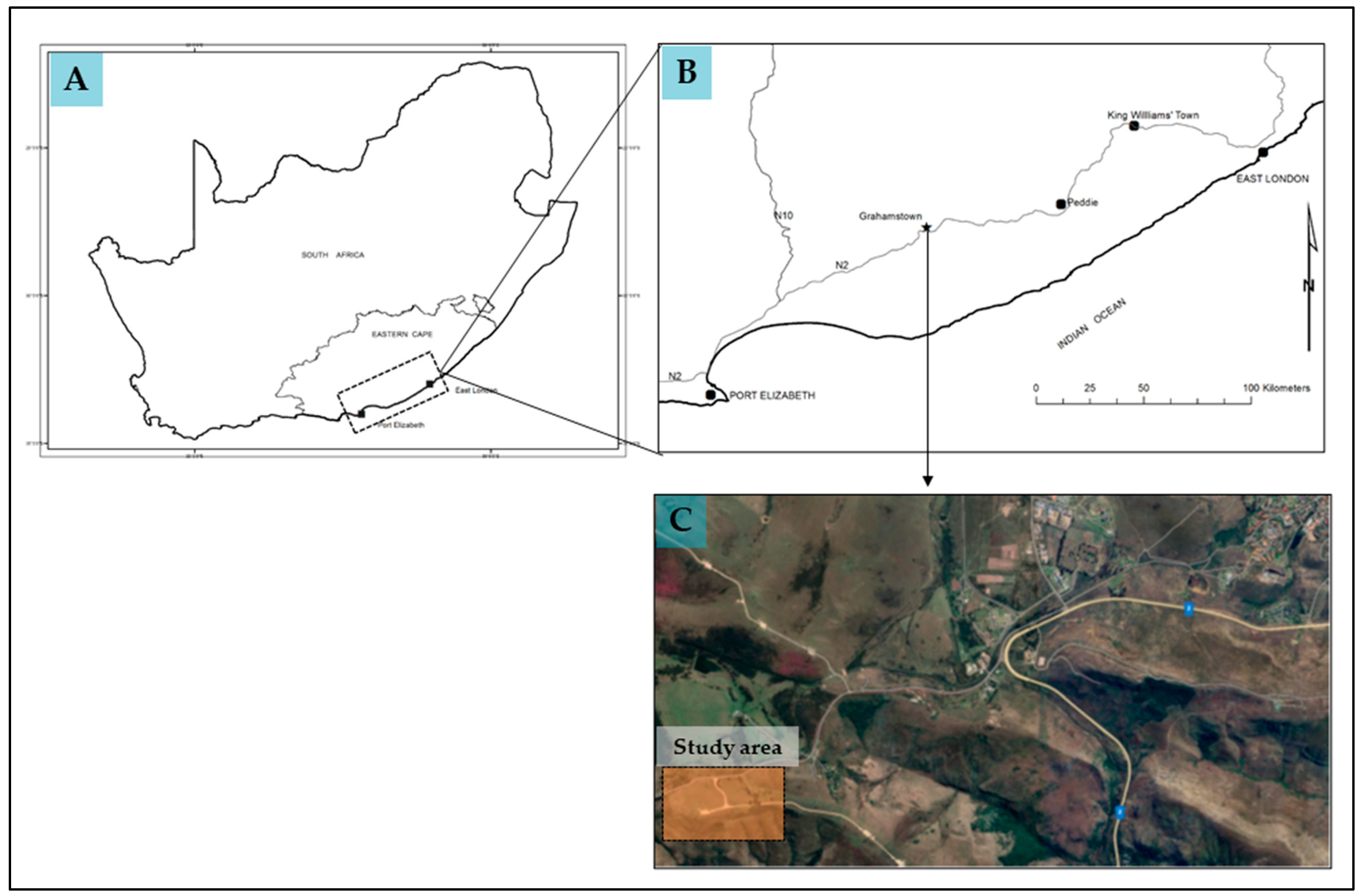
| Cleared | Natural | t-Values | p-Values | |
|---|---|---|---|---|
| Soil pH | 4.43 ± 0.19 | 4.11 ± 0.04 | 1.66 | 0.115 |
| Total nutrient concentrations | ||||
| P Bray II (mg/kg) | 3.66 ± 0.47 | 7.87 ± 1.27 | 3.11 | 0.006 |
| C (%) | 2.90 ± 0.25 | 3.51 ± 0.32 | 1.50 | 0.151 |
| N (%) | 0.23 ± 0.02 | 0.30 ± 0.04 | 1.50 | 0.150 |
| Exchangeable cations (%) | ||||
| K | 6.22 ± 1.63 | 8.77 ± 2.50 | 0.85 | 0.404 |
| Na | 2.49 ± 0.19 | 2.80 ± 0.23 | 1.02 | 0.323 |
| Ca | 38.43 ± 1.20 | 30.28 ± 4.31 | 1.82 | 0.085 |
| Mg | 14.46 ±1.65 | 20.19 ± 0.40 | 3.38 | 0.003 |
| Cleared | Natural | t-Values | p-Values | |
|---|---|---|---|---|
| Species richness | 8.65 ± 0.51 | 10.25 ± 0.63 | 1.96 | 0.057 |
| Shannon–Wiener | 1.25 ± 0.06 | 1.40 ± 0.08 | 1.51 | 0.139 |
| Simpsons index of diversity | 0.72 ± 0.04 | 0.74 ± 0.02 | 0.69 | 0.495 |
| Evenness index | 0.59 ± 0.03 | 0.61 ± 0.02 | 0.68 | 0.679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mthethwa, K.; Ruwanza, S. Topsoil and Vegetation Dynamics 14 Years after Eucalyptus grandis Removal in Eastern Cape Province of South Africa. Plants 2023, 12, 3047. https://doi.org/10.3390/plants12173047
Mthethwa K, Ruwanza S. Topsoil and Vegetation Dynamics 14 Years after Eucalyptus grandis Removal in Eastern Cape Province of South Africa. Plants. 2023; 12(17):3047. https://doi.org/10.3390/plants12173047
Chicago/Turabian StyleMthethwa, Kuhle, and Sheunesu Ruwanza. 2023. "Topsoil and Vegetation Dynamics 14 Years after Eucalyptus grandis Removal in Eastern Cape Province of South Africa" Plants 12, no. 17: 3047. https://doi.org/10.3390/plants12173047






