Integrative Approach to Identifying System-Level Mechanisms of Chung-Sang-Bo-Ha-Hwan’s Influence on Respiratory Tract Diseases: A Network Pharmacological Analysis with Experimental Validation
Abstract
:1. Introduction
2. Results
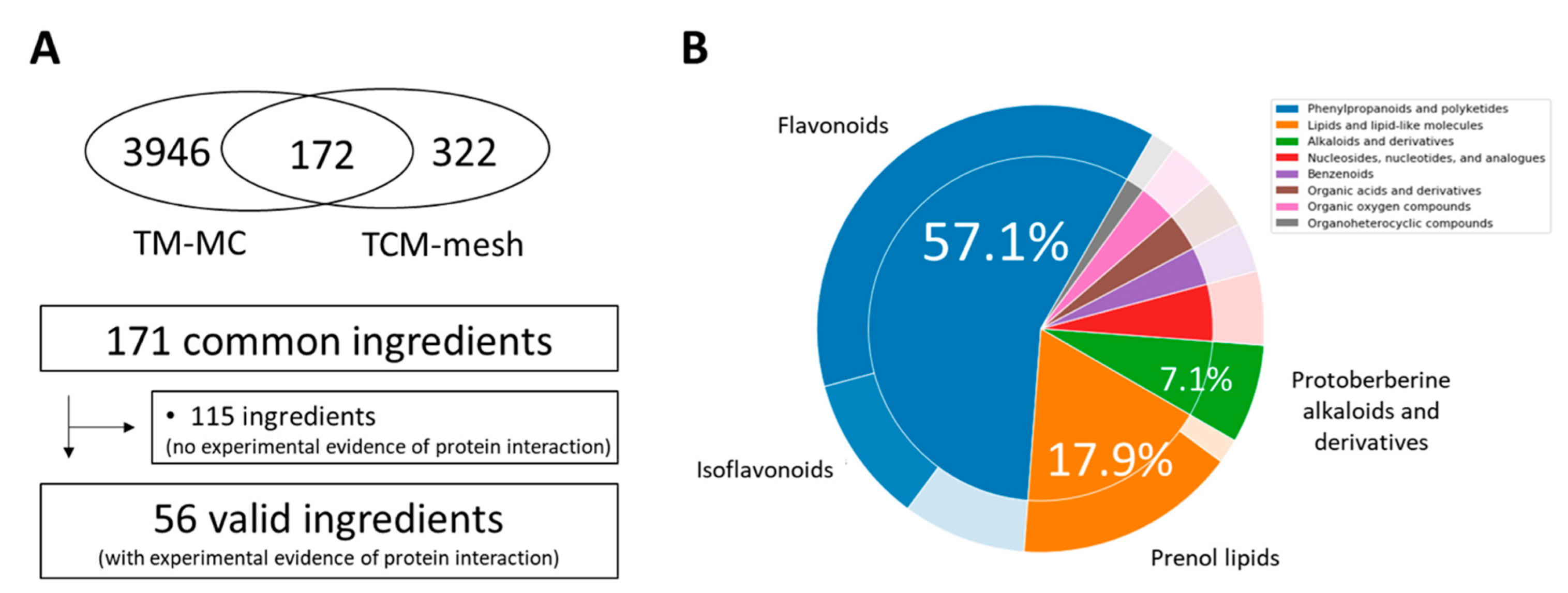
2.1. Identification of CSBHH Compounds and Targets
2.2. Degree of Overlap between CSBHH Targets and Respiratory Tract Disease-Related Proteins
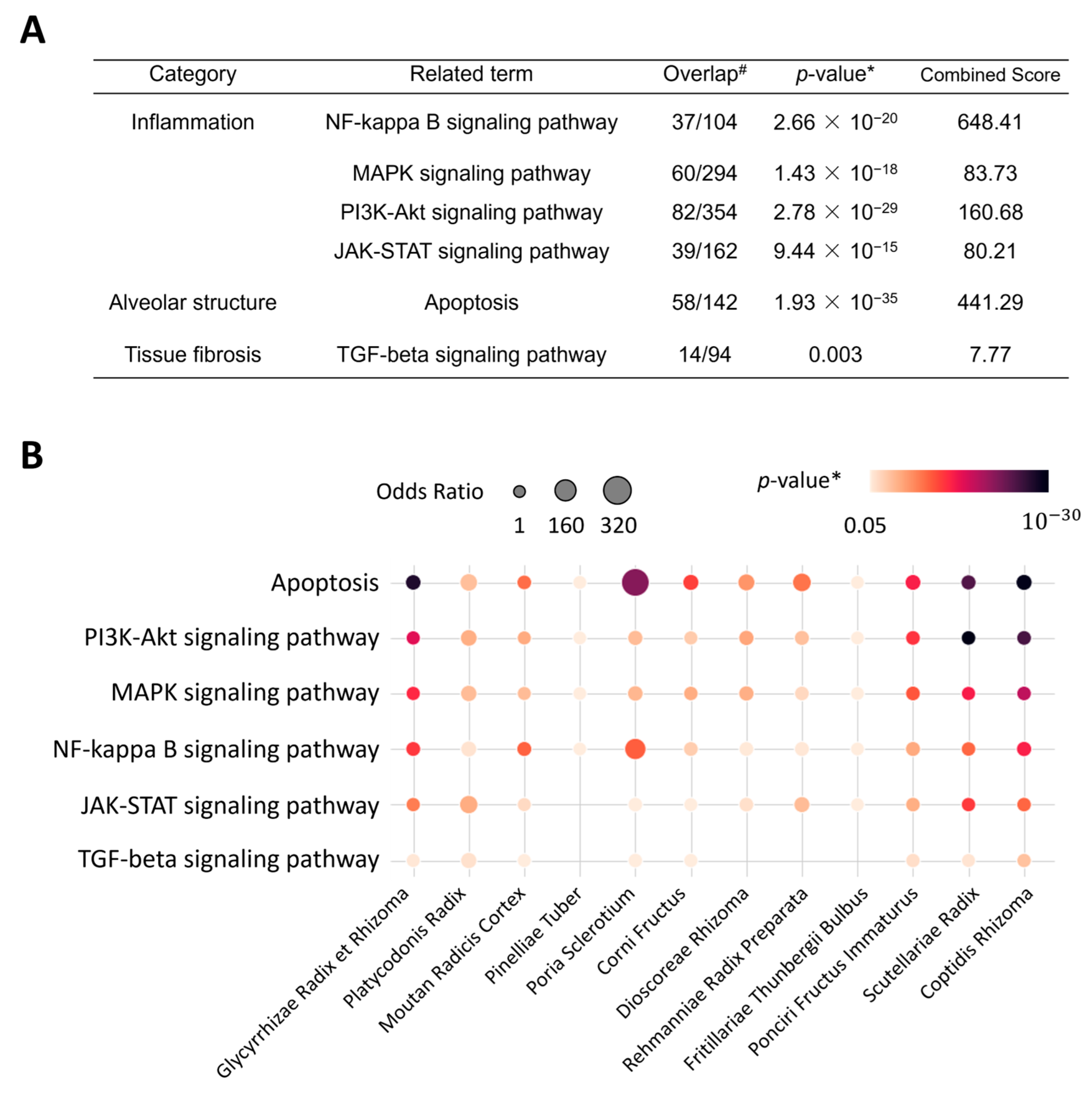
2.3. Enriched Pathways and Biological Processes of CSBHH
2.4. Network Analysis of the Mechanisms of CSBHH against Respiratory Tract Diseases
2.5. Effects of CSBHH on NCI-H292 Cell Viability
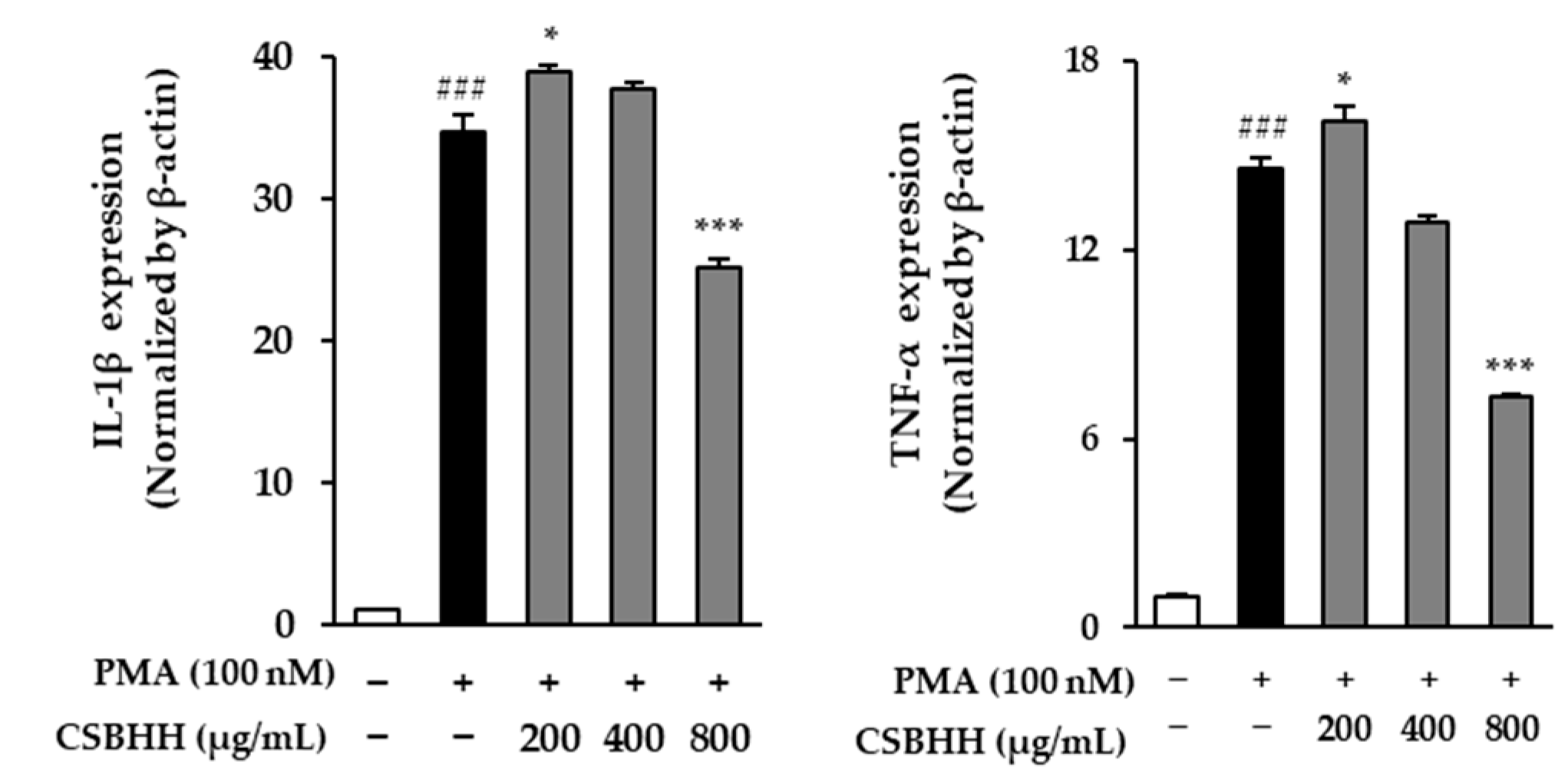
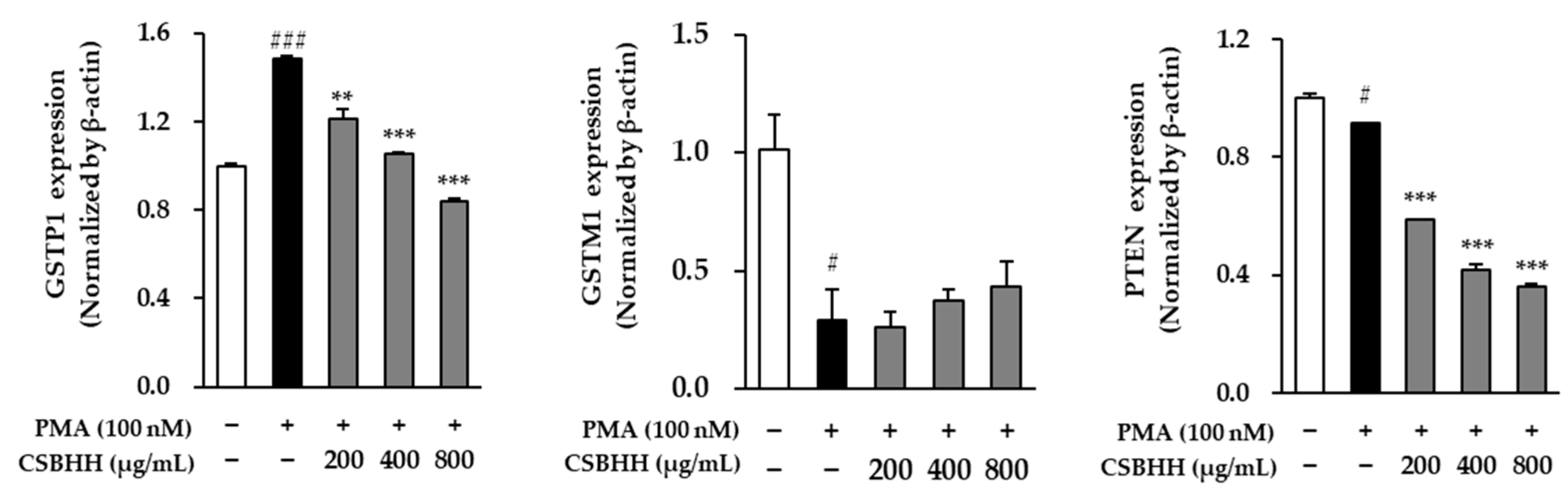
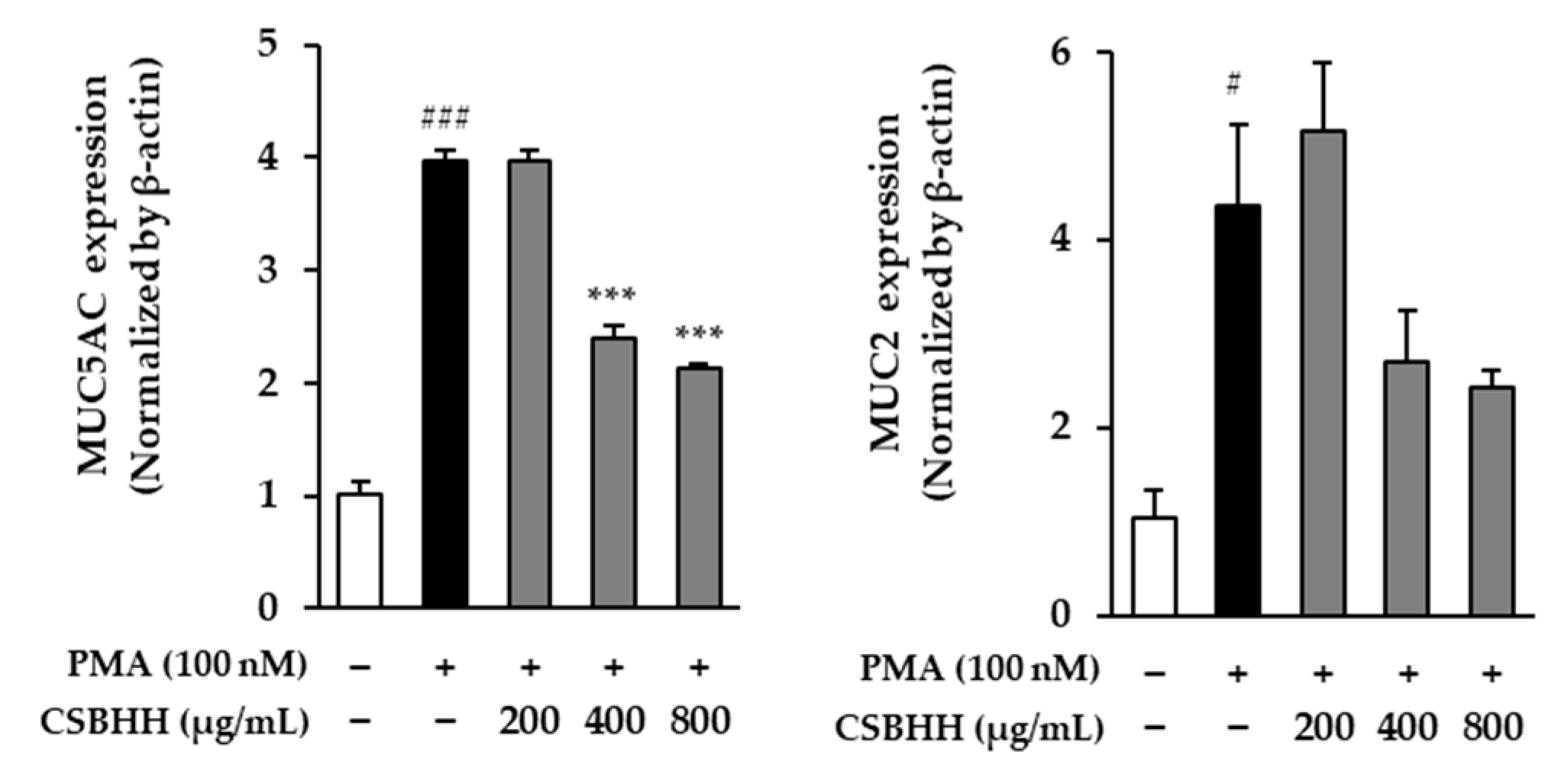
2.6. Effect of CSBHH on PMA-Induced mRNA Expression in NCI-H292 Cells
3. Discussion
4. Materials and Methods
4.1. Compound–Target Network Construction
4.2. Enrichment Analysis
4.3. Disease-Related Proteins and Pathways
4.4. Cell Culture
4.5. Sample Preparation
4.6. Cell Viability
4.7. Total RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Roh, G.S.; Seo, S.-W.; Yeo, S.; Lee, J.M.; Choi, J.-W.; Kim, E.; Shin, Y.; Cho, C.; Bae, H.; Jung, S.-K. Efficacy of a traditional Korean medicine, Chung-Sang-Bo-Ha-Tang, in a murine model of chronic asthma. Int. Immunopharmacol. 2005, 5, 427–436. [Google Scholar] [CrossRef]
- Ha, J.-Y.; You, B.-G. Antitumor and Immunomodulatory Effects of Chungsangbohahwan on Murine Melanoma-induced Lung Metastasis. J. Korean Orient. Oncol. 1998, 4, 159–175. [Google Scholar]
- Jung, S.; Hwang, W.; Ju, C.; Lee, J.; Cho, I.; Jung, H. Clinical effects of chuongsangboha-tang in asthmatic patients. J. Korean Orient. Med. 2002, 23, 151–160. Available online: https://koreascience.kr/article/JAKO200211923035393.pdf (accessed on 26 October 2002).
- Lee, H.; Kim, Y.; Kim, H.J.; Park, S.; Jang, Y.P.; Jung, S.; Jung, H.; Bae, H. Herbal formula, PM014, attenuates lung inflammation in a murine model of chronic obstructive pulmonary disease. Evid.-Based Complement. Altern. Med. 2012, 2012, 769830. [Google Scholar] [CrossRef]
- Jung, K.-H.; Choi, H.-L.; Park, S.; Lee, G.; Kim, M.; Min, J.-K.; Min, B.-I.; Bae, H. The effects of the standardized herbal formula PM014 on pulmonary inflammation and airway responsiveness in a murine model of cockroach allergen-induced asthma. J. Ethnopharmacol. 2014, 155, 113–122. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.; Lee, H.; Shin, D.; Min, D.; Kim, M.; Ryu, B.; Kim, H.W.; Bae, H. A standardized herbal extract PM014 ameliorates pulmonary fibrosis by suppressing the TGF-β1 pathway. Sci. Rep. 2018, 8, 16860. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Lee, C.-Y.; Kim, Y.-S.; Kim, C.-E. The methodological trends of traditional herbal medicine employing network pharmacology. Biomolecules 2019, 9, 362. [Google Scholar] [CrossRef]
- Ruiz, C.; Zitnik, M.; Leskovec, J. Identification of disease treatment mechanisms through the multiscale interactome. Nat. Commun. 2021, 12, 1796. [Google Scholar] [CrossRef]
- Kang, S.-W.; Kim, K.-I.; Bu, Y.; Lee, B.-J.; Jung, H.-J. Therapeutic Potential of Chungsangboha-tang for the Treatment of Asthma: A Review of Preclinical and Clinical Studies. J. Clin. Med. 2022, 11, 4035. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminformatics 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fang, J.; Lu, W.; Wang, Z.; Wang, Q.; Hou, Y.; Jiang, X.; Reizes, O.; Lathia, J.; Nussinov, R. A systems pharmacology approach uncovers wogonoside as an angiogenesis inhibitor of triple-negative breast cancer by targeting hedgehog signaling. Cell Chem. Biol. 2019, 26, 1143–1158.e1146. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Huang, M.-Y.; Yang, Y.; Huang, M.-Q.; Shi, J.-J.; Zou, L.; Lu, J.-J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. 2020, 327, 127029. [Google Scholar] [CrossRef]
- Tao, W.; Su, Q.; Wang, H.; Guo, S.; Chen, Y.; Duan, J.; Wang, S. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. Int. Immunopharmacol. 2015, 27, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, S.; Du, J.; Jinfu, Y.; Shumin, W. Platycodin D attenuates airway inflammation in a mouse model of allergic asthma by regulation NF-κB pathway. Inflammation 2015, 38, 1221–1228. [Google Scholar] [CrossRef]
- Lee, E.G.; Kim, K.H.; Hur, J.; Kang, J.Y.; Lee, H.Y.; Lee, S.Y. Platycodin D attenuates airway inflammation via suppression Th2 transcription factor in a murine model of acute asthma. J. Asthma 2022, 59, 1279–1289. [Google Scholar] [CrossRef]
- Sun, L.; Di, Y.M.; Lu, C.; Guo, X.; Tang, X.; Zhang, A.L.; Xue, C.C.; Fan, G. Additional Benefit of Chinese Medicine Formulae Including Dioscoreae rhizome (Shanyao) for Diabetes Mellitus: Current State of Evidence. Front. Endocrinol. 2020, 11, 553288. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, H.-N.; Kang, K.-S.; Baek, N.-I.; Kim, D.-K.; Kim, Y.-S.; Kim, S.-H.; Jean, B.-H. Methanol extract of Dioscoreae Rhizoma inhibits pro-inflammatory cytokines and mediators in the synoviocytes of rheumatoid arthritis. Int. Immunopharmacol. 2004, 4, 1489–1497. [Google Scholar] [CrossRef]
- Lee, S.-C.; Tsai, C.-C.; Chen, J.-C.; Lin, C.-C.; Hu, M.-L.; Lu, S. The evaluation of reno-and hepato-protective effects of Huai-Shan-Yao (Rhizome Dioscoreae). Am. J. Chin. Med. 2002, 30, 609–616. [Google Scholar] [CrossRef]
- Junchao, Y.; Zhen, W.; Yuan, W.; Liying, X.; Libin, J.; Yuanhong, Z.; Wei, Z.; Ruilin, C.; Lu, Z. Anti-trachea inflammatory effects of diosgenin from Dioscorea nipponica through interactions with glucocorticoid receptor α. J. Int. Med. Res. 2017, 45, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Daştan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R. Diosgenin: An updated pharmacological review and therapeutic perspectives. Oxidative Med. Cell. Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-C.; Yang, Z.-L.; Cui, B.-K.; Yu, C.-J.; Zhou, L.-W. Species diversity and utilization of medicinal mushrooms and fungi in China. Int. J. Med. Mushrooms 2009, 11, 287–302. [Google Scholar] [CrossRef]
- Akihisa, T.; Uchiyama, E.; Kikuchi, T.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-tumor-promoting effects of 25-methoxyporicoic acid A and other triterpene acids from Poria cocos. J. Nat. Prod. 2009, 72, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.-L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; You, H.J.; Jeong, H.G.; Kang, J.S.; Kim, H.M.; Dal Rhee, S.; Jeon, Y.J. Polysaccharide isolated from Poria cocos sclerotium induces NF-κB/Rel activation and iNOS expression through the activation of p38 kinase in murine macrophages. Int. Immunopharmacol. 2004, 4, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-L.; Huang, H.-W.; Su, M.-H.; Lin, H.-C.; Wu, W.-M. The lanostane triterpenoids in Poria cocos play beneficial roles in immunoregulatory activity. Life 2021, 11, 111. [Google Scholar] [CrossRef]
- Chao, C.-L.; Wang, C.-J.; Huang, H.-W.; Kuo, H.-P.; Su, M.-H.; Lin, H.-C.; Teng, C.-W.; Sy, L.B.; Wu, W.-M. Poria cocos modulates Th1/Th2 response and attenuates airway inflammation in an ovalbumin-sensitized mouse allergic asthma Model. Life 2021, 11, 372. [Google Scholar] [CrossRef]
- Ang, L.; Lee, H.W.; Kim, A.; Lee, M.S. Herbal medicine for the management of COVID-19 during the medical observation period: A review of guidelines. Integr. Med. Res. 2020, 9, 100465. [Google Scholar] [CrossRef]
- Yang, X.-L.; Liu, D.; Bian, K.; Zhang, D.-D. Study on in vitro anti-inflammatory activity of total flavonoids from Glycyrrhizae Radix et Rhizoma and its ingredients. J. Chin. Mater. Med. 2013, 38, 99–104. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.-q.; Yuan, B.-c.; Liu, Y. The pharmacological activities of licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Yan, M.; Yu, Y.; Guo, Y. 18β-Glycyrrhetinic acid suppresses allergic airway inflammation through NF-κB and Nrf2/HO-1 signaling pathways in asthma mice. Sci. Rep. 2022, 12, 3121. [Google Scholar] [CrossRef]
- Liu, L.-L.; Zhang, Y.; Zhang, X.-F.; Li, F.-H. Influence of rutin on the effects of neonatal cigarette smoke exposure-induced exacerbated MMP-9 expression, Th17 cytokines and NF-κB/iNOS-mediated inflammatory responses in asthmatic mice model. Korean J. Physiol. Pharmacol. 2018, 22, 481–491. [Google Scholar] [CrossRef]
- Huang, J.; Chen, X.; Xie, A. Formononetin ameliorates IL-13-induced inflammation and mucus formation in human nasal epithelial cells by activating the SIRT1/Nrf2 signaling pathway. Mol. Med. Rep. 2021, 24, 832. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cui, J.; Wang, W.; Tang, W.; Teng, F.; Zhu, X.; Qin, J.; Wuniqiemu, T.; Sun, J.; Wei, Y. Formononetin attenuates airway inflammation and oxidative stress in murine allergic asthma. Front. Pharmacol. 2020, 11, 533841. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Jiang, L.; Wei, M.; Yang, X.; Guan, M.; Xie, X.; Wei, J.; Liu, D.; Wang, D. Attenuation of allergic airway inflammation in a murine model of asthma by Licochalcone A. Immunopharmacol. Immunotoxicol. 2013, 35, 653–661. [Google Scholar] [CrossRef]
- Huang, W.-C.; Liu, C.-Y.; Shen, S.-C.; Chen, L.-C.; Yeh, K.-W.; Liu, S.-H.; Liou, C.-J. Protective effects of licochalcone A improve airway hyper-responsiveness and oxidative stress in a mouse model of asthma. Cells 2019, 8, 617. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi.(Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Yao, J.; Hu, R.; Sun, J.; Lin, B.; Zhao, L.; Sha, Y.; Zhu, B.; You, Q.D.; Yan, T.; Guo, Q.L. Oroxylin a prevents inflammation-related tumor through down-regulation of inflammatory gene expression by inhibiting NF-κB signaling. Mol. Carcinog. 2014, 53, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-G.; Diao, B.-Z.; Zhou, W.; Feng, J.-L. Oroxylin A inhibits allergic airway inflammation in ovalbumin (OVA)-induced asthma murine model. Inflammation 2016, 39, 867–872. [Google Scholar] [CrossRef]
- Lee, A.-Y.; Kang, S.; Park, S.-J.; Huang, J.; Im, D.-S. Anti-allergic effect of oroxylin a from Oroxylum indicum using in vivo and in vitro experiments. Biomol. Ther. 2016, 24, 283. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-K.; Kim, S.-H.; Jung, I.C.; Park, Y.-C. Effects of Scutellaria baicalensis extract on cigarette smoke-induced airway inflammation in a murine model of chronic obstructive pulmonary disease. J. Med. Food 2019, 22, 87–96. [Google Scholar] [CrossRef]
- Shin, H.-D.; Lee, H.J.; Sikder, M.A.; Park, S.H.; Ryu, J.; Hong, J.-H.; Kim, J.-O.; Seok, J.H.; Lee, C.J. Effect of chrysin on gene expression and production of MUC5AC mucin from cultured airway epithelial cells. Tuberc. Respir. Dis. 2012, 73, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-D.; Lee, H.J.; Sikder, M.A.; Ryu, J.; Park, S.H.; Hong, J.-H.; Seok, J.H.; Lee, C.J. Effect of Chrysin on Tumor Necrosis Factor-α-induced Mucin Production, Mucin Gene Expression and Translocation of Nuclear Factor-kappa B in Cultured Airway Epithelial Cells. Yakhak Hoeji 2012, 56, 198–203. [Google Scholar]
- Hariri, B.M.; McMahon, D.B.; Chen, B.; Freund, J.R.; Mansfield, C.J.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Reed, D.R. Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J. Biol. Chem. 2017, 292, 8484–8497. [Google Scholar] [CrossRef] [PubMed]
- Sikder, M.A.; Lee, H.J.; Mia, M.Z.; Park, S.H.; Ryu, J.; Kim, J.H.; Min, S.Y.; Hong, J.H.; Seok, J.H.; Lee, C.J. Inhibition of TNF-α-induced MUC5AC mucin gene expression and production by wogonin through the inactivation of NF-κB signaling in airway epithelial cells. Phytother. Res. 2014, 28, 62–68. [Google Scholar] [CrossRef]
- Sikder, M.A.; Lee, H.J.; Ryu, J.; Park, S.H.; Kim, J.-O.; Hong, J.-H.; Seok, J.H.; Lee, C.J. Apigenin and wogonin regulate epidermal growth factor receptor signaling pathway involved in MUC5AC mucin gene expression and production from cultured airway epithelial cells. Tuberc. Respir. Dis. 2014, 76, 120–126. [Google Scholar] [CrossRef]
- Ryu, E.K.; Kim, T.-H.; Jang, E.J.; Choi, Y.S.; Kim, S.T.; Hahm, K.B.; Lee, H.-J. Wogonin, a plant flavone from Scutellariae radix, attenuated ovalbumin-induced airway inflammation in mouse model of asthma via the suppression of IL-4/STAT6 signaling. J. Clin. Biochem. Nutr. 2015, 57, 105–112. [Google Scholar] [CrossRef]
- Song, K.J.; Shin, Y.-J.; Lee, K.R.; Lee, E.J.; Suh, Y.S.; Kim, K.-S. Expectorant and antitussive effect of Hedera helix and Rhizoma coptidis extracts mixture. Yonsei Med. J. 2015, 56, 819–824. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Lou, G.-H.; Zeng, H.-R.; Hu, J.; Huang, Q.-W.; Peng, W.; Yang, X.-B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019, 57, 193–225. [Google Scholar] [CrossRef]
- Kim, S.; You, D.; Jeong, Y.; Yu, J.; Kim, S.W.; Nam, S.J.; Lee, J.E. Berberine down-regulates IL-8 expression through inhibition of the EGFR/MEK/ERK pathway in triple-negative breast cancer cells. Phytomedicine 2018, 50, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qiu, J.; Zhang, T.; Yang, Y.; Guo, S.; Li, T.; Jiang, K.; Zahoor, A.; Deng, G.; Qiu, C. MicroRNA-188-5p promotes apoptosis and inhibits cell proliferation of breast cancer cells via the MAPK signaling pathway by targeting Rap2c. J. Cell. Physiol. 2020, 235, 2389–2402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, N.; Chai, Y.; Nie, Y.; Liu, K.; Liu, Y.; Yang, Y.; Su, J.; Zhang, C. Apoptosis induction, a sharp edge of berberine to exert anti-cancer effects, focus on breast, lung, and liver cancer. Front. Pharmacol. 2022, 13, 803717. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Liu, S.; Shen, Y.; Li, Q. Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation 2013, 36, 1079–1086. [Google Scholar] [CrossRef]
- Xu, D.; Wan, C.; Wang, T.; Tian, P.; Li, D.; Wu, Y.; Fan, S.; Chen, L.; Shen, Y.; Wen, F. Berberine attenuates cigarette smoke-induced airway inflammation and mucus hypersecretion in mice. Int. J. Clin. Exp. Med. 2015, 8, 8641. [Google Scholar] [PubMed]
- Hong, G.; Kim, Y.-I.; Park, S.J.; Lee, S.Y.; Kim, J.W.; Yoon, S.H.; Lee, K.S.; Byun, M.K.; Kim, H.-R.; Chung, J. Effects of a mixture of ivy leaf extract and Coptidis rhizome on patients with chronic bronchitis and bronchiectasis. Int. J. Environ. Res. Public Health 2021, 18, 4024. [Google Scholar] [CrossRef]
- Fu, P.-K.; Yang, C.-Y.; Tsai, T.-H.; Hsieh, C.-L. Moutan cortex radicis improves lipopolysaccharide-induced acute lung injury in rats through anti-inflammation. Phytomedicine 2012, 19, 1206–1215. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, S. A review of the ethnobotany, phytochemistry and pharmacology of tree peony (Sect. Moutan). South Afr. J. Bot. 2019, 124, 556–563. [Google Scholar] [CrossRef]
- Fu, P.-K.; Wu, C.-L.; Tsai, T.-H.; Hsieh, C.-L. Anti-inflammatory and anticoagulative effects of paeonol on LPS-induced acute lung injury in rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 837513. [Google Scholar] [CrossRef]
- Liu, M.-H.; Lin, A.-H.; Lee, H.-F.; Ko, H.-K.; Lee, T.-S.; Kou, Y.R. Paeonol attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Mediat. Inflamm. 2014, 2014, 651890. [Google Scholar] [CrossRef]
- Bettiol, A.; Lopalco, G.; Emmi, G.; Cantarini, L.; Urban, M.L.; Vitale, A.; Denora, N.; Lopalco, A.; Cutrignelli, A.; Lopedota, A. Unveiling the efficacy, safety, and tolerability of anti-interleukin-1 treatment in monogenic and multifactorial autoinflammatory diseases. Int. J. Mol. Sci. 2019, 20, 1898. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Jang, D.-i.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Fan, J.; Ren, D.; Wang, J.; Liu, X.; Zhang, H.; Wu, M.; Yang, G. Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 2020, 11, 126. [Google Scholar] [CrossRef]
- Lei, X.; Du, L.; Yu, W.; Wang, Y.; Ma, N.; Qu, B. GSTP1 as a novel target in radiation induced lung injury. J. Transl. Med. 2021, 19, 297. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D. Cancer drugs, genetic variation and the glutathione-S-transferase gene family. Am. J. Pharm. 2003, 3, 157–172. [Google Scholar] [CrossRef]
- Wu, W.; Peden, D.; Diaz-Sanchez, D. Role of GSTM1 in resistance to lung inflammation. Free Radic. Biol. Med. 2012, 53, 721–729. [Google Scholar] [CrossRef]
- Adler, V.; Yin, Z.; Fuchs, S.Y.; Benezra, M.; Rosario, L.; Tew, K.D.; Pincus, M.R.; Sardana, M.; Henderson, C.J.; Wolf, C.R. Regulation of JNK signaling by GSTp. EMBO J. 1999, 18, 1321–1334. [Google Scholar] [CrossRef]
- Ruscoe, J.E.; Rosario, L.A.; Wang, T.; Gaté, L.; Arifoglu, P.; Wolf, C.R.; Henderson, C.J.; Ronai, Z.e.; Tew, K.D. Pharmacologic or genetic manipulation of glutathione S-transferase P1–1 (GSTπ) influences cell proliferation pathways. J. Pharmacol. Exp. Ther. 2001, 298, 339–345. [Google Scholar] [PubMed]
- Sau, A.; Filomeni, G.; Pezzola, S.; D’Aguanno, S.; Tregno, F.P.; Urbani, A.; Serra, M.; Pasello, M.; Picci, P.; Federici, G. Targeting GSTP1-1 induces JNK activation and leads to apoptosis in cisplatin-sensitive and-resistant human osteosarcoma cell lines. Mol. BioSystems 2012, 8, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, G.; Yin, J.; Li, L.; Tan, Y.; Wei, H.; Liu, B.; Deng, L.; Tang, J.; Chen, Y. GSTP1 and cancer: Expression, methylation, polymorphisms and signaling. Int. J. Oncol. 2020, 56, 867–878. [Google Scholar] [CrossRef]
- Cho, S.-G.; Lee, Y.H.; Park, H.-S.; Ryoo, K.; Kang, K.W.; Park, J.; Eom, S.-J.; Kim, M.J.; Chang, T.-S.; Choi, S.-Y. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J. Biol. Chem. 2001, 276, 12749–12755. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor suppressor and metabolic regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef]
- Dorion, S.; Lambert, H.; Landry, J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Ask1. J. Biol. Chem. 2002, 277, 30792–30797. [Google Scholar] [CrossRef]
- Gilot, D.; Loyer, P.; Corlu, A.; Glaise, D.; Lagadic-Gossmann, D.; Atfi, A.; Morel, F.; Ichijo, H.; Guguen-Guillouzo, C. Liver protection from apoptosis requires both blockage of initiator caspase activities and inhibition of ASK1/JNK pathway via glutathione S-transferase regulation. J. Biol. Chem. 2002, 277, 49220–49229. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Zhang, L.-L.; Fan, X.-Y.; Wu, S.-S.; Zhang, S.-Q. Poly-L-arginine induces apoptosis of NCI-H292 cells via ERK1/2 signaling pathway. J. Immunol. Res. 2018, 2018, 3651743. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, E.J.; Hur, G.Y.; Jung, K.H.; Jung, H.C.; Lee, S.Y.; Kim, J.H.; Shin, C.; In, K.H.; Kang, K.H. Peroxisome proliferator-activated receptor-γ inhibits cigarette smoke solution-induced mucin production in human airway epithelial (NCI-H292) cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 291, L84–L90. [Google Scholar] [CrossRef]
- Song, Y.-S.; Kim, M.S.; Lee, D.H.; Oh, D.-K.; Yoon, D.-Y. 15-Hydroxyeicosatetraenoic acid inhibits phorbol-12-myristate-13-acetate-induced MUC5AC expression in NCI-H292 respiratory epithelial cells. J. Microbiol. Biotechnol. 2015, 25, 589–597. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Xie, C.; Huang, C.; Zhu, J.; Liang, Z.; Deng, F.; Zhu, M.; Zhu, W.; Wu, R. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother. Res. 2014, 28, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.K.; Ağca, C.A.; Aşkın, H. Quercetin and luteolin improve the anticancer effects of 5-fluorouracil in human colorectal adenocarcinoma in vitro model: A mechanistic insight. Nutr. Cancer 2022, 74, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.-H.; Jung, H.-J. A clinical observation of Chungsangboha-tang’s effects on asthmatic patients. J. Intern. Korean Med. 2011, 32, 10–25. [Google Scholar]
- Davies, J.R.; Herrmann, A.; Russell, W.; Svitacheva, N.; Wickström, C.; Carlstedt, I. Respiratory tract mucins: Structure and expression patterns. In Proceedings of the Mucus Hypersecretion in Respiratory Disease: Novartis Foundation Symposium 248, London, UK, 26–28 February 2002; pp. 76–93. [Google Scholar]
- Gan, G.-L.; Liu, J.; Chen, W.-J.; Ye, Q.-Q.; Xu, Y.; Wu, H.-T.; Li, W. The diverse roles of the mucin gene cluster located on chromosome 11p15. 5 in colorectal cancer. Front. Cell Dev. Biol. 2020, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, S.; Kang, J.; Lin, J.; Lai, K.; Sun, Y.; Xiao, W.; Yang, L.; Yao, W.; Cai, S. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition). Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 399–407. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Ha, H.-K.; Kim, C.-S. Development of an animal model for chronic asthma using Chungsangboha-tang. Korean J. Orient. Med. 1999, 5, 1–15. [Google Scholar]
- Lee, H.; Jung, S.; Jung, H.; Kwan, H. Study on the Effects of Chungsangboha-tang on Allergic Asthma in Respiratory Pattern and Change of Eosinophil Infiltration in Tracheal Mucosa. Kyung-Hee Korean Med. Univ. Collect. 1999, 22, 203–215. [Google Scholar]
- Kim, Y.-D.; Kwon, E.-J.; Park, D.-W.; Song, S.-Y.; Yoon, S.-K.; Baek, S.-H. Interleukin-1β induces MUC2 and MUC5AC synthesis through cyclooxygenase-2 in NCI-H292 cells. Mol. Pharmacol. 2002, 62, 1112–1118. [Google Scholar] [CrossRef]
- Sung, S.; Park, Y.; Jo, J.R.; Jung, N.K.; Song, D.K.; Bae, J.; Keum, D.Y.; Kim, J.B.; Park, G.Y.; Jang, B.C. Overexpression of cyclooxygenase-2 in NCI-H292 human alveolar epithelial carcinoma cells: Roles of p38 MAPK, ERK-1/2, and PI3K/PKB signaling proteins. J. Cell. Biochem. 2011, 112, 3015–3024. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, K.-i.; Hossain, R.; Lee, M.; Hong, J.T.; Lee, H.J.; Lee, C.J. Meclofenamate Suppresses MUC5AC Mucin Gene Expression by Regulating the NF-kB Signaling Pathway in Human Pulmonary Mucoepidermoid NCI-H292 Cells. Biomol. Ther. 2023, 31, 306. [Google Scholar] [CrossRef]
- Bridgeman, M.B.; Wilken, L.A. Essential role of pharmacists in asthma care and management. J. Pharm. Pract. 2021, 34, 149–162. [Google Scholar] [CrossRef]
- Cilag, J.; Abbott, E.; Center, G. Anticholinergic effects of medication in elderly patients. J. Clin. Psychiatry 2001, 62, 11–14. [Google Scholar]
- Billington, C.K.; Penn, R.B.; Hall, I.P. β 2 Agonists. Pharmacol. Ther. Asthma COPD 2017, 237, 23–40. [Google Scholar] [CrossRef]
- Price, D.B.; Trudo, F.; Voorham, J.; Xu, X.; Kerkhof, M.; Ling Zhi Jie, J.; Tran, T.N. Adverse outcomes from initiation of systemic corticosteroids for asthma: Long-term observational study. J. Asthma Allergy 2018, 11, 193–204. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Zhang, R.-z.; Yu, S.-j.; Bai, H.; Ning, K. TCM-Mesh: The database and analytical system for network pharmacology analysis for TCM preparations. Sci. Rep. 2017, 7, 2821. [Google Scholar] [CrossRef]
- Kim, S.-K.; Nam, S.; Jang, H.; Kim, A.; Lee, J.-J. TM-MC: A database of medicinal materials and chemical compounds in Northeast Asian traditional medicine. BMC Complement. Altern. Med. 2015, 15, 218. [Google Scholar] [CrossRef]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2007, 36, D684–D688. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Zhu, F.; Han, B.; Kumar, P.; Liu, X.; Ma, X.; Wei, X.; Huang, L.; Guo, Y.; Han, L.; Zheng, C. Update of TTD: Therapeutic target database. Nucleic Acids Res. 2010, 38, D787–D791. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. Comparative toxicogenomics database (CTD): Update 2021. Nucleic Acids Res. 2021, 49, D1138–D1143. [Google Scholar] [CrossRef]


| Herb Name | Number of Targets | Number of Overlapped Proteins | Odds Ratio # | p-Value |
|---|---|---|---|---|
| Glycyrrhizae Radix et Rhizoma | 235 | 14 | 5.35 | 4.4 × 10−8 |
| Platycodonis Radix | 13 | 3 | 20.73 | 9.6 × 10−6 |
| Moutan Radicis Cortex | 162 | 10 | 5.55 | 1.7 × 10−6 |
| Pinelliae Tuber | 264 | 3 | 1.02 | 0.34 |
| Poria Sclerotium | 20 | 2 | 8.98 | 1.3 × 10−3 |
| Corni Fructus | 65 | 5 | 6.91 | 8.0 × 10−5 |
| Dioscoreae Rhizoma | 26 | 4 | 13.82 | 8.5 × 10−6 |
| Rehmanniae Radix Preparata | 22 | 0 | 0.00 | 0.22 |
| Fritillariae Thunbergii Bulbus | 251 | 2 | 0.72 | 0.53 |
| Ponciri Fructus Immaturus | 90 | 4 | 3.99 | 3.2 × 10−3 |
| Scutellariae Radix | 302 | 16 | 4.76 | 3.3 × 10−8 |
| Coptidis Rhizoma | 186 | 12 | 5.80 | 1.3 × 10−7 |
| Total (CSBHH) | 1088 | 34 | 2.80 | 2.6 × 10−9 |
| Active Ingredient | Pubchem ID | Related Herb | Overlap # | p-Value |
|---|---|---|---|---|
| Oroxylin | 5320315 | Scutellariae Radix | 8/70 | 6.90 × 10−8 |
| Berberine | 2353 | Coptidis Rhizoma | 10/141 | 4.26 × 10−7 |
| Paeonol | 11092 | Moutan Radicis Cortex | 5/31 | 9.70 × 10−7 |
| Chrysin | 5281607 | Scutellariae Radix | 7/82 | 3.26 × 10−6 |
| Diosgenin | 99474 | Dioscoreae Rhizoma | 4/25 | 6.92 × 10−6 |
| Platycodin D | 162859 | Platycodonis Radix | 3/13 | 9.60 × 10−6 |
| Rutin | 5280805 | Glycyrrhizae Radix et Rhizoma | 5/46 | 1.08 × 10−5 |
| Formononetin | 5280378 | Glycyrrhizae Radix et Rhizoma | 4/29 | 1.49 × 10−5 |
| Wogonin | 5281703 | Scutellariae Radix | 7/108 | 2.53 × 10−5 |
| Licochalcone A | 5318998 | Glycyrrhizae Radix et Rhizoma | 4/39 | 6.62 × 10−5 |
| Gene | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| IL-1β | CTGTCCTGCGTGTTGAAAGA | TTCTGCTTGAGAGGTGCTGA |
| TNF-α | TTCCCCAGGGACCTCTCTCTAATC | GAGGGTTTGCTACAACATGGGCTAC |
| GSTP1 | GGCAACTGAAGCCTTTTGAG | GGCTAGGACCTCATGGATCA |
| GSTM1 | CTGGGCATGATCTGCTACAATC | CAAAAGTGATCTTGTTTCCTGCAA |
| PTEN | TGGCTAAGTGAAGATGACAATCATG | TGCACATATCATTAC ACCAGTTCGT |
| MUC5AC | TCCACCATATACCGCCACAGA | TGGACGGACAGTCACTGTCAAC |
| MUC2 | TGCCTGGCCCTGTCTTTG | CAGCTCCAGCATGAGTGC |
| β-actin | AGGAGAAGCTGTGCTACGTC | GGATGTCCACGTCACACTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Kim, K.-S.; Lee, W.-Y.; Kim, C.-E.; Lee, S. Integrative Approach to Identifying System-Level Mechanisms of Chung-Sang-Bo-Ha-Hwan’s Influence on Respiratory Tract Diseases: A Network Pharmacological Analysis with Experimental Validation. Plants 2023, 12, 3024. https://doi.org/10.3390/plants12173024
Park S-Y, Kim K-S, Lee W-Y, Kim C-E, Lee S. Integrative Approach to Identifying System-Level Mechanisms of Chung-Sang-Bo-Ha-Hwan’s Influence on Respiratory Tract Diseases: A Network Pharmacological Analysis with Experimental Validation. Plants. 2023; 12(17):3024. https://doi.org/10.3390/plants12173024
Chicago/Turabian StylePark, Sa-Yoon, Kang-Sub Kim, Won-Yung Lee, Chang-Eop Kim, and Sullim Lee. 2023. "Integrative Approach to Identifying System-Level Mechanisms of Chung-Sang-Bo-Ha-Hwan’s Influence on Respiratory Tract Diseases: A Network Pharmacological Analysis with Experimental Validation" Plants 12, no. 17: 3024. https://doi.org/10.3390/plants12173024





