Water Deficit at Vegetative Stage Induces Tolerance to High Temperature during Anthesis in Rice
Abstract
:1. Introduction
2. Results
2.1. Analysis of Variance
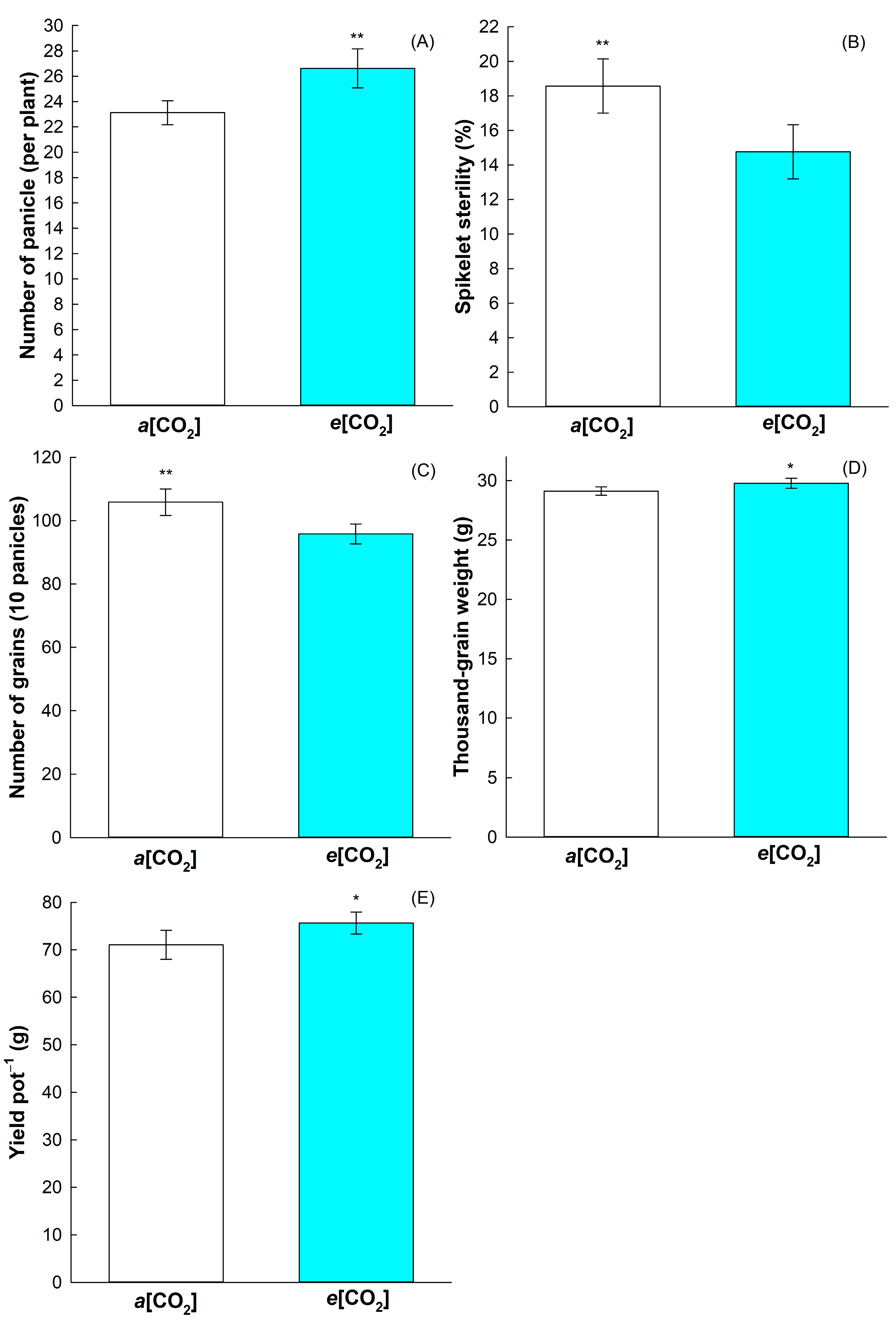
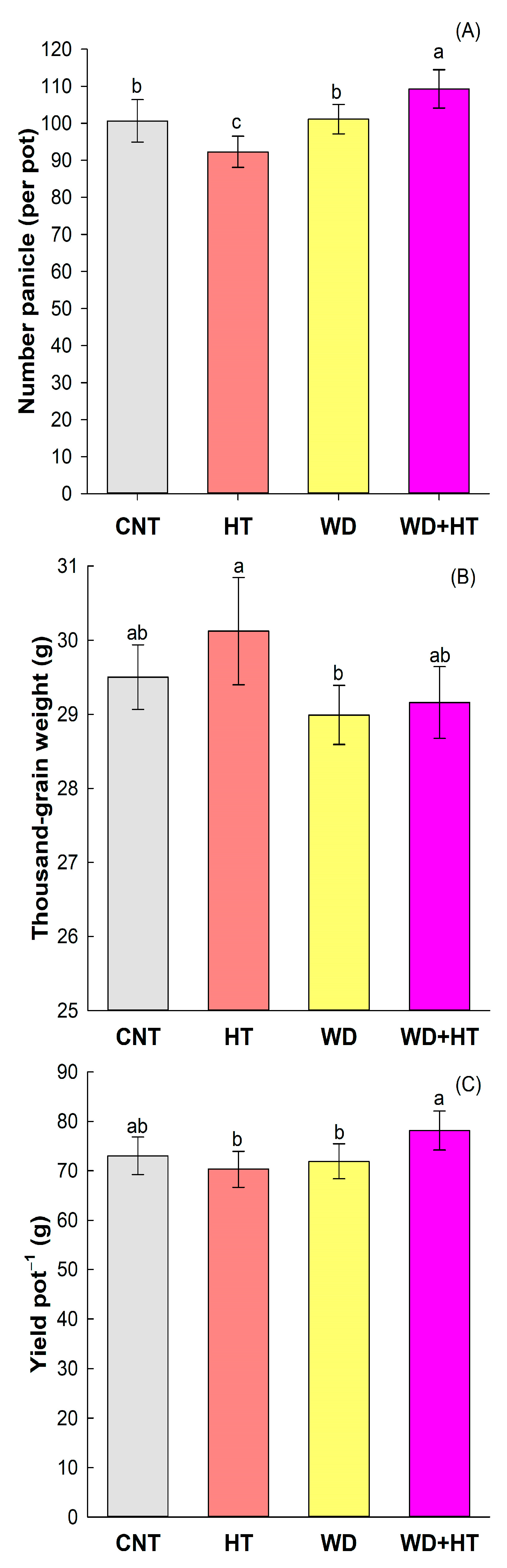
2.2. Effect on Rice Yield Components
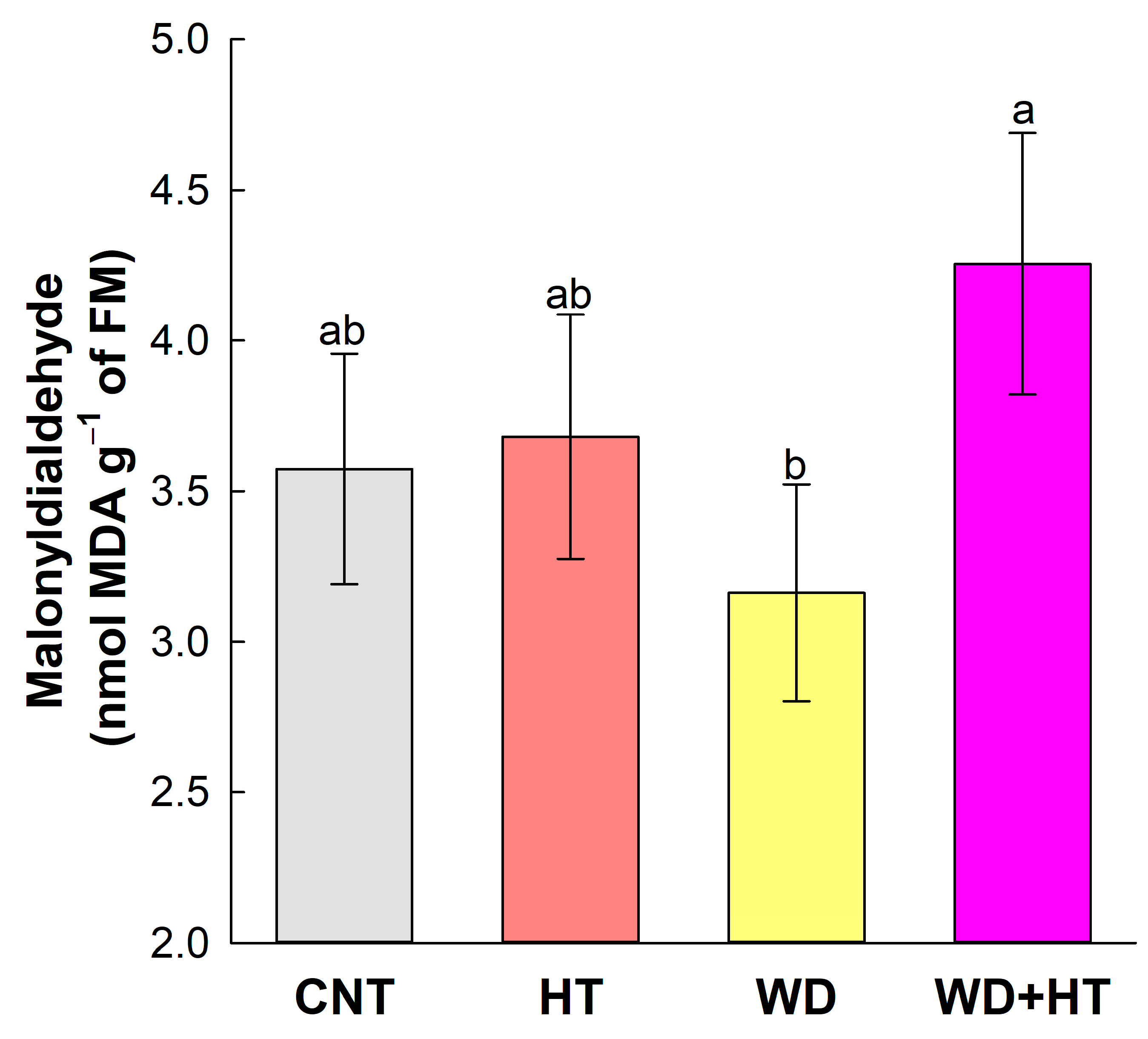
2.3. Effects on Photosynthesis and the Antioxidant Mechanism
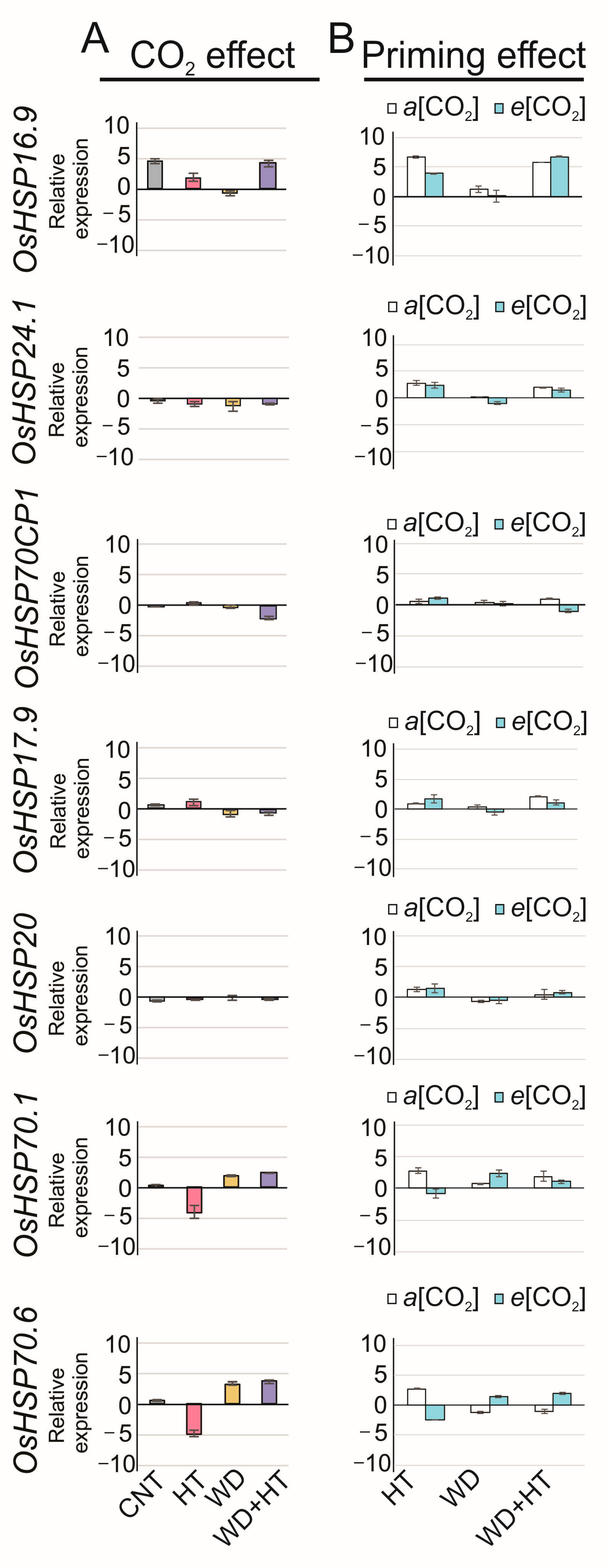
2.4. Effects on HSP Transcriptional Regulation
3. Discussion
3.1. Rice Yield Is Positively Affected by e[CO2]
3.2. Priming with Water Deficit Increases Rice Yield
3.3. Tolerance to Heat Stress Induced by Priming with Water Deficit
4. Materials and Methods
4.1. General Experimental Conditions
4.2. Procedures for Establishing Water and Temperatures Stresses
4.3. Evaluations of the Priming and CO2 Effects
4.3.1. Yield Components and Biomass Accumulation
4.3.2. Biochemical Analysis
Quantification of Hydrogen Peroxide Content and Lipid Peroxidation
Antioxidant Enzymes Activity
Quantification of Chlorophyll a, Chlorophyll b, and Carotenoids
4.3.3. Gene Expression Analysis
RNA Extraction and cDNA Synthesis
Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
| Gene | ID | Oligonucleotide—(5′-3′) | References | |
|---|---|---|---|---|
| SMALL HEAT-SHOCK PROTEIN 16.9A | OsHSP16.9A | Forward | CATTCTCCCTCGACCTCTGG | [48] |
| Reverse | GGTGCCACTTGTCGTTCTTGT | |||
| SMALL HEAT-SHOCK PROTEIN 17.9A | OsHSP17.9A | Forward | GGCAGCATCTTCCCGTCCTT | [48] |
| Reverse | CGTCGTCCACCTCCACCTTG | |||
| SMALL HEAT-SHOCK PROTEIN 20 | OsHSP20 | Forward | AAGTTCCTCCGCAGGTTCC | [62] |
| Reverse | GAGCACGCCGTTCTCCAT | |||
| SMALL HEAT-SHOCK PROTEIN 24.15 | OsHSP24.15 | Forward | GATCAAGGCGGAGATGAAGAAC | [63] |
| Reverse | ACTCGACGTTGACCTGGAAGA | |||
| HEAT-SHOCK PROTEIN 70CP1 | OsHSP70CP1 | Forward | CCAGGCGATGTGAAGGGTAA | [64] |
| Reverse | CAGCATCGATGACATCACCT | |||
| HEAT-SHOCK PROTEIN 70.1 | OsHSP70.1 | Forward | TCGAGCTCTCCGGCATCCCT | [65] |
| Reverse | TCACGTAGGACCACCACCAGCT | |||
| HEAT-SHOCK PROTEIN 70.6 | OsHSP70.6 | Forward | GATCAACGTCTGCTTCGACA | [65] |
| Reverse | AGTCGACCTCCTCGATCTTG | |||
| 18S ribosomal RNA | Os18S | Forward | CTACGTCCCTGCCCTTTGTACA | [61] |
| Reverse | ACACTTCACCGGACCATTCAA | |||
| Elongation Factor 1-α | OsEF1α | Forward | TTTCACTCTTGGTGTGAAGCAGAT | [61] |
| Reverse | GACTTCCTTCACGATTTCATCGTAA | |||
| Ubiquitin 5 | OsUBQ5 | Forward | ACCACTTCGACCGCCACTACT | [61] |
| Reverse | ACGCCTAAGCCTGCTGGTT | |||
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.M.; Nayyar, H. Individual and Combined Effects of Transient Drought and Heat Stress on Carbon Assimilation and Seed Filling in Chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic Engineering and Breeding of Drought-Resistant Crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Nahar, S.; Sahoo, L.; Tanti, B. Screening of Drought Tolerant Rice through Morpho-Physiological and Biochemical Approaches. Biocatal. Agric. Biotechnol. 2018, 15, 150–159. [Google Scholar] [CrossRef]
- Satake, T.; Yoshida, S. High Temperature-Induced Sterility in Indica Rices at Flowering. Jpn. J. Crop Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Chen, L.; Li, P.; Cao, C. The Different Influences of Drought Stress at the Flowering Stage on Rice Physiological Traits, Grain Yield, and Quality. Sci. Rep. 2019, 9, 3742. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Chen, T.; Feng, B.; Fu, W.; Yan, J.; Islam, M.R.; Jin, Q.; Tao, L.; Fu, G. Heat Stress Induces Spikelet Sterility in Rice at Anthesis through Inhibition of Pollen Tube Elongation Interfering with Auxin Homeostasis in Pollinated Pistils. Rice 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant Adaptations to the Combination of Drought and High Temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Planeta Arroz 2023. Available online: https://planetaarroz.com.br/lavoura-laboratorio/ (accessed on 10 July 2023).
- Hossain, M.A.; Li, Z.-G.; Hoque, T.S.; Burritt, D.J.; Fujita, M.; Munné-Bosch, S. Heat or Cold Priming-Induced Cross-Tolerance to Abiotic Stresses in Plants: Key Regulators and Possible Mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef]
- Walter, J.; Jentsch, A.; Beierkuhnlein, C.; Kreyling, J. Ecological Stress Memory and Cross Stress Tolerance in Plants in the Face of Climate Extremes. Environ. Exp. Bot. 2013, 94, 3–8. [Google Scholar] [CrossRef]
- Kim, J.M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin Changes in Response to Drought, Salinity, Heat, and Cold Stresses in Plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef]
- Xiong, H.; Yu, J.; Miao, J.; Li, J.; Zhang, H.; Wang, X.; Liu, P.; Zhao, Y.; Jiang, C.; Yin, Z.; et al. Natural Variation in OsLG3 Increases Drought Tolerance in Rice by Inducing ROS Scavenging. Plant Physiol. 2018, 178, 451–467. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, L.; Liu, Y.; Yang, Z.; Huang, B. Protein Phosphorylation Associated with Drought Priming-Enhanced Heat Tolerance in a Temperate Grass Species. Hortic. Res. 2020, 7, 207. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Larsen, X.; Zhu, F.; Song, F.; Liu, F. Drought Priming at Vegetative Growth Stage Enhances Nitrogen-Use Efficiency under Post-Anthesis Drought and Heat Stress in Wheat. J. Agron. Crop Sci. 2017, 203, 29–40. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, C.; Xu, L.; Huang, B. Improved Heat Tolerance through Drought Preconditioning Associated with Changes in Lipid Composition, Antioxidant Enzymes, and Protein Expression in Kentucky Bluegrass. Crop Sci. 2012, 52, 807–817. [Google Scholar] [CrossRef]
- Lv, C.; Huang, Y.; Sun, W.; Yu, L.; Zhu, J. Response of Rice Yield and Yield Components to Elevated [CO2]: A Synthesis of Updated Data from FACE Experiments. Eur. J. Agron. 2020, 112, 125961. [Google Scholar] [CrossRef]
- Zhang, G.; Sakai, H.; Usui, Y.; Tokida, T.; Nakamura, H.; Zhu, C.; Fukuoka, M.; Kobayashi, K.; Hasegawa, T. Grain Growth of Different Rice Cultivars under Elevated CO2 Concentrations Affects Yield and Quality. Field Crop. Res. 2015, 179, 72–80. [Google Scholar] [CrossRef]
- Sloan, J.M.; Mujab, A.A.M.; Mashitah, J.; Zulkarami, B.; Wilson, M.J.; Toh, L.S.; Nur Zahirah, A.J.; Afiq, K.; Asyraf, A.T.; Zhu, X.G.; et al. Elevated CO2 Priming as a Sustainable Approach to Increasing Rice Tiller Number and Yield Potential. Rice 2023, 16, 16. [Google Scholar] [CrossRef]
- Dias de Oliveira, E.; Palta, J.A.; Bramley, H.; Stefanova, K.; Siddique, K.H.M. Elevated CO2 Reduced Floret Death in Wheat under Warmer Average Temperatures and Terminal Drought. Front. Plant Sci. 2015, 6, 1010. [Google Scholar] [CrossRef]
- Kobata, T.; Yoshida, H.; Masiko, U.; Honda, T. Spikelet Sterility Is Associated with a Lack of Assimilate in High-Spikelet-Number Rice. Agron. J. 2013, 105, 1821–1831. [Google Scholar] [CrossRef]
- Wang, J.; Hasegawa, T.; Li, L.; Lam, S.K.; Zhang, X.; Liu, X.; Pan, G. Changes in Grain Protein and Amino Acids Composition of Wheat and Rice under Short-term Increased [CO2] and Temperature of Canopy Air in a Paddy from East China. New Phytol. 2019, 222, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, J.; Yang, H.; Zhu, J.; Liu, H.; Dong, G.; Liu, G.; Han, Y.; Wang, Y. The Impact of Free-Air CO2 Enrichment (FACE) and N Supply on Yield Formation of Rice Crops with Large Panicle. Field Crop. Res. 2006, 98, 141–150. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Yang, L. Response of Rice Yield Traits to Elevated Atmospheric CO2 Concentration and Its Interaction with Cultivar, Nitrogen Application Rate and Temperature: A Meta-Analysis of 20 Years FACE Studies. Sci. Total Environ. 2021, 764, 142797. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of Nitrogen Application Rate on the Photosynthetic Pigment, Leaf Fluorescence Characteristics, and Yield of Indica Hybrid Rice and Their Interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, Y.H.; Liu, T.; He, Y.H. Leaf Defense System of Robinia Pseudoacacia L. Seedlings Exposed to 3 Years of Elevated Atmospheric CO2 and Cd-Contaminated Soils. Sci. Total Environ. 2017, 605–606, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Q.; Shi, Y.; Li, X.; Hou, L.; Xing, G.; Ahammed, G.J. Elevated CO2 Improves Antioxidant Capacity, Ion Homeostasis, and Polyamine Metabolism in Tomato Seedlings under Ca(NO3)2-Induced Salt Stress. Sci. Hortic. 2020, 273, 109644. [Google Scholar] [CrossRef]
- Zhu, C.; Ziska, L.; Zhu, J.; Zeng, Q.; Xie, Z.; Tang, H.; Jia, X.; Hasegawa, T. The Temporal and Species Dynamics of Photosynthetic Acclimation in Flag Leaves of Rice (Oryza sativa) and Wheat (Triticum aestivum) under Elevated Carbon Dioxide. Physiol. Plant. 2012, 145, 395–405. [Google Scholar] [CrossRef]
- Kumari, S.; Agrawal, M.; Tiwari, S. Impact of Elevated CO2 and Elevated O3 on Beta vulgaris L.: Pigments, Metabolites, Antioxidants, Growth and Yield. Environ. Pollut. 2013, 174, 279–288. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Z.; Jespersen, D.; Huang, B. Photosynthesis and Protein Metabolism Associated with Elevated CO2-Mitigation of Heat Stress Damages in Tall Fescue. Environ. Exp. Bot. 2014, 99, 75–85. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Rice (Oryza sativa L.) with Reduced Chlorophyll Content Exhibit Higher Photosynthetic Rate and Efficiency, Improved Canopy Light Distribution, and Greater Yields than Normally Pigmented Plants. Field Crop. Res. 2017, 200, 58–70. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming Crops for the Future: Rewiring Stress Memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Tian, Z.; Hu, J.; Shao, Y.; Liu, R.; Jiang, D.; Yuan, J.; Dai, T. Drought Priming during the Vegetative Stage Can Enhance Post-Anthesis Drought Tolerance by Improving Photosynthetic Capacity in Winter Wheat. Arid Land Res. Manag. 2019, 33, 183–199. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Osmolyte Accumulation Plays Important Roles in the Drought Priming Induced Tolerance to Post-Anthesis Drought Stress in Winter Wheat (Triticum aestivum L.). Environ. Exp. Bot. 2019, 166, 103804. [Google Scholar] [CrossRef]
- Abid, M.; Shao, Y.; Liu, S.; Wang, F.; Gao, J.; Jiang, D.; Tian, Z.; Dai, T. Pre-Drought Priming Sustains Grain Development under Post-Anthesis Drought Stress by Regulating the Growth Hormones in Winter Wheat (Triticum aestivum L.). Planta 2017, 246, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Azhar, A.; Salam, E.; Galani, S. Differential expression of molecular chaperon (Hsp70) and antioxidant enzymes: Inducing thermotolerance in rice (Oryza sativa L.). Pakistan J. Bot. 2017, 49, 229–238. [Google Scholar]
- Mohammed, M.; Srividhya, S.W.; Shanthi, A.P. Identification of Differentially Expressed Genes under Heat Stress Conditions in Rice (Oryza sativa L.). Mol. Biol. Rep. 2020, 47, 1935–1948. [Google Scholar] [CrossRef]
- Xue, D.; Jiang, H.; Hu, J.; Zhang, X.; Guo, L.; Zeng, D.; Dong, G.; Sun, G.; Qian, Q. Plant Physiology and Biochemistry Characterization of Physiological Response and Identi Fi Cation of Associated Genes under Heat Stress in Rice Seedlings. Plant Physiol. Biochem. 2012, 61, 46–53. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, C.; Yao, S. The Impact of High-Temperature Stress on Rice: Challenges and Solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Karwa, S.; Taunk, J.; Maurya, S.; Das, A.; Krishna, G.K.; Arya, S.S.; Kumar, A.; Kumar, S.; Kumar, P.; Chinnusamy, V.; et al. Spermidine Exogenous Application Mollifies Reproductive Stage Heat Stress Ramifications in Rice. Front. Plant Sci. 2022, 13, 1027662. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Liu, F.; Jacobsen, S.; Jiang, D.; Wollenweber, B. Drought Priming at Vegetative Growth Stages Improves Tolerance to Drought and Heat Stresses Occurring during Grain Filling in Spring Wheat. Plant Growth Regul. 2015, 75, 677–687. [Google Scholar] [CrossRef]
- Anwar Hossain, M.; Golam Mostofa, M.; Fujita, M. Heat-Shock Positively Modulates Oxidative Protection of Salt and Drought-Stressed Mustard (Brassica campestris L.) Seedlings. J. Plant Sci. Mol. Breed. 2013, 2, 2. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Kim, Y.K.; Grover, A. Rice SHsp Genes: Genomic Organization and Expression Profiling under Stress and Development. BMC Genom. 2009, 10, 393. [Google Scholar] [CrossRef]
- Usman, M.G.; Rafii, M.Y.; Martini, M.Y.; Yusuff, O.A.; Ismail, M.R.; Miah, G. Molecular Analysis of Hsp70 Mechanisms in Plants and Their Function in Response to Stress. Biotechnol. Genet. Eng. Rev. 2017, 33, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.M.; Iqbal, A.; Wattoo, F.M.; Khan, M.A.; Zhang, H. HSP70 Mediated Stress Modulation. Plants 2018, 15, 281–290. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic Acid and Jasmonic Acid Are Involved in Drought Priming-Induced Tolerance to Drought in Wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Chen, X.; Lin, S.; Liu, Q.; Huang, J.; Zhang, W.; Lin, J.; Wang, Y.; Ke, Y.; He, H. Expression and Interaction of Small Heat Shock Proteins (SHsps) in Rice in Response to Heat Stress. Biochim. Biophys. Acta-Proteins Proteom. 2014, 1844, 818–828. [Google Scholar] [CrossRef]
- Shahnejat-Bushehri, S.; Mueller-Roeber, B.; Balazadeh, S. Arabidopsis NAC Transcription Factor JUNGBRUNNEN1 Affects Thermomemoryassociated Genes and Enhances Heat Stress Tolerance in Primed and Unprimed Conditions. Plant Signal. Behav. 2012, 7, 1518–1521. [Google Scholar] [CrossRef]
- Goswami, A.; Banerjee, R.; Raha, S. Mechanisms of Plant Adaptation/Memory in Rice Seedlings under Arsenic and Heat Stress: Expression of Heat-Shock Protein Gene HSP70. AoB Plants 2010, 2010, plq023. [Google Scholar] [CrossRef]
- Wu, T.Y.; Juan, Y.T.; Hsu, Y.H.; Wu, S.H.; Liao, H.T.; Fung, R.W.M.; Charng, Y.Y. Interplay between Heat Shock Proteins HSP101 and HSA32 Prolongs Heat Acclimation Memory Posttranscriptionally in Arabidopsis. Plant Physiol. 2013, 161, 2075–2084. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon Dioxide Enrichment Alleviates Heat Stress by Improving Cellular Redox Homeostasis through an ABA-Independent Process in Tomato Plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef] [PubMed]
- EMBRAPA–Empresa Brasileira de Pesquisa Agropecuária. In Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2018; ISBN 978-85-7035-198-2.
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 2018, 22, 867–880. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. Erratum: A Method for the Extraction of Chlorophyll from Leaf Tissue without Maceration. Can. J. Bot. 1980, 58, 403. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of Housekeeping Genes as Internal Control for Studying Gene Expression in Rice by Quantitative Real-Time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef]
- Guo, L.M.; Li, J.; He, J.; Liu, H.; Zhang, H.M. A Class I Cytosolic HSP20 of Rice Enhances Heat and Salt Tolerance in Different Organisms. Sci. Rep. 2020, 10, 1383. [Google Scholar] [CrossRef]
- Ye, S.F.; Yu, S.W.; Shu, L.B.; Wu, J.H.; Wu, A.Z.; Luo, L.J. Expression Profile Analysis of 9 Heat Shock Protein Genes throughout the Life Cycle and under Abiotic Stress in Rice. Chin. Sci. Bull. 2012, 57, 336–343. [Google Scholar] [CrossRef]
- Kim, S.R.; An, G. Rice Chloroplast-Localized Heat Shock Protein 70, OsHsp70CP1, Is Essential for Chloroplast Development under High-Temperature Conditions. J. Plant Physiol. 2013, 170, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; Kundnani, P.; Grover, A. Functional Analysis of Hsp70 Superfamily Proteins of Rice (Oryza sativa). Cell Stress Chaperones 2013, 18, 427–437. [Google Scholar] [CrossRef] [PubMed]




| Variable | [CO2] | Stresses | [CO2] x Treatments |
|---|---|---|---|
| SDW | 0.058 | 0.930 | 0.065 |
| RDW | 0.141 | 0.05 | 0.684 |
| Panicles vase−1 | 0.001 ** | 0.596 | 0.682 |
| Grains panicle−1 | 0.000 ** | 0.000 ** | 0.467 |
| Sterility (%) | 0.004 ** | 0.821 | 0.642 |
| TGW | 0.017 * | 0.022* | 0.937 |
| Yield pot−1 | 0.016 * | 0.27* | 0.669 |
| H2O2 | 0.010 ** | 0.451 | 0.491 |
| MDA | 0.468 | 0.019 | 0.675 |
| SOD | 0.001 ** | 0.192 | 0.976 |
| CAT | 0.026 * | 0.064 | 0.164 |
| APX | 0.083 | 0.588 | 0.780 |
| Chlo a | 0.004 ** | 0.562 | 0.221 |
| Chlo b | 0.007 ** | 0.518 | 0.212 |
| Chlo T | 0.004 ** | 0.551 | 0.219 |
| Carot | 0.102 | 0.363 | 0.077 |
| Treatments | Description |
|---|---|
| CNT a[CO2] | Control condition—400 ppm of CO2; irrigated and 28/25 °C day/night (optimal conditions) |
| HT a[CO2] | Heat—400 ppm of CO2; irrigated and with heat stress at R4 stage (35/28 °C day/night) |
| WD a[CO2] | Water deficit—400 ppm of CO2; water deficit at V4–5 stage and optimal temperature |
| WD + HT a[CO2] | Priming—400 ppm of CO2; water deficit at V4–5 stage and heat stress at R4 stage (35/28 °C day/night) |
| CNT e[CO2] | Control condition—700 ppm of CO2; irrigated and optimal temperature |
| HT e[CO2] | Heat—700 ppm of CO2; irrigated and with heat stress at R4 stage (35/28 °C day/night) |
| WD e[CO2] | Water deficit—700 ppm of CO2; water deficit at V4–5 stage and optimal temperature |
| WD + HT e[CO2] | Priming—700 ppm of CO2; water deficit at V4–5 stage and heat stress at R4 stage (35/28 °C day/night) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feijó, A.d.R.; Viana, V.E.; Balbinot, A.; Fipke, M.V.; Souza, G.M.; do Amarante, L.; Avila, L.A.d. Water Deficit at Vegetative Stage Induces Tolerance to High Temperature during Anthesis in Rice. Plants 2023, 12, 3133. https://doi.org/10.3390/plants12173133
Feijó AdR, Viana VE, Balbinot A, Fipke MV, Souza GM, do Amarante L, Avila LAd. Water Deficit at Vegetative Stage Induces Tolerance to High Temperature during Anthesis in Rice. Plants. 2023; 12(17):3133. https://doi.org/10.3390/plants12173133
Chicago/Turabian StyleFeijó, Anderson da Rosa, Vívian Ebeling Viana, Andrisa Balbinot, Marcus Vinicius Fipke, Gustavo Maia Souza, Luciano do Amarante, and Luis Antonio de Avila. 2023. "Water Deficit at Vegetative Stage Induces Tolerance to High Temperature during Anthesis in Rice" Plants 12, no. 17: 3133. https://doi.org/10.3390/plants12173133








