In Vitro Analysis of Cytotoxic Activities of Monotheca buxifolia Targeting WNT/β-Catenin Genes in Breast Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Percentage Yield
2.2. Total Phenolic/Total Flavonoid Contents
2.3. Fourier Transform Infrared Spectrophotometer (FTIR) and Gas Chromatography Mass Spectrometry (GCMS) Analyses
2.4. Radical Scavenging Assays
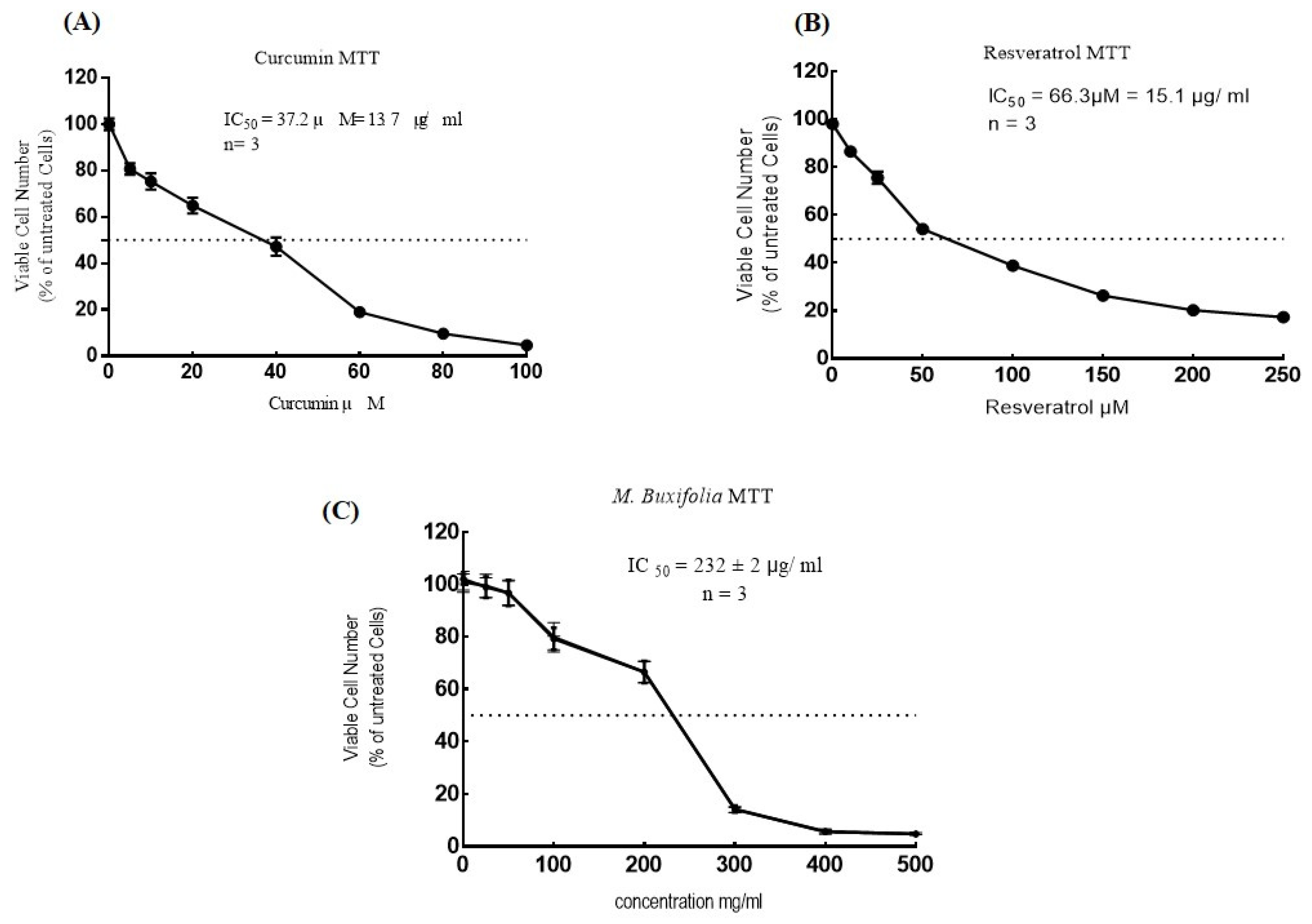
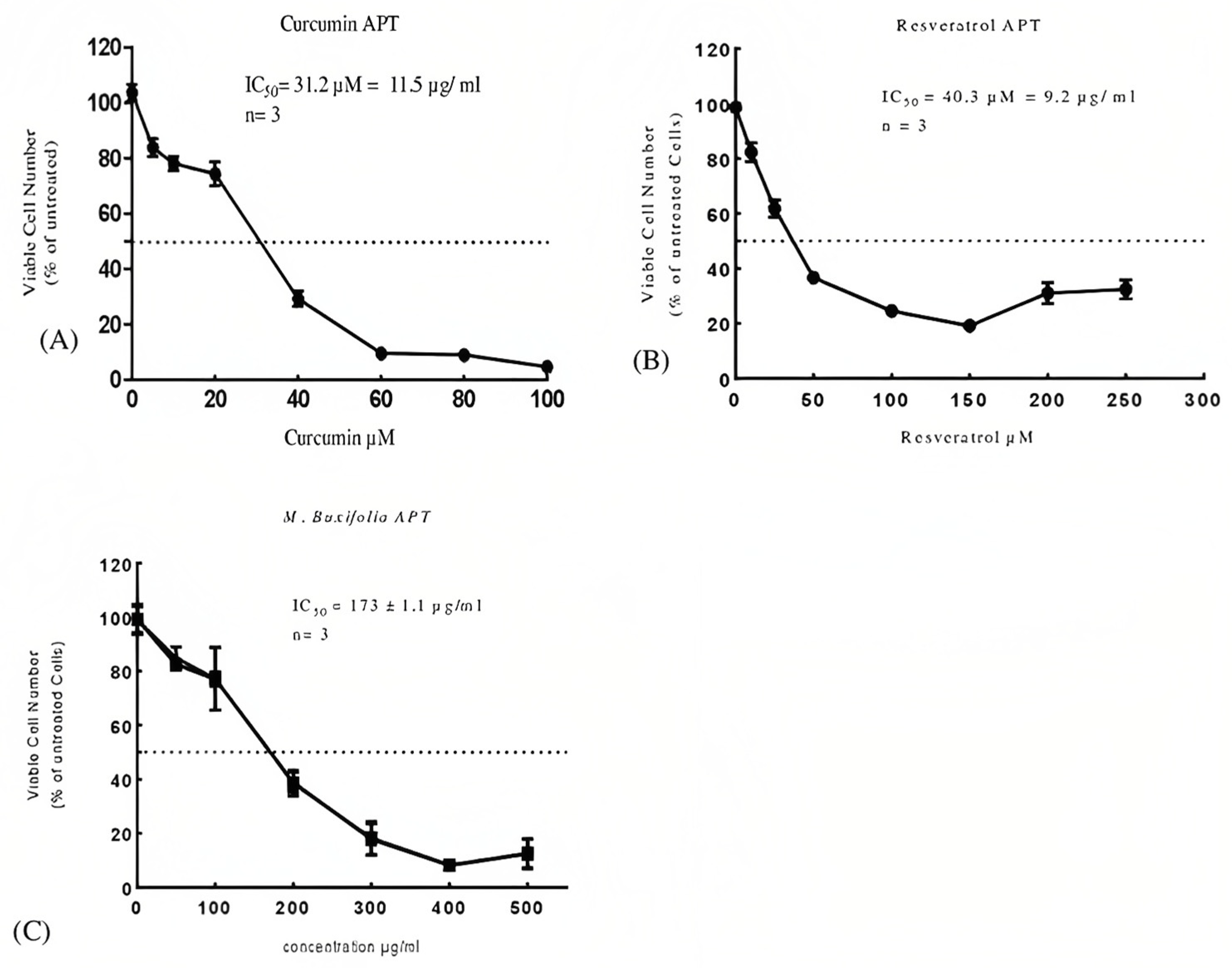
2.5. Cell Viability Assays
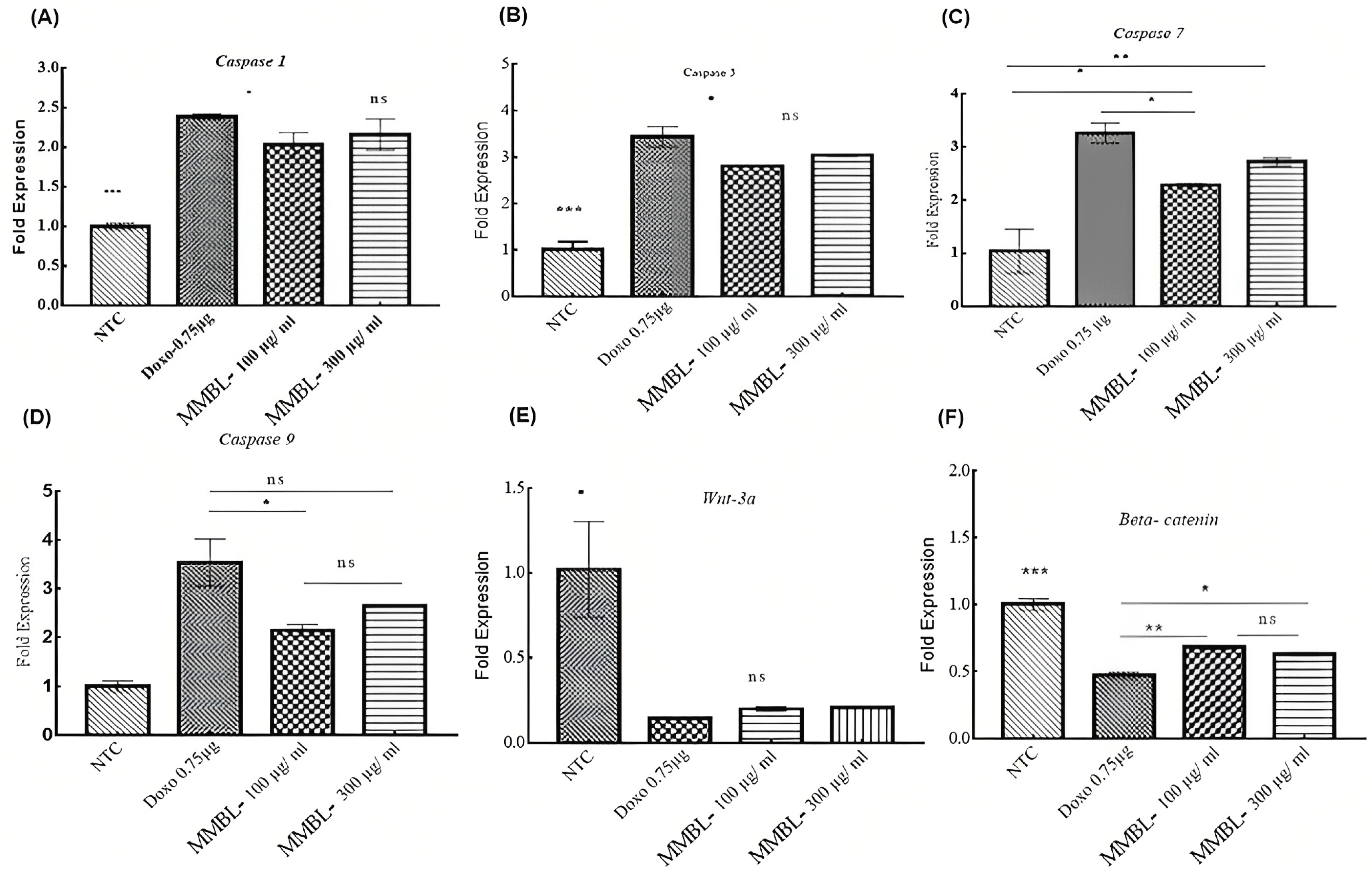
2.6. Real-Time Fold Expression of Targeted Genes
2.6.1. Upregulation of Caspase-1, -3, -7, and -9
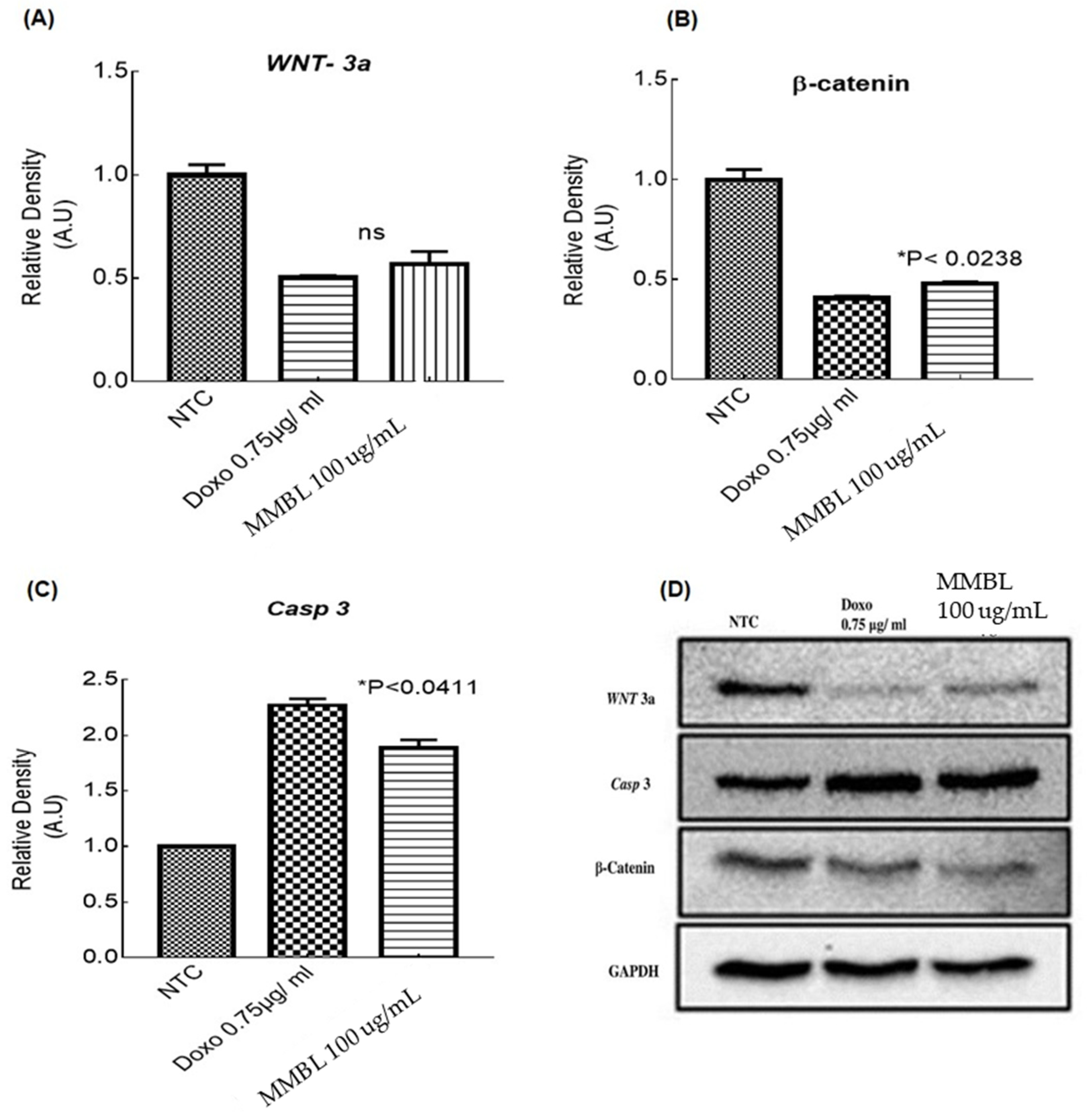
2.6.2. Downregulation of WNT-3a and β-Catenin
2.7. MMBL-Induced Apoptosis in MCF-7 Cells
2.8. Western Blotting
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Collection
4.1.2. Extract Preparation
4.2. Total Phenolic Contents and Total Flavonoid Contents
4.3. Characterization
4.3.1. Fourier Transform Infrared Spectrophotometer (FTIR)
4.3.2. Gas Chromatography Mass Spectrometry
4.4. Radical Scavanging Assays
4.4.1. DPPH Assay
4.4.2. Total Antioxidant Capacity (TAC) Assay
4.5. Cell Culture and Cell Viability Analysis
4.5.1. MTT Assay
4.5.2. Acid Phosphatase Assay
4.6. Real-Time PCR Targeting WNT/β-Catenin Genes
4.6.1. Selection of Doses
4.6.2. RNA Extraction Using TRIzol Method
4.6.3. Expression Profiling of Caspases-1, -3, -7, -9, WNT-3a, and β-Catenin Genes
4.7. Apoptosis Detection by Annexin V and PI Staining
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, Y.; Deng, J.; Wu, X. Germline mutations and blood malignancy. Oncol. Rep. 2021, 45, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, S.; Shah, N.; Maqbool, S.A.; Soomro, N.M. Estimates of past and future time trends in age-specific breast cancer incidence among women in Karachi, Pakistan: 2004–2025. BMC Public Health 2019, 19, 1001. [Google Scholar] [CrossRef]
- Špaková, I.; Rabajdová, M.; Mičková, H.; Graier, W.F.; Mareková, M. Effect of hypoxia factors gene silencing on ROS production and metabolic status of A375 malignant melanoma cells. Sci. Rep. 2021, 11, 10325. [Google Scholar] [CrossRef]
- Koni, M.; Pinnarò, V. The Wnt Signalling Pathway: A Tailored Target in Cancer. Available online: https://www.mdpi.com/861384 (accessed on 6 February 2022).
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef] [Green Version]
- Blagodatski, A.; Poteryaev, D.; Katanaev, V.L. Targeting the Wnt pathways for therapies. Mol. Cell. Ther. 2014, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Franzoi, M.A.; Agostinetto, E.; Perachino, M.; Del Mastro, L.; de Azambuja, E.; Vaz-Luis, I.; Partridge, A.H.; Lambertini, M. Evi-dence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021, 22, e303–e313. [Google Scholar] [CrossRef]
- Fierascu, R.; Fierascu, I.; Baroi, A.; Ortan, A. Selected Aspects Related to Medicinal and Aromatic Plants as Alternative Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 1521. [Google Scholar] [CrossRef]
- Ali, F.; Khan, N.; Abd_Allah, E.F.; Ahmad, A. Species Diversity, Growing Stock Variables and Carbon Mitigation Potential in the Phytocoenosis of Monotheca buxifolia Forests along Altitudinal Gradient across Pakistan. Appl. Sci. 2022, 12, 1292. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, S.; Naheed, S.; Ahmad, Z.; Mehmmod, T. Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of Monotheca Buxifolia. Univ. Wah J. Sci. Technol. (UWJST) 2019, 3, 15–22. [Google Scholar]
- Moretti, V. Poultry CC-H of Processed Meats and, 2008 U. Omega-3 and Trans Fatty Acids. Available online: https://books.google.com.pk/books?hl=en&lr=&id=s3qptENwh30C&oi=fnd&pg=PA233&dq=sinclair+et+al+2002+9,12,15-Octadecatrienoic+acid,+cancer+&ots=xAj4Jw_QgY&sig=pylQ8vySWBBZk97LPDjreuNzZss (accessed on 6 February 2022).
- Kusumah, D.; Wakui, M.; Murakami, M.; Xie, X.; Yukihito, K.; Maeda, I. Linoleic acid, α-linolenic acid, and monolinolenins as antibacterial substances in the heat-processed soybean fermented with Rhizopus oligosporus. Biosci. Biotechnol. Biochem. 2020, 84, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Gunda, H.; Yelwa, A.; Shehu, A. Isolation and Characterization of Bioactive Compound from. 2021. Available online: https://www.ajol.info/index.php/bajopas/article/view/218951/206556 (accessed on 6 February 2022).
- Godwin, A.; Akinpelu, B. Identification of n-Hexane Fraction Constituents of Archidium ohioense (Schimp. Ex Mull) Extract Using GC-MS Technique. 2015. Available online: https://www.academia.edu/download/57223492/Akinpelu662014BJPR13590.pdf (accessed on 6 February 2022).
- Hadi, H.; Vettor, R. Antioxidants MR-, 2018 U. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? 2018. Available online: https://www.mdpi.com/253814 (accessed on 6 February 2022).
- Moreno-Anzúrez, N.; Marquina, S.; Alvarez, L. A Cytotoxic and Anti-Inflammatory Campesterol Derivative from Genetically Transformed Hairy Roots of Lopezia racemosa Cav.(Onagraceae). 2017. Available online: https://www.mdpi.com/175318 (accessed on 6 February 2022).
- Awad, A.B.; Barta, S.L.; Fink, C.S.; Bradford, P.G. β-Sitosterol enhances tamoxifen effectiveness on breast cancer cells by affecting ceramide metabolism. Mol. Nutr. Food Res. 2008, 52, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Aljari, S.; Abutaha, N. Acute and Subacute Toxicity Studies of a New Herbal Formula Induced Apoptosis in the Highly Metastatic MDA-MB-231 Cells. Available online: https://www.sciencedirect.com/science/article/pii/S1018364721003086 (accessed on 29 April 2022).
- Basu, S.; Ellinger, B.; Rizzo, S.; Deraeve, C.; Schürmann, M.; Preut, H.; Arndt, H.-D.; Waldmann, H. Biology-oriented synthesis of a natural-product inspired oxepane collection yields a small-molecule activator of the Wnt-pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 6805–6810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailafiya, M.; Yusuf, A. Antimicrobial Activity of Stigmasterol from the Stem Bark of Neocarya macrophylla. 2018. Available online: https://journals.co.za/doi/abs/10.4102/jomped.v2i1.38 (accessed on 6 February 2022).
- Marwat, S.; Usman, K. Medico-Ethnobotanical Studies of Edible Wild Fruit Plants Species from the Flora of North Western PAKISTAN (DI Khan District). Available online: https://academicjournals.org/journal/JMPR/article-abstract/70C437720880 (accessed on 6 February 2022).
- Batool, R.; Khan, M.R.; Sajid, M.; Ali, S.; Zahra, Z. Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus (Schott & Endl.) R. Br. BMC Chem. 2019, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Zhang, X.; Zhang, J. Antioxidant Activity In Vitro Guided Screening and Identification of Flavonoids Antioxidants in the Extract from Tetrastigma hemsleyanum Diels et Gilg. Int. J. Anal. Chem. 2021, 2021, 7195125. [Google Scholar] [CrossRef]
- Hassan, S.; Rehman, A.U.; Jamal, S.B.; Liu, Y.; Lu, S. Bioactive compounds from Monotheca Buxifolia inhibit the growth of hepato-cellular carcinoma. Preprints 2020, 2020090326. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Huang, C.; Liu, Y.; Kuang, H.; Pang, Q. Involvement of NLRP3/Caspase-1/GSDMD-Dependent Pyroptosis in BPA-Induced Apoptosis of Neuroblastoma Cells. 2021. Available online: https://www.researchsquare.com/article/rs-722221/latest.pdf (accessed on 23 January 2022).
- Pang, X.; Gao, X.; Liu, F.; Jiang, Y.; Wang, M. Xanthoangelol Modulates Caspase-1-Dependent Pyroptotic Death among Hepatocellular Carcinoma Cells with High Expression of GSDMD. 2021. Available online: https://www.sciencedirect.com/science/article/pii/S1756464621002267 (accessed on 26 December 2021).
- Al-oqail, M.M.; Al-sheddi, E.S.; Farshori, N.N.; Al-massarani, S.M.; Al-turki, E.A.; Ahmad, J.; Al-khedhairy, A.A.; Siddiqui, M.A. Corn Silk ( Zea mays L.) Induced Apoptosis in Human Breast Cancer (MCF-7) Cells via the ROS-Mediated Mitochondrial Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 9789241. [Google Scholar] [CrossRef] [Green Version]
- Yaoxian, W.; Hui, Y.; Yunyan, Z.; Yanqin, L.; Xin, G.; Xiaoke, W. Emodin induces apoptosis of human cervical cancer hela cells via intrinsic mitochondrial and extrinsic death receptor pathway. Cancer Cell Int. 2013, 13, 71. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, G.; Anto, R.J.; Srinivas, P.; Vidhyalakshmi, S.; Senan, V.P.; Karunagaran, D. Emodin induces apoptosis of human cervical cancer cells through poly(ADP-ribose) polymerase cleavage and activation of caspase-9. Eur. J. Pharmacol. 2003, 473, 117–125. [Google Scholar] [CrossRef]
- Li, B.; Wang, S.; Wang, S. MiR-195 suppresses colon cancer proliferation and metastasis by targeting WNT3A. Mol. Genet. Genom. 2018, 293, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Al-Malky, H.S.; Osman, A.M.; Damanhouri, Z.A.; Alkreathy, H.M.; Al Aama, J.Y.; Ramadan, W.S.; Al Qahtani, A.A.; Al Mahdi, H.B. Modulation of doxorubicin-induced expression of the multidrug resistance gene in breast cancer cells by diltiazem and protection against cardiotoxicity in experimental animals. Cancer Cell Int. 2019, 19, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taparia, S.S.; Khanna, A. Procyanidin-rich extract of natural cocoa powder causes ROS-mediated caspase-3 dependent apoptosis and reduction of pro-MMP-2 in epithelial ovarian carcinoma cell lines. Biomed. Pharmacother. 2016, 83, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Halimah, E.; Hendriani, R.; Indradi, B. Beta-sitosterol from sablo ( Acalypha wilkesiana Muell. Arg.) leaves induce apoptosis in MCF-7 Breast cancer cell lines. J. Adv. Pharm. Educ. Res. 2023, 13, 111–115. [Google Scholar] [CrossRef]
- Sangpairoj, K.; Settacomkul, R.; Siangcham, T.; Meemon, K.; Niamnont, N.; Sornkaew, N.; Tamtin, M.; Sobhon, P.; Vivithanaporn, P. Hexadecanoic acid-enriched extract of Halymenia durvillei induces apoptotic and autophagic death of human triple-negative breast cancer cells by upregulating ER stress. Asian Pac. J. Trop. Biomed. 2022, 12, 132. [Google Scholar]
- AmeliMojarad, M.; AmeliMojarad, M.; Pourmahdian, A. The inhibitory role of stigmasterol on tumor growth by inducing apop-tosis in Balb/c mouse with spontaneous breast tumor (SMMT). BMC Pharmacol. Toxicol. 2022, 23, 42. [Google Scholar] [CrossRef]
- Prasad, C.P.; Rath, G.; Mathur, S.; Bhatnagar, D.; Ralhan, R. Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/β-catenin signaling. Chem. Interact. 2009, 181, 263–271. [Google Scholar] [CrossRef]
- Amado, N.G.; Predes, D.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Dudenhoeffer, A.C.; Borges, H.; Mendes, F.A.; Abreu, J.G. Isoquercitrin Suppresses Colon Cancer Cell Growth in Vitro by Targeting the Wnt/β-Catenin Signaling Pathway. J. Biol. Chem. 2014, 289, 35456–35467. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, Y.; Wang, X.-Q.; Meng, Y.; Zhang, Q.; Zhu, J.-Y.; Chen, J.-Q.; Cao, W.-S.; Wang, X.-Q.; Xie, C.-F.; et al. Phenethyl isothiocyanate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Phytother. Res. 2018, 32, 2447–2455. [Google Scholar] [CrossRef]
- Maithili Karpaga Selvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R. Curcumin Attenuates Oxidative Stress and Activation of Redox-Sensitive Kinases in High Fructose- and High-Fat-Fed Male Wistar Rats. Sci Pharm. 2014, 83, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Yuan, Q.; Meng, X. Resveratrol ameliorates cognitive dysfunction in aging by rescuing synaptic plasticity and attenuating oxidative stress injury. Neural Regen. Res. 2019, 14, 238–246. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, Y.M.; Lee, C.-K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Tabassum, S.; Erum, S. Screening and Characterization of Selected Drugs Having Antibacterial Potential. 2018. Available online: https://www.researchgate.net/profile/Muhammad-Farrukh-Nisar/publication/324521592_Screening_and_characterization_of_selected_drugs_having_antibacterial_potential/links/5ad25166458515c60f51db28/Screening-and-characterization-of-selected-drugs-having-antibacterial-potential.pdf (accessed on 25 December 2021).
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Shahid, M.; Ahmad, W.; Ullah, I.; Ahmad, A.; Syed, N.-I. GC-MS Analysis and Gastroprotective Evaluations of Crude Extracts, Isolated Saponins, and Essential Oil from Polygonum hydropiper L. Front. Chem. 2017, 5, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.; Rehman, J.U.; Khan, S.A.; Bhatty, M.K. The Constituents of Unsaponifiable from Monotheca buxifolia Seed Oil. Fette, Seifen, Anstrichm. 1986, 88, 266–268. [Google Scholar] [CrossRef]
- Llorent-Martínez, E. Lathyrus Species: Phytochemical Composition, Antioxidant Activity, Enzyme Inhibitory Properties, Cytotoxic Effects, and In Silico Studies of L. czeczottianus and L. nissolia. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2017.00083/full (accessed on 25 December 2021).
- Akinyele, O.; Wallace, H. Characterising the Response of Human Breast Cancer Cells to Polyamine Modulation. Biomolecules 2021, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sinai, P. An Acid Phosphatase Assay for Quantifying the Growth of Adherent and Non-Adherent Cells. 1996. Available online: https://www.sciencedirect.com/science/article/pii/S0003269796903838 (accessed on 25 December 2021).
- Rio, D.; Ares, M. Purification of RNA Using TRIzol (TRI Reagent). Available online: http://cshprotocols.cshlp.org/content/2010/6/pdb.prot5439.short (accessed on 25 December 2021).
- Livak, K.J.; Schnittger, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| RT (min) | Compound Name | CAS No. | Abundance (%) | Mol. Formula | Mol. Weight (a.m.u.) | Compound Nature | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|---|
| 16.60 | Heptadecanoic acid | 000506-12-7 | 1.30 | C17H34O2 | 270.5 |  | Antidiabetic | [13] |
| 17.30 | 8,11-Octadecadienoic acid, meth. | 056599-58-7 | 1.62 | C19H34O2 | 294.5 |  | Antihypertensive, anticoagulant | [14] |
| 17.58 | Phytol | 070928-44-8 | 2.46 | C20H40O | 296.5 | 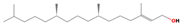 | Antimicrobial, anti-inflammatory, cytotoxic, diuretic | [15] |
| 18.25 | 9,12-Octadecadienoic acid (Z,Z)- | 000060-33-3 | 19.54 | C18H32O2 | 280.4 | 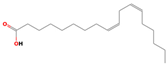 | Hepatoprotective, antihistaminic, hypocholesterolemic, anti-eczemic | [16] |
| 18.32 | 9,12,15-Octadecatrienoic acid, | 000301-00-8 | 5.50 | C18H30O2 | 278.4 |  | Antihistaminic, anticoronary, insectifuge, anti-eczemic, anti-acne | [17] |
| 20.94 | Linoleic acid ethyl ester | 000544-35-4 | 1.22 | C20H36O2 | 308.4986 | 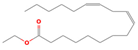 | Hypocholesterolemic, hepatoprotective, insectifuge, anti-eczemic, anti-acne antihistaminic, anticoronary | [18] |
| 23.78 | 1,2-Benzenedicarboxylic acid, m. | 004376-20-9 | 6.17 | C8H6O4 | 166.1308 |  | Antibacterial | [19] |
| 32.12 | D,.alpha.-Tocopherol | 1000128-08 | 0.54 | C29H50O2 | 430.7 | 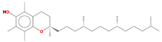 | Antioxidant, anti-inflammatory, anti-cancer | [20] |
| 33.93 | Campesterol | 000474-62-4 | 2.32 | C28H48O | 400.7 | 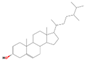 | Cytotoxic, anti-inflammatory | [21] |
| 34.66 | Stigmasterol | 000083-48-7 | 2.89 | C29H48O | 412.7 |  | Antioxidant, antimicrobial, anti-cancer, anti-arthritic, anti-inflammatory, antigungal | [22] |
| 36.23 | Sitosterol | 000083-47- | 15.95 | C29H50O | 414.7 | 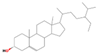 | Anti-osteoarthritic, anti-cancer, anti-inflammatory, anti-neurological, antioxidant, and antimicrobial | [19] |
| 15.84 | n-Hexadecanoic acid | 000057-10-3 | 6.68 | C16H32O2 | 256.4 |  | Cytotoxic, antioxidant | [20] |
| 15.96 | Hexadecanoic acid, ethyl ester | 000628-97-7 | 3.45 | C18H36O2 | 284.4772 |  | Antioxidant, hypocholesterolemic, nematicide, pesticide, lubricant, antiandrogenic, flavor, hemolytic 5-alpha reductase inhibitor | [21] |
| Sample | MTT IC50 μg/mL | APT IC50 μg/mL |
|---|---|---|
| MMBL | 232 μg/mL | 173 μg/mL |
| Curcumin | 13.7 μg/mL | 11.5 μg/mL |
| Resveratrol | 15.1 μg/mL | 9.2 μg/mL |
| Treatments/Samples | Targeting Casp-1 | Targeting Casp-3 | ||
|---|---|---|---|---|
| Mean of ∆∆CT | Fold Expression 2−∆∆Cq | Mean of ∆∆CT | Fold Expression 2−∆∆Cq | |
| NTC | 0.00 ± 0.06 | 1.00 ± 0.04 | 0.00 ± 0.25 | 1.01 ± 0.17 |
| Doxorubicin 0.75 μg | −1.25 ± 0.02 | 2.38 ± 0.03 | −1.78 ± 0.09 | 3.44 ± 0.22 |
| MMBL 100 μg/mL | −1.05 ± 0.01 | 2.03 ± 0.15 | −1.48 ± 0.02 | 2.79 ± 0.04 |
| MMBL 300 μg/mL | −1.17 ± 0.09 | 2.16 ± 0.19 | −1.61 ± 0.01 | 3.04 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sher, A.; Tabassum, S.; Wallace, H.M.; Khan, A.; Karim, A.M.; Gul, S.; Kang, S.C. In Vitro Analysis of Cytotoxic Activities of Monotheca buxifolia Targeting WNT/β-Catenin Genes in Breast Cancer Cells. Plants 2023, 12, 1147. https://doi.org/10.3390/plants12051147
Sher A, Tabassum S, Wallace HM, Khan A, Karim AM, Gul S, Kang SC. In Vitro Analysis of Cytotoxic Activities of Monotheca buxifolia Targeting WNT/β-Catenin Genes in Breast Cancer Cells. Plants. 2023; 12(5):1147. https://doi.org/10.3390/plants12051147
Chicago/Turabian StyleSher, Ambreen, Sobia Tabassum, Heather Mann Wallace, Asifullah Khan, Asad Mustafa Karim, Sarah Gul, and Se Chan Kang. 2023. "In Vitro Analysis of Cytotoxic Activities of Monotheca buxifolia Targeting WNT/β-Catenin Genes in Breast Cancer Cells" Plants 12, no. 5: 1147. https://doi.org/10.3390/plants12051147






