Physiological and Biochemical Mechanisms of Wood Vinegar-Induced Stress Response against Tomato Fusarium Wilt Disease
Abstract
:1. Introduction
2. Results
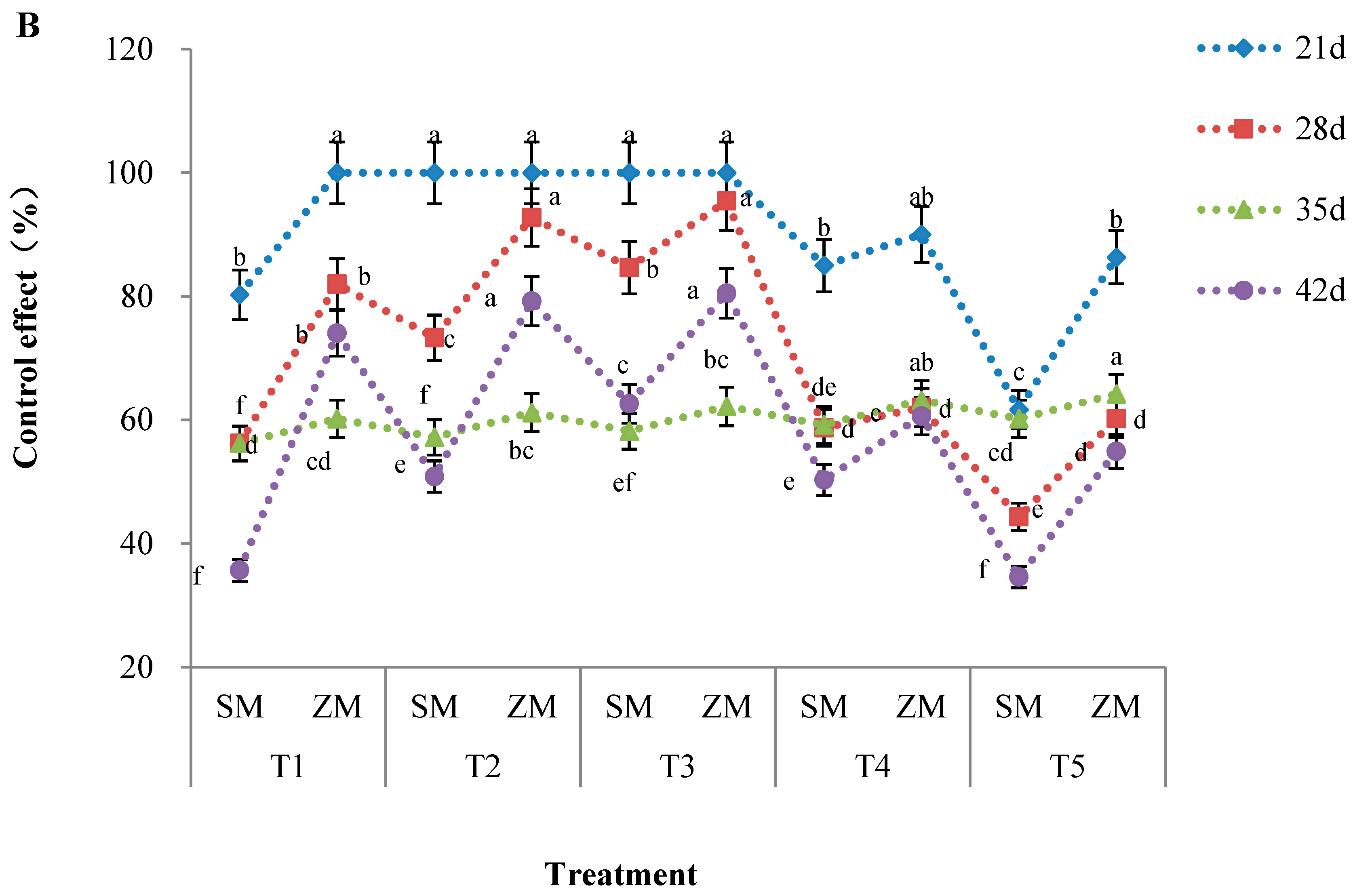
2.1. Disease Severity
2.2. Plant Growth
2.3. Nutritional Quality and Yield
2.4. Malondialdehyde (MDA) Content in Tomato Leaves
2.5. Hydrogen Peroxide (H2O2) Content in Tomato Leaves
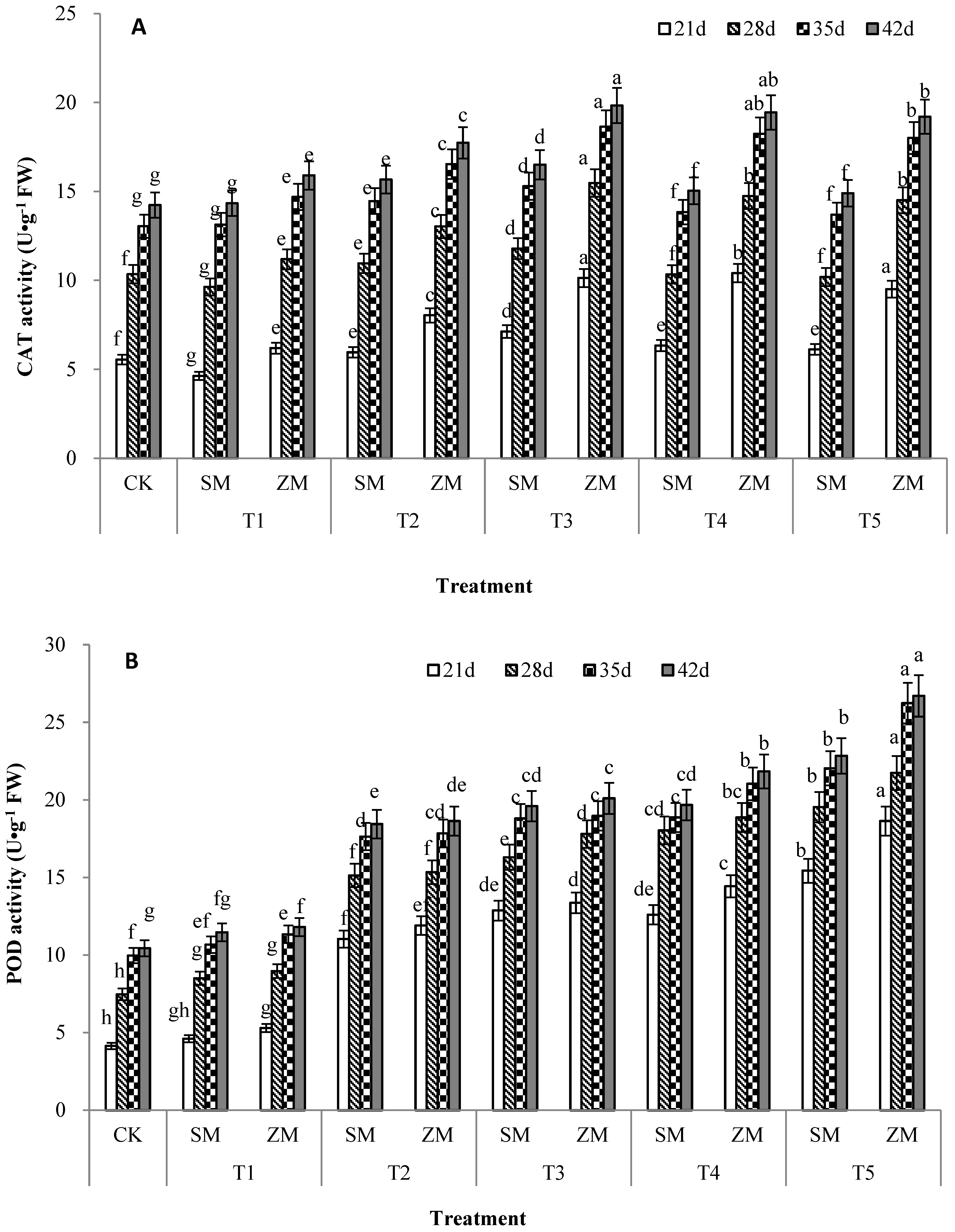
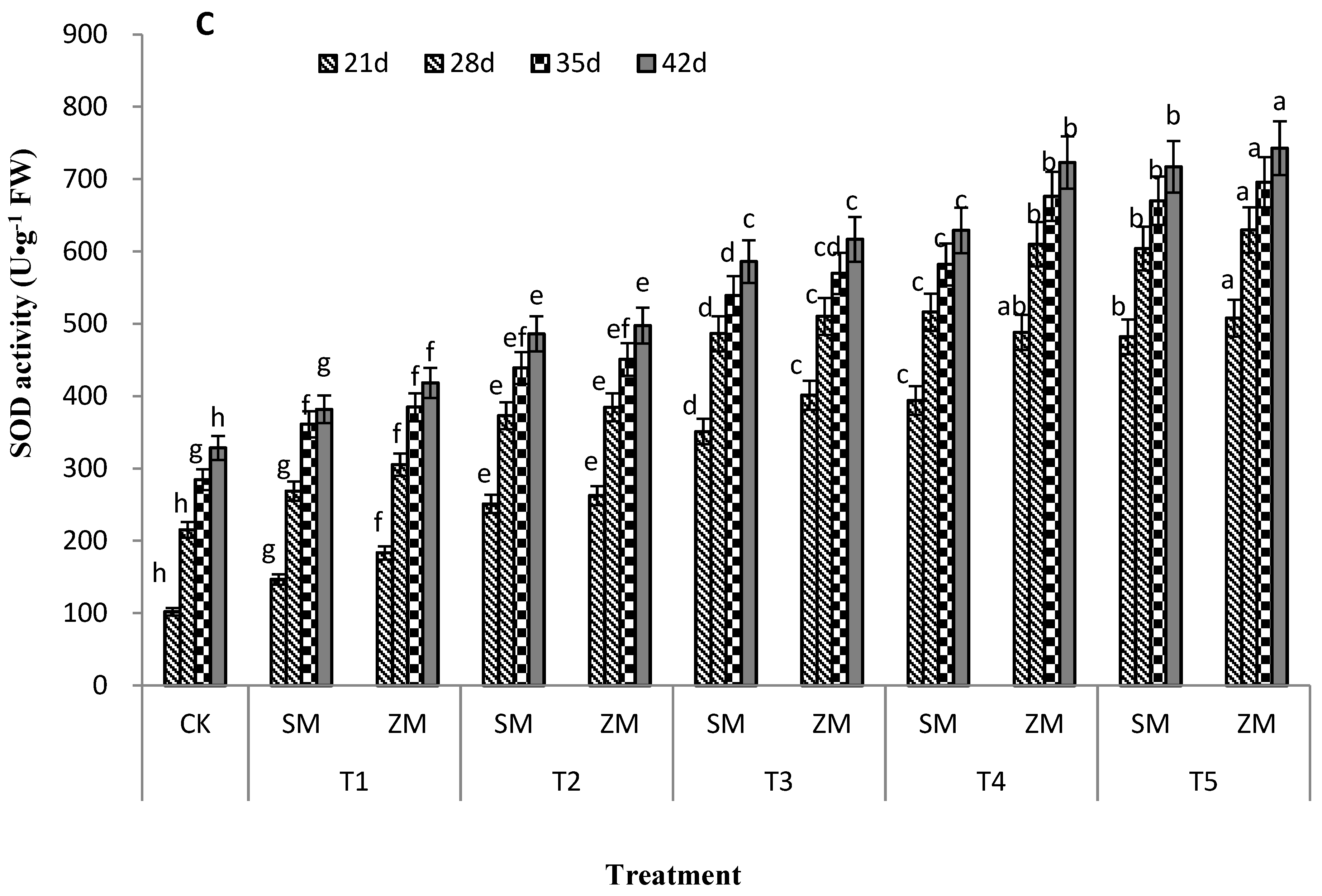
2.6. Activities of Antioxidant Enzymes in Tomato Leaves
2.7. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Wood Vinegar Material
4.1.2. Plant Material
4.1.3. Experimental Soil
4.1.4. Pathogen Strain Tested
4.2. Experimental Design
4.3. Indicators and Methods of Determination
4.3.1. Determination of Disease Severity Index
4.3.2. Determination of Fruit Nutritional Quality
4.3.3. Determination of MDA and H2O2 Contents in Tomato Leaves
4.3.4. Determination of Antioxidant Enzyme Activity in Leaves
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar Role in the Sustainability of Agriculture and Environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Sun, S.; Gao, J.; Zhang, H.; Zhang, Z.; Wang, Z. Disinfection and Removal Performance for Escherichia coli, Toxic Heavy Metals and Arsenic by Wood Vinegar-Modified Zeolite. Ecotoxicol. Environ. Saf. 2019, 174, 129–136. [Google Scholar] [CrossRef]
- Zhang, F.; Shao, J.; Yang, H.; Guo, D.; Chen, Z.; Zhang, S.; Chen, H. Effects of Biomass Pyrolysis Derived Wood Vinegar on Microbial Activity and Communities of Activated Sludge. Bioresour. Technol. 2019, 279, 252–261. [Google Scholar] [CrossRef]
- Mathew, S.; Zakaria, Z.A. Pyroligneous Acid—The Smoky Acidic Liquid from Plant Biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef]
- Marumoto, S.; Yamamoto, S.P.; Nishimura, H.; Onomoto, K.; Yatagai, M.; Yazaki, K.; Fujita, T.; Watanabe, T. Identification of a Germicidal Compound against Picornavirus in Bamboo Pyroligneous Acid. J. Agric. Food Chem. 2012, 60, 9106–9111. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, C.; Hou, X.; Wu, M.; Yao, Y.; Li, F. Pyrolysis of Arundo donax L. to Produce Pyrolytic Vinegar and Its Effect on the Growth of Dinoflagellate Karenia Brevis. Bioresour. Technol. 2018, 247, 273–281. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, Prospects and Potential Application of Pyroligneous Acid in Agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Xue, R.; Cui, E.L.; Hu, G.Q.; Zhu, M.Q. The composition, physicochemical properties, antimicrobial and antioxidant activity of wood vinegar prepared by pyrolysis of Eucommia ulmoides Oliver branches under different refining methods and storage conditions. Ind. Crops Prod. 2022, 178, 114586. [Google Scholar] [CrossRef]
- Ofoe, R.; Qin, D.; Gunupuru, L.R.; Thomas, R.H.; Abbey, L. Effect of Pyroligneous Acid on the Productivity and Nutritional Quality of Greenhouse Tomato. Plants 2022, 11, 1650. [Google Scholar] [CrossRef]
- Gao, T.; Bian, R.; Joseph, S.; Taherymoosavi, S.; Shi, J. Wheat Straw Vinegar: A More Cost-Effective Solution than Chemical Fungicides for Sustainable Wheat Plant Protection. Sci. Total Environ. 2020, 725, 138359. [Google Scholar] [CrossRef]
- Bouket, A.C.; Narmani, A.; Tavasolee, A.; Elyasi, G.; Abdi, A.; Naeimi, S.; Sharifi, K.; Oszako, T.; Alenezi, F.N.; Belbahri, L. In Vitro Evaluation of Wood Vinegar (Pyroligneous Acid) VOCs Inhibitory Effect against a Fungus-like Microorganism Ovatisporangium (Phytopythium) Isolate Recovered from Tomato Fields in Iran. Agronomy 2022, 12, 1609. [Google Scholar] [CrossRef]
- Vignesh, K.; Rajamohan, K.; Balabaskar, P.; Ramaswamy, A.; Udhayakumar, R. Survey on the Incidence of Fusarium Wilt of Tomato Incited by Fusarium oxysporum f. Sp. Lycopersici (FOL) in Major Tomato Growing Areas of Krishnagiri District. Plant Arch. 2021, 21, 2369–2376. [Google Scholar]
- Bharti, P.; Jyoti, P.; Kapoor, P.; Sharma, V.; Shanmugam, V.; Yadav, S.K. Host-Induced Silencing of Pathogenicity Genes Enhances Resistance to Fusarium oxysporum Wilt in Tomato. Mol. Biotechnol. 2017, 59, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Upadhyay, R.S. Effects of Arsenic on Reactive Oxygen Species and Antioxidant Defense System in Tomato Plants. Toxicol. Environ. Chem. 2014, 96, 1374–1383. [Google Scholar] [CrossRef]
- Mylona, P.V.; Polidoros, A.N.; Scandalios, J.G. Modulation of Antioxidant Responses by Arsenic in Maize. Free Radic. Biol. Med. 1998, 25, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Lamo, F.J.D.; Spijkers, S.B.; Takken, F.L.W. Protection to Tomato Wilt Disease Conferred by the Nonpathogen Fusarium oxysporum Fo47 Is More Effective Than That Conferred by Avirulent Strains. Phytopathology 2020, 111, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Xueuan, R.; Dandan, S.; Zhuo, L.; Qingjun, K. Effect of Mint Oil against Botrytis Cinerea on Table Grapes and Its Possible Mechanism of Action. Eur. J. Plant Pathol. 2018, 151, 321–328. [Google Scholar] [CrossRef]
- Marín, A.; Cháfer, M.; Atarés, L.; Chiralt, A.; Torres, R.; Usall, J.; Teixidó, N. Effect of Different Coating-Forming Agents on the Efficacy of the Biocontrol Agent Candida sake CPA-1 for Control of Botrytis cinerea on Grapes. Biol. Control 2016, 96, 108–119. [Google Scholar] [CrossRef]
- Hua, C.; Li, Y.; Wang, X.; Kai, K.; Su, M.; Shi, W.; Zhang, D.; Liu, Y. The Effect of Low and High Molecular Weight Chitosan on the Control of Gray Mold (Botrytis cinerea) on Kiwifruit and Host Response. Sci. Hortic. 2019, 246, 700–709. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of Pesticide Pollution at the Global Scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Solla, A.; Moreno, G.; Malewski, T.; Jung, T.; Oszako, T. Phosphite Spray for the Control of Oak Decline Induced by Phytophthora in Europe. For. Ecol. Manag. 2021, 485, 118938. [Google Scholar] [CrossRef]
- Belbahri, L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests 2021, 12, 1579. [Google Scholar]
- Cherifsilini, H.; Thissera, B.; Bouket, A.C.; Saadaoui, N.; Silini, A.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Luptakova, L.; Yahiaoui, B. Durum Wheat Stress Tolerance Induced by Endophyte Pantoea Agglomerans with Genes Contributing to Plant Functions and Secondary Metabolite Arsenal. Int. J. Mol. Sci. 2019, 20, 3989. [Google Scholar] [CrossRef]
- Cheffi, M.; Bouket, A.C.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea L. Root Endophyte Bacillus Velezensis OEE1 Counteracts Oomycete and Fungal Harmful Pathogens and Harbours a Large Repertoire of Secreted and Volatile Metabolites and Beneficial Functional Genes. Microorganisms 2019, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.F.; Jiang, E.C.; Xiong, L.M.; Xu, X.W.; Zhao, C.; Wang, G.; Ma, Q. Components Characteristics of Wood Vinegar from Rice Husk Continuous Pyrolysis and Catalytic Cracking. Appl. Mech. Mater. 2013, 291–294, 368–374. [Google Scholar] [CrossRef]
- Oramahi, H.A.; Yoshimura, T. Antifungal and Antitermitic Activities of Wood Vinegar from Vitex pubescens Vahl. J. Wood Sci. 2013, 59, 344–350. [Google Scholar] [CrossRef]
- Liu, X.; Cui, R.; Shi, J.; Jiang, Q.; Li, X. Separation and Microencapsulation of Antibacterial Compounds from Wood Vinegar. Process Biochem. 2021, 110, 275–281. [Google Scholar] [CrossRef]
- Velmurugan, N.; Han, S.S.; Lee, Y.S. Antifungal Activity of Neutralized Wood Vinegar with Water Extracts of Pinus densiflora and Quercus serrata Saw Dusts. Int. J. Environ. Res. 2009, 3, 167–176. [Google Scholar]
- Xiao, H.; Cheng, W.J.; Zhang, P.; Zhang, H.; Jiao, R.M.; Wang, L.Y.; Zhao, J.; Pan, J. Control Effects of Combination of Wood Vinegar and Fungicide on Tomato Fusarium Wilt and Gray Mold. Jiangsu Agric. Sci. 2021, 49, 82–87. [Google Scholar]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood Vinegar as a Complex Growth Regulator Promotes the Growth, Yield, and Quality of Rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Benzon, H.R.L.; Lee, S.C. Potential of Wood Vinegar in Enhancing Fruit Yield and Antioxidant Capacity in Tomato. Korean J. Plant Resour. 2016, 29, 704–711. [Google Scholar] [CrossRef]
- Zhang, L.; Garcia-Perez, P.; Arikan, B.; Elbasan, F.; Alp, F.N.; Balci, M.; Zengin, G.; Yildiztugay, E.; Lucini, L. The exogenous application of wood vinegar induces a tissue- and dose-dependent elicitation of phenolics and functional traits in onion (Allium cepa L.). Food Chem. 2023, 405, 134926. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Baharom, N.A.; Rahman, M.H.A.; Shahrun, M.S.; Suherman, F.H.S.; Masdar, S.N.H. Chemical Composition and Antimicrobial Activities of Wood Vinegars from Carambola, Coconut Shells and Mango against Selected Plant Pathogenic Microorganisms. Malays. J. Microbiol. 2020, 16, 438–445. [Google Scholar]
- De Souza Araújo, E.; Pimenta, A.S.; Feijó, F.M.C.; Castro, R.V.O.; Fasciotti, M.; Monteiro, T.V.C.; De Lima, K.M.G. Antibacterial and Antifungal Activities of Pyroligneous Acid from Wood of Eucalyptus urograndis and Mimosa tenuiflora. J. Appl. Microbiol. 2018, 124, 85–96. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Qin, Q.Q.; Diao, C.Q.; Yu, J.B.; Wang, X. Effects of Different Wood Vinegar on Growth and Physiology of Flue-Cured Tobacco Under Drought Stress. Southwest China J. Agric. Sci. 2020, 33, 2232–2236. [Google Scholar]
- De Alché, J.D.A. Concise Appraisal of Lipid Oxidation and Lipoxidation in Higher Plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Nian, F.Z.; Chen, B.D.; Zhu, Y.G.; Yue, X.R.; Zhang, N.M.; Xia, Y.S. Synergistic Reduction of Arsenic Uptake and Alleviation of Leaf Arsenic Toxicity in Maize (Zea mays L.) by Arbuscular Mycorrhizal Fungi (AMF) and Exogenous Iron through Antioxidant Activity. J. Fungi 2023, 9, 677. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Kalaba, M.H.; Hashem, A.H.; Sharaf, M.H.; Attia, M.S. Biostimulation of Tomato Growth and Biocontrol of Fusarium Wilt Disease Using Certain Endophytic Fungi. Bot. Stud. 2022, 63, 34. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Christensen, M.J.; Gao, P.; Li, Y.; Duan, T. An Arbuscular Mycorrhizal Fungus and Epichloë festucae Var. Lolii Reduce Bipolaris sorokiniana Disease Incidence and Improve Perennial Ryegrass Growth. Mycorrhiza 2018, 28, 159–169. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, Y.F.; Wei, H.; Li, X.X.; Zheng, H.T.; Dong, X.Y.; Xu, T.F.; Meng, J.F. Inhibition Efficiency of Wood Vinegar on Grey Mould of Table Grapes. Food Biosci. 2020, 38, 100755. [Google Scholar] [CrossRef]
- Habibi, G. Chapter 19-Hydrogen Peroxide (H2O2) Generation, Scavenging and Signaling in Plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 557–584. [Google Scholar]
- Latef, A.A.H.A.; Kordrostami, M.; Zakir, A.; Zaki, H.; Saleh, O.M. Eustress with H2O2 Facilitates Plant Growth by Improving Tolerance to Salt Stress in Two Wheat Cultivars. Plants 2019, 8, 303. [Google Scholar] [CrossRef]
- Yu, G.B.; Chen, R.N.; Chen, Q.S.; Chen, F.Q.; Liu, H.L.; Ren, C.Y.; Zhang, Y.X.; Yang, F.J.; Wei, J.P. Jasmonic acid promotes glutathione assisted degradation of chlorothalonil during tomato growth. Ecotoxicol. Environ. Saf. 2022, 233, 113296. [Google Scholar] [CrossRef]
- Li, X.; Riaz, M.; Song, B.; Liang, X.; Liu, H. Exogenous salicylic acid alleviates fomesafen toxicity by improving photosynthetic characteristics and antioxidant defense system in sugar beet. Ecotoxicol. Environ. Saf. 2022, 238, 113587. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sgherri, C.; Ranieri, A.; Quartacci, M.F. Antioxidative Responses in Vitis Vinifera Infected by Grapevine Fanleaf Virus. J. Plant Physiol. 2013, 170, 121–128. [Google Scholar] [CrossRef]
- Samiksha, S.; Parul, P.; Rachana, S.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Breusegem, F.V. ROS Signaling: The New Wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Herrero, J.; Esteban-Carrasco, A.; Zapata, J.M. Looking for Arabidopsis thaliana Peroxidases Involved in Lignin Biosynthesis. Plant Physiol. Biochem. 2013, 67, 77–86. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root Proteomics Reveals the Effects of Wood Vinegar on Wheat Growth and Subsequent Tolerance to Drought Stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, X.; Zhao, Z.; Zhang, S.; Liu, S. Antioxidant Activities and Chemical Profiles of Pyroligneous Acids from Walnut Shell. J. Anal. Appl. Pyrolysis 2010, 88, 149–154. [Google Scholar] [CrossRef]
- Loo, A.Y.; Jain, K.; Darah, I. Antioxidant and Radical Scavenging Activities of the Pyroligneous Acid from a Mangrove Plant, Rhizophora apiculata. Food Chem. 2007, 104, 300–307. [Google Scholar] [CrossRef]
- Mihutagrimm, I.; Erb, W.A.; Rowe, R.C. Fusarium Crown and Root Rot of Tomato in Greenhouse Rock Wool Systems: Sources of Inoculum and Disease Management with Benomyl. Plant Dis. 1990, 74, 996–1002. [Google Scholar]
- Xia, W.; Guang, X.; Li, Y.X. Study on Determination Conditions of Total Sugar from Potato Starch by Anthrone Colorimetry. Food Res. Dev. 2013, 34, 86–88. [Google Scholar]
- Fernandez, S.R.; Zhang, Y.; Parsons, C.M. Determination of Protein Solubility in Oilseed Meals Using Coomassie Blue Dye Binding. Poult. Sci. 1993, 72, 1925–1930. [Google Scholar] [CrossRef]
- Harris, L.J.; Olliver, M. Vitamin Methods: The Reliability of the Method for Estimating Vitamin C by Titration against 2:6-Dichlorophenolindophenol. 1. Control Tests with Plant Tissues. Biochem. J. 1942, 36, 155–182. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Prange, F.R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Borusiewicz, A.; Łozowicka, B. Fluazinam and its mixtures induce diversified changes of crucial biochemical and antioxidant profile in leafy vegetable. Sci. Hortic. 2022, 298, 110988. [Google Scholar] [CrossRef]



| Treatment | Plant Height (cm) | Stem Diameter (mm) | Leaf Area (cm2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 d | 28 d | 35 d | 42 d | 21 d | 28 d | 35 d | 42 d | 21 d | 28 d | 35 d | 42 d | ||
| Control | CK | 4.63 f | 5.69 g | 7.63 f | 10.03 f | 2.62 d | 3.72 f | 3.97 d | 4.96 g | 1.00 e | 1.11 g | 1.27 f | 1.57 f |
| T1 | SM | 5.59 de | 7.09 ef | 9.03 e | 10.93 e | 2.63 d | 3.73 f | 3.98 d | 4.94 g | 1.24 de | 1.37 e | 1.52 e | 1.82 e |
| ZM | 6.06 c | 7.56 de | 9.50 de | 11.37 de | 2.70 cd | 3.86 e | 4.04 cd | 5.12 e | 1.43 cd | 1.56 d | 1.71 d | 2.01 d | |
| T2 | SM | 5.84 cd | 7.77 d | 9.80 d | 11.70 d | 2.95 bc | 4.05 d | 4.30 b | 5.29 d | 1.13 de | 1.26 f | 1.42 e | 1.71 e |
| ZM | 6.89 b | 8.53 c | 10.47 c | 12.43 c | 2.97 b | 4.13 c | 4.32 b | 5.47 c | 1.83 b | 1.96 b | 2.10 b | 2.41 b | |
| T3 | SM | 7.47 a | 9.27 b | 11.27 b | 13.40 b | 3.26 ab | 4.56 b | 4.81 a | 5.80 b | 1.66 bc | 1.79 c | 1.93 c | 2.24 c |
| ZM | 7.71 a | 10.24 a | 12.37 a | 14.37 a | 3.54 a | 4.77 a | 4.89 a | 5.91 a | 2.22 a | 2.28 a | 2.43 a | 2.73 a | |
| T4 | SM | 5.46 e | 6.96 f | 8.90 e | 10.80 e | 2.67 cd | 3.77 f | 4.02 cd | 5.01 fg | 1.07 de | 2.03 b | 2.18 b | 2.41 b |
| ZM | 5.86 cd | 7.36 def | 9.30 de | 11.20 de | 2.75 c | 3.85 e | 4.10 c | 5.09 ef | 1.91 ab | 2.04 b | 2.19 b | 2.49 b | |
| T5 | SM | 4.36 f | 5.46 g | 7.40 f | 9.30 g | 1.83 f | 2.93 h | 3.18 f | 4.17 i | 0.89 e | 1.05 g | 1.17 f | 1.47 f |
| ZM | 3.46 g | 4.36 h | 6.03 g | 8.03 h | 2.14 e | 3.24 g | 3.46 e | 4.45 h | 1.08 de | 1.28 ef | 1.42 e | 1.72 e | |
| Treatment | Soluble Sugar (%) | Soluble Protein (mg·g−1) | Vitamin C (mg·100 g−1) | Organic Acid (%) | Yield (g) | |
|---|---|---|---|---|---|---|
| Control | CK | 7.87 ± 0.04 i | 1.24 ± 0.11 h | 15.26 ± 0.62 i | 4.73 ± 0.11 b | 248.65 ± 29.75 f |
| T1 | SM | 8.03 ± 0.02 h | 1.42 ± 0.06 g | 17.16 ± 0.45 h | 2.21 ± 0.06 g | 342.23 ± 33.29 bcde |
| ZM | 8.85 ± 0.02 e | 2.13 ± 0.11 f | 22.84 ± 0.70 f | 5.22 ± 0.14 a | 363.34 ± 45.09 bcd | |
| T2 | SM | 8.17 ± 0.03 g | 2.11 ± 0.11 f | 18.92 ± 0.22 g | 3.12 ± 0.11 e | 358.60 ± 36.06 bcd |
| ZM | 9.04 ± 0.04 d | 3.48 ± 0.10 d | 30.20 ± 0.63 d | 3.51 ± 0.06 d | 391.09 ± 25.70 bc | |
| T3 | SM | 8.75 ± 0.04 f | 3.30 ± 0.06 e | 22.97 ± 0.22 f | 2.58 ± 0.06 f | 432.23 ± 35.25 b |
| ZM | 9.58 ± 0.01 c | 4.28 ± 0.08 b | 33.09 ± 0.32 c | 3.76 ± 0.09 d | 553.55 ± 84.66 a | |
| T4 | SM | 8.82 ± 0.04 e | 3.45 ± 0.06 d | 25.02 ± 0.72 e | 3.41 ± 0.57 de | 316.61 ± 51.75 cdef |
| ZM | 9.81 ± 0.04 b | 4.85 ± 0.10 a | 35.49 ± 1.15 b | 3.76 ± 0.14 d | 251.63 ± 40.84 ef | |
| T5 | SM | 8.99 ± 0.04 d | 3.63 ± 0.08 c | 25.89 ± 0.44 e | 4.39 ± 0.01 c | 376.19 ± 44.06 bcd |
| ZM | 10.04 ± 0.04 a | 4.96 ± 0.06 a | 39.54 ± 0.95 a | 3.56 ± 0.06 d | 288.57 ± 80.34 def | |
| Disease Index | Plant Height | Stem Diameter | Leaf Area | MDA | H2O2 | CAT | POD | |
|---|---|---|---|---|---|---|---|---|
| Plant Height | −0.69 ** | |||||||
| Stem Diameter | −0.59 ** | 0.81 ** | ||||||
| Leaf Area | −0.71 ** | 0.85 ** | 0.93 ** | |||||
| MDA | 0.82 ** | −0.67 ** | −0.73 ** | −0.69 ** | ||||
| H2O2 | 0.51 ** | −0.52 ** | −0.47 ** | −0.39 ** | 0.79 ** | |||
| CAT | −0.40 * | 0.34 | 0.19 | 0.21 | −0.63 ** | −0.72 ** | ||
| POD | 0.01 | −0.15 | −0.20 | −0.31 | −0.29 | −0.63 ** | 0.52 ** | |
| SOD | −0.02 | −0.20 | −0.20 | −0.33 | −0.35 * | −0.62 ** | 0.48 ** | 0.94 ** |
| Concentration of ZM | Disease Index | Plant Height | Stem Diameter | Leaf Area | MDA | H2O2 | CAT | POD | |
|---|---|---|---|---|---|---|---|---|---|
| Disease Index | 0.73 ** | ||||||||
| Plant Height | −0.54 * | −0.85 ** | |||||||
| Stem Diameter | −0.43 | −0.77 ** | 0.97 ** | ||||||
| Leaf Area | −0.19 | −0.57 * | 0.88 ** | 0.89 ** | |||||
| MDA | 0.32 | 0.57 * | −0.88 ** | −0.88 ** | −0.96 ** | ||||
| H2O2 | −0.48 | 0.14 | −0.41 | −0.53 * | −0.62 * | 0.43 | |||
| CAT | 0.83 ** | 0.27 | 0.01 | 0.13 | 0.31 | −0.14 | −0.86 ** | ||
| POD | 0.96 ** | 0.63 * | −0.46 | −0.35 | −0.15 | 0.32 | −0.56 * | 0.83 ** | |
| SOD | 0.98 ** | 0.69 ** | −0.42 | −0.31 | −0.04 | 0.15 | −0.53 * | 0.86 ** | 0.91 ** |
| Concentration of SM | Disease Index | Plant Height | Stem Diameter | Leaf Area | MDA | H2O2 | CAT | POD | |
|---|---|---|---|---|---|---|---|---|---|
| Disease Index | 0.04 | ||||||||
| Plant Height | −0.45 | −0.84 ** | |||||||
| Stem Diameter | −0.50 | −0.85 ** | 0.97 ** | ||||||
| Leaf Area | −0.46 | −0.59 * | 0.86 ** | 0.81 ** | |||||
| MDA | −0.04 | 0.89 ** | −0.85 ** | −0.79 ** | −0.76 ** | ||||
| H2O2 | −0.34 | 0.81 ** | −0.62 * | −0.57 * | −0.46 | 0.88 ** | |||
| CAT | 0.29 | −0.85 ** | 0.74 ** | 0.70 ** | 0.64 * | −0.84 ** | −0.71 ** | ||
| POD | 0.89 ** | −0.23 | −0.18 | −0.24 | −0.26 | −0.23 | −0.39 | 0.42 | |
| SOD | 0.98 ** | −0.07 | −0.33 | −0.40 | −0.36 | −0.14 | −0.41 | 0.21 | 0.94 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Fu, K.; Shen, Y.; Li, R.; Su, Y.; Deng, Y.; Xia, Y.; Zhang, N. Physiological and Biochemical Mechanisms of Wood Vinegar-Induced Stress Response against Tomato Fusarium Wilt Disease. Plants 2024, 13, 157. https://doi.org/10.3390/plants13020157
Zhou H, Fu K, Shen Y, Li R, Su Y, Deng Y, Xia Y, Zhang N. Physiological and Biochemical Mechanisms of Wood Vinegar-Induced Stress Response against Tomato Fusarium Wilt Disease. Plants. 2024; 13(2):157. https://doi.org/10.3390/plants13020157
Chicago/Turabian StyleZhou, Hongyin, Kejian Fu, Yan Shen, Runhe Li, Youbo Su, Yishu Deng, Yunsheng Xia, and Naiming Zhang. 2024. "Physiological and Biochemical Mechanisms of Wood Vinegar-Induced Stress Response against Tomato Fusarium Wilt Disease" Plants 13, no. 2: 157. https://doi.org/10.3390/plants13020157





