Influence of Organic and Inorganic Fertilizers on Tea Growth and Quality and Soil Properties of Tea Orchards’ Top Rhizosphere Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Seedling Establishment and Treatment Design
2.3. Determination of Different Amino Acid and Catechin Contents by HPLC
2.4. Determination of Chlorophyll
2.5. Plant Growth Parameters
2.6. Soil Macro− and Micronutrients
2.7. Statistical Analysis
3. Results
3.1. Plant Growth Parameters
3.2. Catechin Contents in Tea Young Shoots, Stems, and Roots
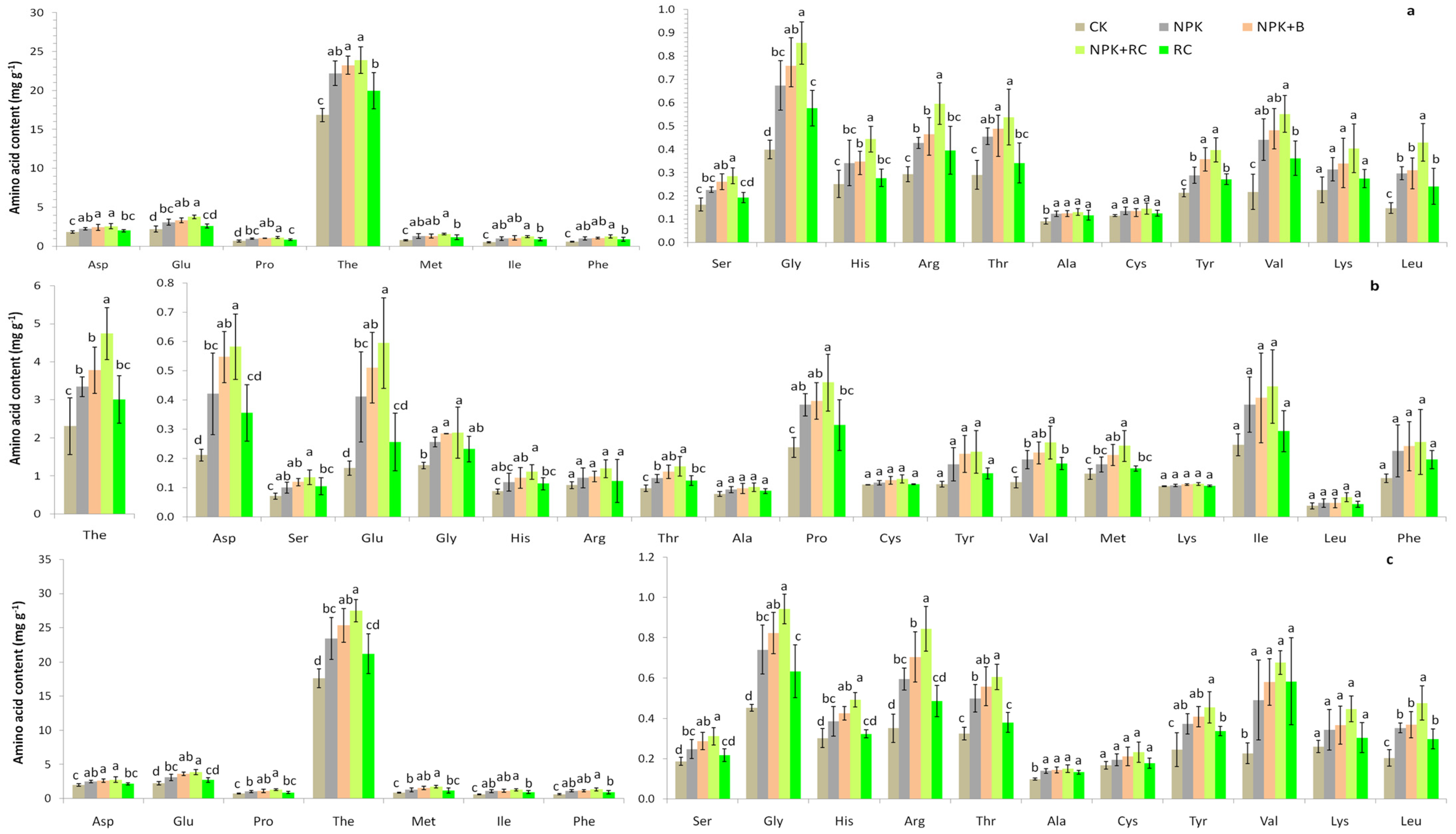
3.3. Targeted Metabolites’ Amino Acid Concentrations in Tea Young Shoots, Stems, and Roots
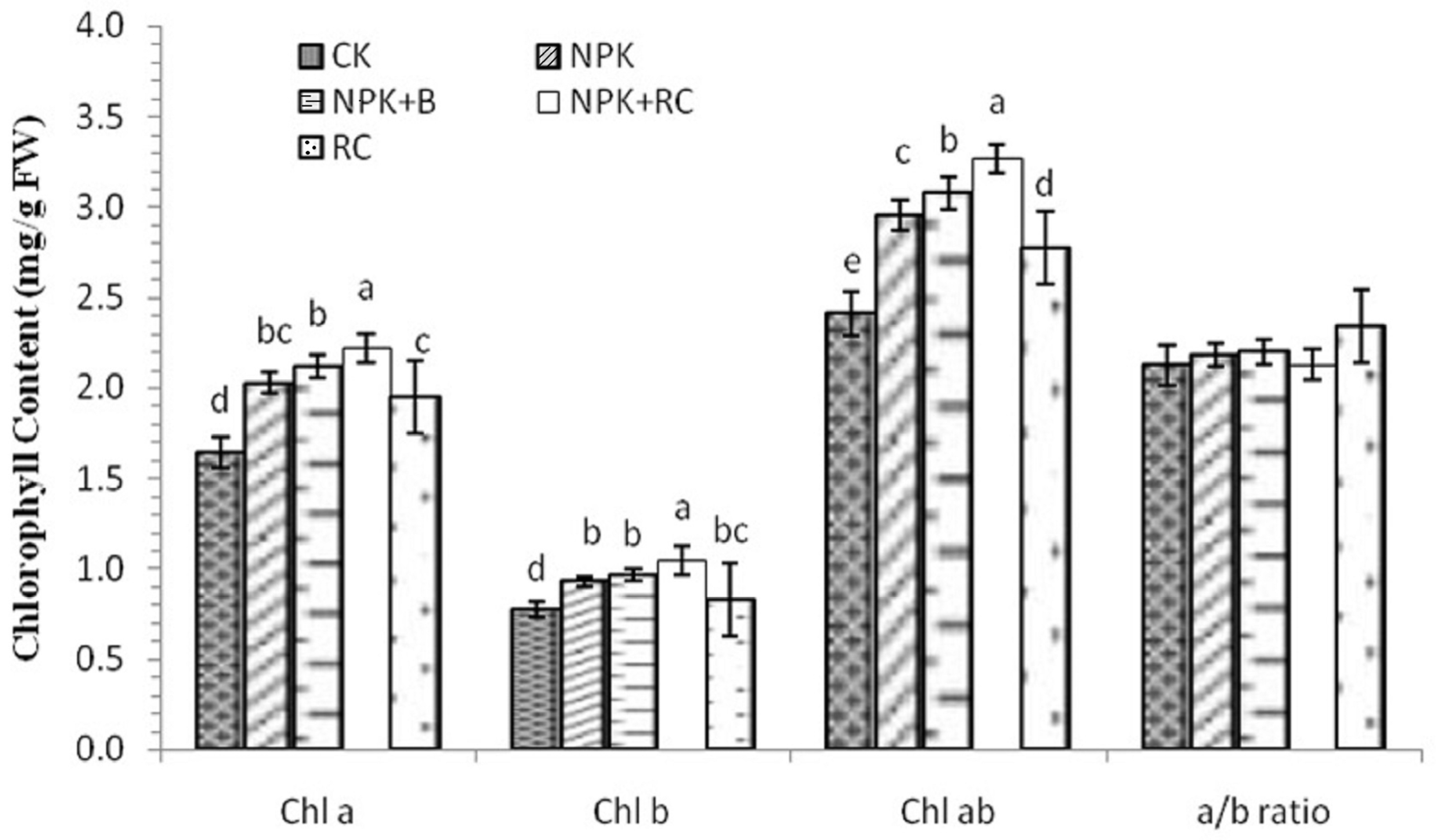
3.4. Chlorophyll Contents of Leaves
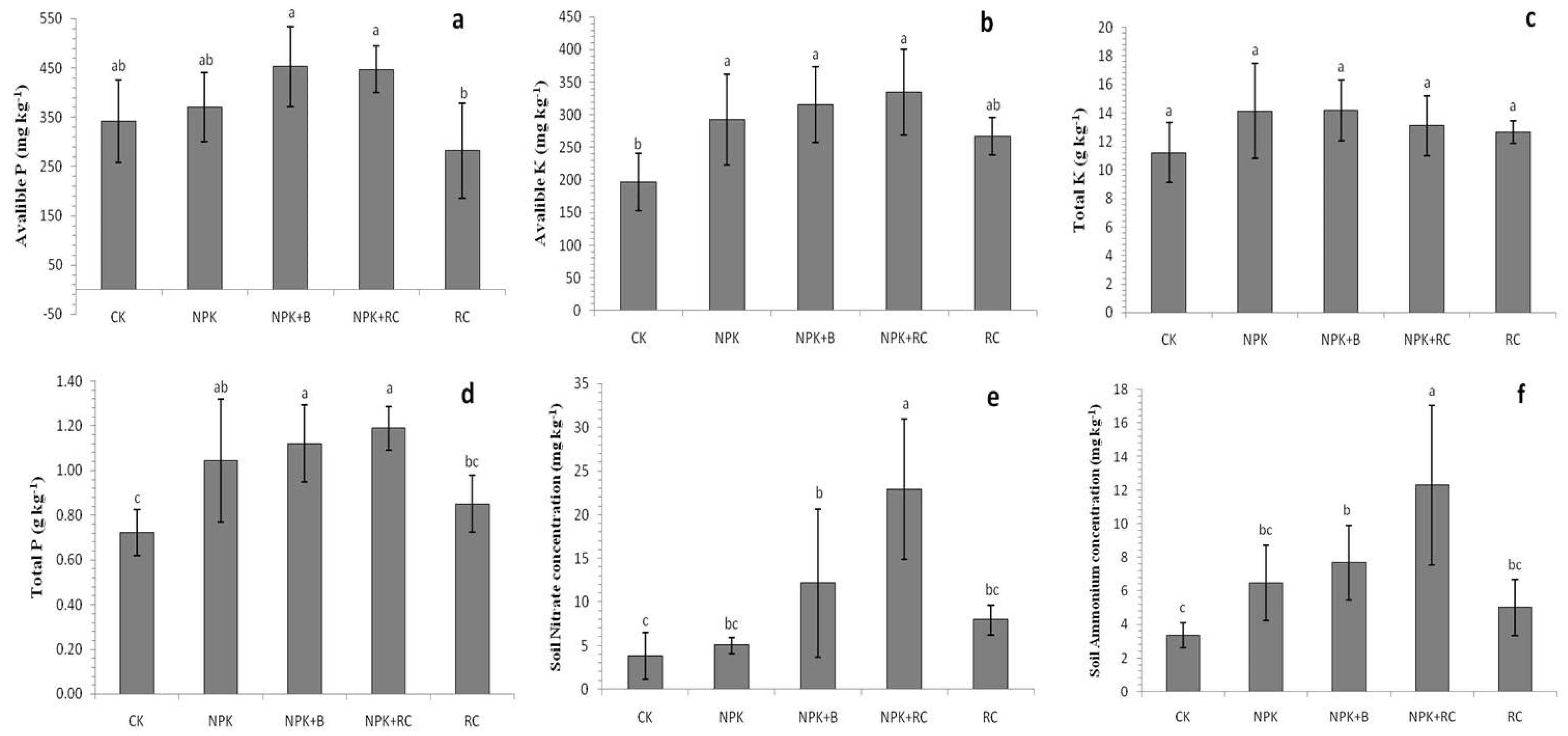
3.5. Soil pH and NH4+ and NO3− Concentrations
3.6. Soil Micro and Macronutrients
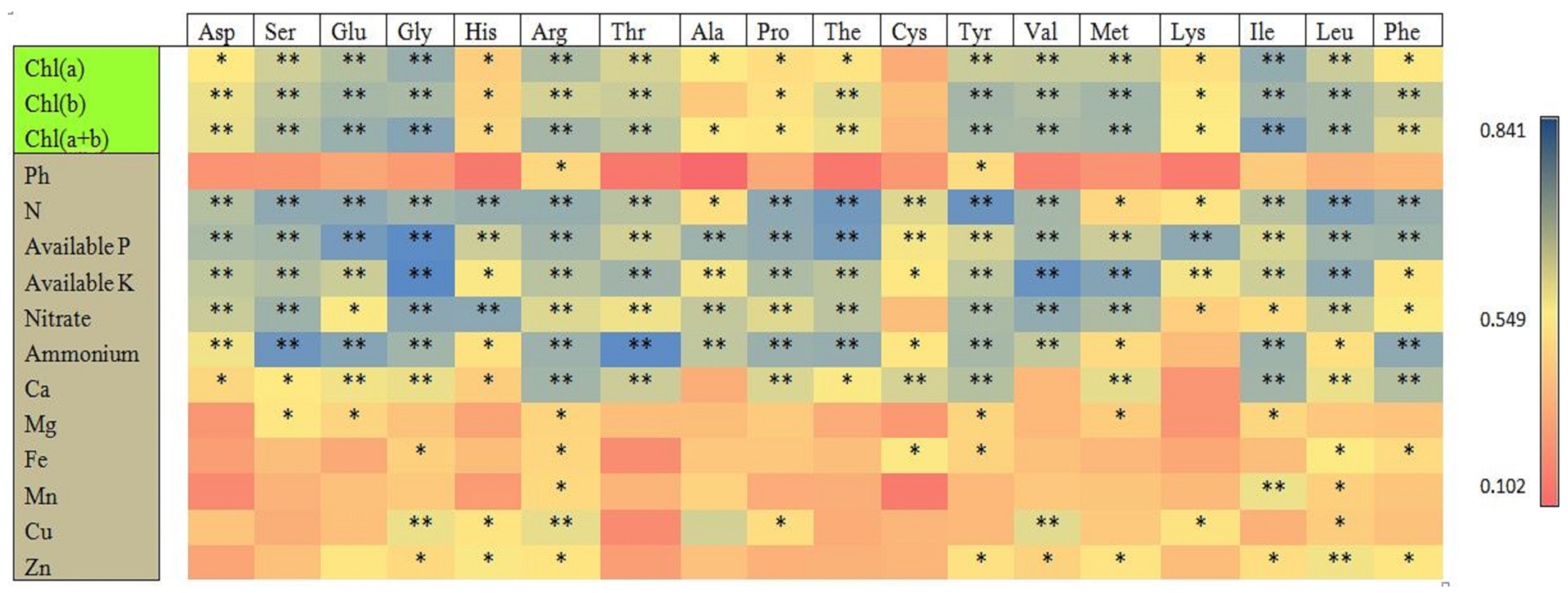
3.7. Pearson’s Correlation Analysis of Soil Chemical Properties and Plant Growth
3.8. Pearson’s Correlation Analysis of Chlorophyll Content and Soil Chemical Properties with Different Catechin Contents in Tea Young Shoots
3.9. Pearson’s Correlation Analysis of Chlorophyll Contents and Soil Chemical Properties with Different Targeted Amino Acids of Tea Young Shoots
4. Discussion
4.1. Effects of Different Fertilization Amendments on Plant Growth
4.2. Effect of Integrated Fertilization on Catechin Contents in Roots, Stems, and Young Shoots of Tea
4.3. Effect of Integrated Fertilization on Amino Acid Concentration in Tea Young Shoots, Stems, and Roots
4.4. Chlorophyll Content of Leaves as Affected by Organic and Inorganic Fertilizer Management
4.5. Effect of Integrated Fertilization on Soil pH and NH4+ and NO3− Concentrations
4.6. Effect of Integrated Fertilization on Soil Macro and Micronutrients
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, H.-G.; Lee, Y.-R.; Lee, M.-S.; Hwang, K.H.; Kim, E.-H.; Park, J.S.; Hong, Y.-S. Metabolic phenotyping of various tea (Camellia sinensis L.) cultivars and understanding of their intrinsic metabolism. Food Chem. 2017, 233, 321–330. [Google Scholar] [CrossRef]
- Fraser, K.; Harrison, S.; Lane, G.; Otter, D.; Quek, S.Y.; Rasmussen, S. Analysis of Low Molecular Weight Metabolites in Tea Using Mass Spectrometry-Based Analytical Methods. Crit. Rev. Food Sci. Nutr. 2014, 54, 924–937. [Google Scholar] [CrossRef]
- Kavitha, S.; Prapagar, K.; Gunarathne, G.P. Nutrient Availability of Tea Growing Soil Influenced by Different Rates of Dolomite. J. Tea Sci. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Lin, W.; Manhong, L.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 2019, 14, e0217018. [Google Scholar] [CrossRef] [PubMed]
- Too, J.; Thomas, K.; Kanyiri, W.; Wachira, F. Effect of Sunlight Exposure and Different Withering Durations on Theanine Levels in Tea (Camellia sinensis). Food Nutr. Sci. 2015, 6, 1014–1021. [Google Scholar]
- Horanni, R.; Engelhardt, U.H. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J. Food Compos. Anal. 2013, 31, 94–100. [Google Scholar] [CrossRef]
- Fang, R.; Redfern, S.P.; Kirkup, D.; Porter, E.A.; Kite, G.C.; Terry, L.A.; Berry, M.J.; Simmonds, M.S.J. Variation of theanine, phenolic, and methylxanthine compounds in 21 cultivars of Camellia sinensis harvested in different seasons. Food Chem. 2017, 220, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, J.; Guo, Z.; Wang, X. Determination of caffeine content and main catechins contents in green tea (Camellia sinensis L.) using taste sensor technique and multivariate calibration. J. Food Compos. Anal. 2010, 23, 353–358. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals—Promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2007, 120, 451–458. [Google Scholar] [CrossRef]
- Cavet, M.E.; Harrington, K.L.; Vollmer, T.R.; Ward, K.W.; Zhang, J.-Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar] [PubMed]
- Cho, Y.-S.; Oh, J.J.; Oh, K.-H. Synergistic anti-bacterial and proteomic effects of epigallocatechin gallate on clinical isolates of imipenem-resistant Klebsiella pneumoniae. Phytomedicine 2011, 18, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.; Mullen, W.; Burns, J.; Lean, M.; Brighenti, F.; Crozier, A. HPLC-MSn Analysis of Phenolic Compounds and Purine Alkaloids in Green and Black Tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Lin, L.; Harnly, J.M.; Yu, L.; Li, Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem. 2011, 126, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Mackey, B.; Kim, H.-J.; Lee, I.-S.; Lee, K.-R.; Lee, S.-U.; Kozukue, E.; Kozukue, N. Structure–Activity Relationships of Tea Compounds against Human Cancer Cells. J. Agric. Food Chem. 2007, 55, 243–253. [Google Scholar] [CrossRef]
- Zheng, X.-Q.; Nie, Y.; Gao, Y.; Huang, B.; Ye, J.-H.; Lu, J.-L.; Liang, Y.-R. Screening the cultivar and processing factors based on the flavonoid profiles of dry teas using principal component analysis. J. Food Compos. Anal. 2018, 67, 29–37. [Google Scholar] [CrossRef]
- Daglia, M.; Antiochia, R.; Sobolev, A.P.; Mannina, L. Untargeted and targeted methodologies in the study of tea (Camellia sinensis L.). Food Res. Int. 2014, 63, 275–289. [Google Scholar] [CrossRef]
- Ji, L.; Wu, Z.; You, Z.; Yi, X.; Ni, K.; Guo, S.; Ruan, J. Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: A 10-year field trial in a tea plantation. Agric. Ecosyst. Environ. 2018, 268, 124–132. [Google Scholar] [CrossRef]
- Gu, S.; Hu, Q.; Cheng, Y.; Bai, L.; Liu, Z.; Xiao, W.; Gong, Z.; Wu, Y.; Feng, K.; Deng, Y.; et al. Application of organic fertilizer improves microbial community diversity and alters microbial network structure in tea (Camellia sinensis) plantation soils. Soil Tillage Res. 2019, 195, 104356. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Y.; Gerendas, J.; Härdter, R.; Sattelmacher, B. Effect of Nitrogen Form and Root-zone pH on Growth and Nitrogen Uptake of Tea (Camellia sinensis) Plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Arafat, Y.; Tayyab, M.; Khan, M.U.; Chen, T.; Amjad, H.; Awais, S.; Lin, X.; Lin, W.; Lin, S. Long-Term Monoculture Negatively Regulates Fungal Community Composition and Abundance of Tea Orchards. Agronomy 2019, 9, 466. [Google Scholar] [CrossRef]
- Ji, L.; Ni, K.; Wu, Z.; Zhang, J.; Yi, X.; Yang, X.; Ling, N.; You, Z.; Guo, S.; Ruan, J. Effect of organic substitution rates on soil quality and fungal community composition in a tea plantation with long-term fertilization. Biol. Fertil. Soils 2020, 56, 633–646. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Tang, Y.-J.; Zhang, Q. Long-term effects of biochar amendment on rhizosphere and bulk soil microbial communities in a karst region, southwest China. Appl. Soil Ecol. 2019, 140, 126–134. [Google Scholar] [CrossRef]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, L.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Wallace, H.M.; Xu, C.-Y.; Zwieten, L.; Weng, Z.H.; Xu, Z.; Che, R.; Tahmasbian, I.; Hu, H.-W.; Bai, S.H. The effects of short term, long term and reapplication of biochar on soil bacteria. Sci. Total Environ. 2018, 636, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Naeem, I.; Masood, N.; Turan, V.; Iqbal, M. Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Ecotoxicol. Environ. Saf. 2021, 208, 111723. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, T.; Zhu, Q.; Yan, T.; Li, D.; Xue, J.; Wu, Y. Increases in bacterial community network complexity induced by biochar-based fertilizer amendments to karst calcareous soil. Geoderma 2019, 337, 691–700. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, T.; Van Zwieten, L.; Zhu, Q.; Yan, T.; Xue, J.; Wu, Y. Soil Microbial Community Structure Shifts Induced by Biochar and Biochar-Based Fertilizer Amendment to Karst Calcareous Soil. Soil Sci. Soc. Am. J. 2019, 83, 398–408. [Google Scholar] [CrossRef]
- Zhang, M.; Riaz, M.; Zhang, L.; Xia, H.; El-desouki, Z.; Jiang, C. Response of fungal communities in different soils to biochar and chemical fertilizers under simulated rainfall conditions. Sci. Total Environ. 2019, 691, 654–663. [Google Scholar] [CrossRef]
- Oladele, S.O.; Adeyemo, A.J.; Awodun, M.A. Influence of rice husk biochar and inorganic fertilizer on soil nutrients availability and rain-fed rice yield in two contrasting soils. Geoderma 2019, 336, 1–11. [Google Scholar] [CrossRef]
- Faloye, O.T.; Ajayi, A.E.; Alatise, M.O.; Ewulo, B.S.; Horn, R. Nutrient uptake, maximum yield production, and economic return of maize under deficit irrigation with biochar and inorganic fertiliser amendments. Biochar 2019, 1, 375–388. [Google Scholar] [CrossRef]
- Rafael, R.B.A.; FernÁNdez-Marcos, M.L.; Cocco, S.; Ruello, M.L.; Fornasier, F.; Corti, G. Benefits of Biochars and NPK Fertilizers for Soil Quality and Growth of Cowpea (Vigna unguiculata L. Walp.) in an Acid Arenosol. Pedosphere 2019, 29, 311–333. [Google Scholar] [CrossRef]
- Yan, S.; Zhao, J.; Ren, T.; Liu, G. Correlation between soil microbial communities and tobacco aroma in the presence of different fertilizers. Ind. Crops Prod. 2020, 151, 112454. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Gajbhiye, N.A.; Kalariya, K.A.; Manivel, P. Sustainable fertilization through co-application of biochar and chemical fertilizers improves yield, quality of Andrographis paniculata and soil health. Ind. Crops Prod. 2019, 140, 111607. [Google Scholar] [CrossRef]
- Lustosa Filho, J.F.; Carneiro, J.S.d.S.; Barbosa, C.F.; de Lima, K.P.; Leite, A.d.A.; Melo, L.C.A. Aging of biochar-based fertilizers in soil: Effects on phosphorus pools and availability to Urochloabrizantha grass. Sci. Total Environ. 2020, 709, 136028. [Google Scholar] [CrossRef]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Shi, X.Z.; Yu, D.S.; Xu, S.X.; Warner, E.D.; Wang, H.J.; Sun, W.X.; Zhao, Y.C.; Gong, Z.T. Cross-reference for relating Genetic Soil Classification of China with WRB at different scales. Geoderma 2010, 155, 344–350. [Google Scholar] [CrossRef]
- Bao, S.D. Analysis of Soil Agricultural Chemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2005; pp. 263–271. (In Chinese) [Google Scholar]
- Grant, A. Growing Tea from Seed—Tips for Germinating Tea Seeds. [Online] Gardening Know How. 2019. Available online: https://www.gardeningknowhow.com/edible/herbs/tea-plant/growing-tea-from-seed.htm (accessed on 18 November 2023).
- Ni, Z.; Kim, E.-D.; Chen, Z.J. Chlorophyll and starch assays. Protocol Exchange. Protoc. Exch. 2009, 10, 1038. [Google Scholar]
- Bass, A.M.; Bird, M.I.; Kay, G.; Muirhead, B. Soil properties, greenhouse gas emissions and crop yield under compost, biochar and co-composted biochar in two tropical agronomic systems. Sci. Total Environ. 2016, 550, 459–470. [Google Scholar] [CrossRef]
- Kamau, S.; Karanja, N.K.; Ayuke, F.O.; Lehmann, J. Short-term influence of biochar and fertilizer-biochar blends on soil nutrients, fauna and maize growth. Biol. Fertil. Soils 2019, 55, 661–673. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Helliwell, R. Effect of biochar on plant growth. Arboric. J. 2015, 37, 238–242. [Google Scholar] [CrossRef]
- Berihun, T.; Tolosa, S.; Tadele, M.; Kebede, F. Effect of Biochar Application on Growth of Garden Pea (Pisum sativum L.) in Acidic Soils of Bule Woreda Gedeo Zone Southern Ethiopia. Int. J. Agron. 2017, 2017, 6827323. [Google Scholar] [CrossRef]
- Wilson, K. Biochar for Forest Restoration in Western United States; Wilson Biochar Associates, South Umpqua Rural Community Partnership (SURCP): Tiller, OR, USA, 2015. [Google Scholar]
- Khaliq, A.; Abbasi, M.K.; Hussain, T. Effects of integrated use of organic and inorganic nutrient sources with effective microorganisms (EM) on seed cotton yield in Pakistan. Bioresour. Technol. 2006, 97, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Ramana, S.; Biswas, A.K.; Singh, A.B.; Yadava, R.B.R. Relative efficacy of different distillery effluents on growth, nitrogen fixation and yield of groundnut. Bioresour. Technol. 2002, 81, 117–121. [Google Scholar] [CrossRef]
- Hao, X.; Chang, C. Effect of 25 annual cattle manure applications on soluble and exchangeable cations in soil. Soil Sci. 2002, 167, 126–134. [Google Scholar] [CrossRef]
- Ashihara, H.; Deng, W.-W.; Mullen, W.; Crozier, A. Distribution and biosynthesis of flavan-3-ols in Camellia sinensis seedlings and expression of genes encoding biosynthetic enzymes. Phytochemistry 2010, 71, 559–566. [Google Scholar] [CrossRef]
- Bagchi, A.; Swain, D.; Mitra, A. Comparative assessment of organic and inorganic tea leaf extract feeding on anxiety behaviour status of colchicine-induced rat model of Alzheimer’s disease. Inflammopharmacology 2022, 30, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Stafford, H.A. Pathway to Proanthocyanindins (Condensed Tannins), Flavan−3−ols, and Unsubstituted Flavans; CRC Press: New York, NY, USA, 1990; pp. 63–99. [Google Scholar]
- Wellmann, F.; Griesser, M.; Schwab, W.; Martens, S.; Eisenreich, W.; Matern, U.; Lukacin, R. Anthocyanidin synthase from Gerbera hybridacatalyzes the conversion of (+)-catechin to cyanidin and a novel procyanidin. FEBS Lett. 2006, 580, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Li, F.; Yang, T.; Feng, L.; Zhang, S.; Li, F.; Li, W.; Xu, G.; Bao, S.; Wan, X.; et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020, 101, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, C.; Yang, T.; Ma, J.; Zhang, S.; Wei, C.; Wan, X.; Zhang, Z. Seasonal Theanine Accumulation and Related Gene Expression in the Roots and Leaf Buds of Tea Plants (Camellia sinensis L.). Front. Plant Sci. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Traversi, M.; Faraloni, C.; Valagussa, M.; Pozzi, A. Influence of biochar application on nutritional quality of tomato (Lycopersicon esculentum). Crop Pasture Sci. 2015, 66, 747–755. [Google Scholar] [CrossRef]
- Xu, L.; Yi, M.; Yi, H.; Guo, E.; Zhang, A. Manure and mineral fertilization change enzyme activity and bacterial community in millet rhizosphere soils. World J. Microbiol. Biotechnol. 2017, 34, 8. [Google Scholar] [CrossRef] [PubMed]
- Kropp, B.R.; Albee-Scott, S. Inocybetauensis, a new species from the Samoan Archipelago with biogeographic evidence for a Paleotropical origin. Fungal Biol. 2010, 114, 790–796. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Wan, Q.; Ruan, J. Transcriptome analysis using RNA-Seq revealed the effects of nitrogen form on major secondary metabolite biosynthesis in tea (Camellia sinensis) plants. Acta Physiol. Plant. 2018, 40, 127. [Google Scholar] [CrossRef]
- Ruan, J.Y.; Gerendas, J.; Härdter, R.; Sattelmacher, B. Effect of root zone pH and form and concentration of nitrogen on accumulation of quality-related components in green tea. J. Sci. Food Agric. 2007, 87, 1505–1516. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.; Xia, E.-H.; Gao, L.-Z. Metabolomics and Transcriptomics Analyses Reveal Nitrogen Influences on the Accumulation of Flavonoids and Amino Acids in Young Shoots of Tea Plant (Camellia sinensis L.) Associated with Tea Flavor. J. Agric. Food Chem. 2018, 66, 9828–9838. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, Y.; Wang, P.; Zhao, M.; Li, L.; Hu, X.; Chen, F. Simultaneous determination of free amino acids in Pu-erh tea and their changes during fermentation. Food Chem. 2016, 194, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Q.; Zhang, Z.; Wan, X. A novel colorimetric determination of free amino acids content in tea infusions with 2,4-dinitrofluorobenzene. J. Food Compos. Anal. 2009, 22, 137–141. [Google Scholar] [CrossRef]
- Thippeswamy, R.; Gouda, K.; Rao, D.; Martin, A.; Gowda, R. Determination of Theanine in Commercial Tea by Liquid Chromatography with Fluorescence and Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2006, 54, 7014–7019. [Google Scholar] [CrossRef]
- Yi, Y.; Xing, J.; Wan, M.; Yu, L.; Lu, Y.; Jian, Y. Effect of Cu on microstructure, crystallography and mechanical properties in Fe-B-C-Cu alloys. Mater. Sci. Eng. A 2017, 708, 274–284. [Google Scholar] [CrossRef]
- Ghosh, P.; Kumar, A.; Bandyopadhyay, K.; Manna, M.; Mandal, K.G.; Misra, A.K.; Hati, K. Comparative effectiveness of cattle manure, poultry manure, phosphocompost and fertilizer-NPK on three cropping systems in vertisols of semi-arid tropics. II. Dry matter yield, nodulation, chlorophyll content and enzyme activity. Bioresour. Technol. 2004, 95, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Amujoyegbe, B.J.; Opabode, J.T.; Olayinka, A. Effect of Organic and Inorganic Fertilizer on Yield and Chlorophyll Content of Maize (Zea mays L.) and Sorghum Sorghum bicolor (L.) Moench. Afr. J. Biotechnol. 2007, 6, 1869–1873. [Google Scholar]
- Nayek, S.; Choudhury, I.; Haque; Nishika, J.; Roy, S. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 2231–2606. [Google Scholar]
- Wang, L.F.; Park, S.C.; Chung, J.O.; Baik, J.H.; Park, S.K. The Compounds Contributing to the Greenness of Green Tea. J. Food Sci. 2004, 69, S301–S305. [Google Scholar] [CrossRef]
- Baba, R.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of Chinese green tea infusions by aroma extract dilution analysis. J. Agric. Food Chem. 2014, 62, 8308–8313. [Google Scholar] [CrossRef]
- Yang, W.; Li, C.; Wang, S.; Zhou, B.; Mao, Y.; Rensing, C.; Xing, S. Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl. Soil Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Liu, C.; Wei, C.; Liang, T. Organic fertilizer reduced carbon and nitrogen in runoff and buffered soil acidification in tea plantations: Evidence in nutrient contents and isotope fractionations. Sci. Total Environ. 2021, 762, 143059. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Shen, C.; Fan, L.; Li, X.; Zhang, L.; Zhang, L.; Han, W. Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Ruan, J.; Zhang, F.; Wong, M.H. Effect of nitrogen form and phosphorus source on the growth, nutrient uptake and rhizosphere soil property of Camellia sinensis L. Plant Soi. 2000, 223, 65–73. [Google Scholar] [CrossRef]
- Wang, L.; Butterly, C.R.; Chen, Q.; Mu, Z.; Wang, X.; Xi, Y.; Zhang, J.; Xiao, X. Surface Amendments Can Ameliorate Subsoil Acidity in Tea Garden Soils of High-Rainfall Environments. Pedosphere 2016, 26, 180–191. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification—A critical review. Sci. Total Environ. 2017, 581–582, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Riaz, M.; Zhang, L.; El-desouki, Z.; Jiang, C. Biochar Induces Changes to Basic Soil Properties and Bacterial Communities of Different Soils to Varying Degrees at 25 mm Rainfall: More Effective on Acidic Soils. Front. Microbiol. 2019, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Tong, C.; Hu, K.; Zhou, B.; Xing, S.; Mao, Y. Biochar-fertilizer interaction modifies N-sorption, enzyme activities and microbial functional abundance regulating nitrogen retention in rhizosphere soil. Sci. Total Environ. 2020, 739, 140065. [Google Scholar] [CrossRef]
- Mahmood, F.; Khan, I.; Ashraf, U.; Shahzad, T.; Hussain, S.; Shahid, M.; Abid, M.; Ullah, S. Effects of organic and inorganic manures on maize and their residual impact on soil physico-chemical properties. J. Soil Sci. Plant Nutr. 2017, 17, 22–32. [Google Scholar] [CrossRef]
- Birkhofer, K.; Bezemer, T.M.; Bloem, J.; Bonkowski, M.; Christensen, S.; Dubois, D.; Ekelund, F.; Fließbach, A.; Gunst, L.; Hedlund, K.; et al. Long-term organic farming fosters below and aboveground biota: Implications for soil quality, biological control and productivity. Soil Biol. Biochem. 2008, 40, 2297–2308. [Google Scholar] [CrossRef]
- Adeniyan, O.N.; Ojo, A.O.; Akinbode; Adediran, J.A. Comparative study of different organic manures and NPK fertilizer for improvement of soil chemical properties and dry matter yield of maize in two different soils. J. Soil Sci. Environ. Manag. 2011, 2, 9–13. [Google Scholar]
- Kimetu, J.; Lehmann, J. Stability and Stabilisation of Biochar and Green Manure in Soil with Different Organic Carbon Contents. Soil Res. 2010, 48, 577–585. [Google Scholar] [CrossRef]
- Shi, R.; Hong, Z.; Li, J.; Jiang, J.; Baquy, M.A.-A.; Xu, R.-K.; Qian, W. Mechanisms for Increasing the pH Buffering Capacity of an Acidic Ultisol by Crop Residue-Derived Biochars. J. Agric. Food Chem. 2017, 65, 8111–8119. [Google Scholar] [CrossRef] [PubMed]
- Busari, M.A.; Salako, F.K.; Tuniz, C. Stable isotope technique in the evaluation of tillage and fertilizer effects on soil carbon and nitrogen sequestration and water use efficiency. Eur. J. Agron. 2016, 73, 98–106. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; He, H.; Xie, H.; Yan, Y.; Zhu, P.; Ren, J.; Wang, L. Carbon and nitrogen pools in different aggregates of a Chinese Mollisol as influenced by long-term fertilization. J. Soils Sediments 2010, 10, 1018–1026. [Google Scholar] [CrossRef]
- Shahzad, T.; Chenu, C.; Genet, P.; Barot, S.; Perveen, N.; Mougin, C.; Fontaine, S. Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol. Biochem. 2015, 80, 146–155. [Google Scholar] [CrossRef]
- Adediran, J.A.; Taiwo, L.B.; Akande, M.O.; Sobulo, R.A.; Idowu, O.J. Application of Organic and Inorganic Fertilizer for Sustainable Maize and Cowpea Yields in Nigeria. J. Plant Nutr. 2005, 27, 1163–1181. [Google Scholar] [CrossRef]




| Soil Properties | Means |
|---|---|
| Soil texture | Clay |
| Clay (%) | 63.0 ± 2.2 |
| Sand (%) | 9.0 ± 0.2 |
| Silt (%) | 28.0 ± 1.0 |
| EC (μS cm−1) | 108.0 ± 4.6 |
| pH | 4.4 ± 0.01 |
| Available K (mg kg−1) | 114.0 ± 2.7 |
| Available P (mg kg−1) | 30.0 ± 0.06 |
| NO3− (mg kg−1) | 16.0 ± 0.4 |
| NH4+(mg kg−1) | 33.0 ± 2.2 |
| P (g kg−1) | 0.8 ± 0.01 |
| N (g kg−1) | 2.7 ± 0.01 |
| K (g kg−1) | 12.0 ± 0.1 |
| C (g kg−1) | 18.0 ± 0.02 |
| Treatments | Plant Height (cm) | No. of Leaves per Plant−1 | Leaf Fresh Weight (g Plant−1) | Stem Fresh Weight (g Plant−1) | Stem Mid Girth (mm) |
|---|---|---|---|---|---|
| CK | 22.63 ± 1.80 c | 46.0 ± 8.2 c | 6.22 ± 1.23 d | 2.73 ± 0.95 c | 2.18 ± 0.09 c |
| NPK | 32.75 ± 3.30 b | 71.0 ± 22.8 b | 12.04 ± 0.80 bc | 4.65 ± 1.50 bc | 2.76 ± 0.17 b |
| NPK+B | 34.00 ± 4.08 b | 80.0 ± 19.9 b | 13.62 ± 1.52 b | 5.87 ± 1.48 ab | 2.83 ± 0.19 ab |
| NPK+RC | 42.75 ± 4.57 a | 111.0 ± 10.5 a | 18.47 ± 3.98 a | 7.16 ± 1.32 a | 3.03 ± 0.10 a |
| RC | 30.50 ± 6.66 b | 58.0 ± 9.1 bc | 10.38 ± 1.28 c | 4.54 ± 1.23 bc | 2.58 ± 0.14 b |
| Treatments | pH | N (%) | C (%) | CN Ratio | Ca (mg kg−1) | Mg (mg kg−1) | Fe (mg kg−1) | Mn (mg kg−1) | Cu (mg kg−1) | Zn (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| CK | 4.41 ± 0.09 bc | 0.19 ± 0.005 c | 1.56 ± 0.03 c | 9.57 ± 0.33 a | 154.66 ± 54 c | 24.73 ± 3.4 b | 267.63 ± 31 b | 10.48 ± 2.5 c | 1.01 ± 0.06 b | 1.74 ± 0.13 b |
| NPK | 4.35 ± 0.03 c | 0.21 ± 0.03 bc | 1.61 ± 0.06 c | 8.78 ± 0.93 ab | 202.57 ± 41 bc | 34.00 ± 5.8 ab | 358.39 ± 32 a | 16.48 ± 2.3 bc | 1.18 ± 0.11 b | 1.68 ± 0.22 b |
| NPK+B | 4.55 ± 0.17 ab | 0.23 ± 0.02 ab | 2.01 ± 0.07 a | 7.61 ± 0.96 b | 275.61 ± 34 ab | 30.70 ± 3.4 ab | 336.12 ± 24 ab | 17.99 ± 8.6 b | 1.27 ± 0.21 b | 1.70 ± 0.08 b |
| NPK+RC | 4.64 ± 0.13 a | 0.26 ± 0.03 a | 2.05 ± 0.04 a | 7.74 ± 0.19 b | 290.44 ± 43 ab | 36.68 ± 11.5 a | 393.99 ± 40 a | 18.39 ± 4.3 b | 1.46 ± 0.43 b | 1.98 ± 0.24 b |
| RC | 4.70 ± 0.09 a | 0.20 ± 0.01 c | 1.77 ± 0.06 b | 7.69 ± 1.08 b | 324.30 ± 116 a | 42.45 ± 10.9 a | 399.85 ± 100 a | 27.39 ± 3.8 a | 2.14 ± 0.59 a | 2.43 ± 0.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoor; Ma, L.; Ni, K.; Ruan, J. Influence of Organic and Inorganic Fertilizers on Tea Growth and Quality and Soil Properties of Tea Orchards’ Top Rhizosphere Soil. Plants 2024, 13, 207. https://doi.org/10.3390/plants13020207
Manzoor, Ma L, Ni K, Ruan J. Influence of Organic and Inorganic Fertilizers on Tea Growth and Quality and Soil Properties of Tea Orchards’ Top Rhizosphere Soil. Plants. 2024; 13(2):207. https://doi.org/10.3390/plants13020207
Chicago/Turabian StyleManzoor, Lifeng Ma, Kang Ni, and Jianyun Ruan. 2024. "Influence of Organic and Inorganic Fertilizers on Tea Growth and Quality and Soil Properties of Tea Orchards’ Top Rhizosphere Soil" Plants 13, no. 2: 207. https://doi.org/10.3390/plants13020207





