“Mortality, or not mortality, that is the question …”: How to Treat Removals in Tree Survival Analysis of Central European Managed Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
2.2. Study Design and Statistical Analysis
3. Results
3.1. Survival Analysis
3.2. PCA and Kruskal–Wallis Tests Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmermann, V.; Potočić, N.; Ognjenović, M.; Kirchner, T. Tree crown condition in 2020. In Forest Condition in Europe: The 2021 Assessment; ICP Forests Technical Report under the UNECE Convention on Long-range Transboundary Air Pollution (Air Convention); Michael, A., Kirchner, T., Prescher, A.-K., Schwärzel, K., Eds.; Thünen Institute: Eberswalde, Germany, 2021. [Google Scholar] [CrossRef]
- Senf, C.; Pflugmacher, D.; Zhiqiang, Y.; Sebald, J.; Knorn, J.; Neumann, M.; Hostert, P.; Seidl, R. Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat. Commun. 2018, 9, 4978. [Google Scholar] [CrossRef]
- Neumann, M.; Mues, V.; Moreno, A.; Hasenauer, H.; Seidl, R. Climate variability drives recent tree mortality in Europe. Glob. Chang. Biol. 2017, 23, 4788–4797. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, W.A. Forest Inventory and Analysis: Tree Mortality; FIA Fact Sheet Series; Forest Inventory and Analysis National Program: Washington, DC, USA, 2003; 2p. [Google Scholar]
- Cobb, R.C.; Haas, S.E.; Kruskamp, N.; Dillon, W.W.; Swiecki, T.J.; Rizzo, D.M.; Frankel, S.J.; Meentemeyer, R.K. The Magnitude of Regional-Scale Tree Mortality Caused by the Invasive Pathogen Phytophtora ramorum. Earth’s Future 2020, 8, e2020EF001500. [Google Scholar] [CrossRef]
- Hicke, J.A.; Meddens, A.J.H.; Koldeni, C.A. Recent Tree Mortality in the Western United States from Bark Beetles and Forest Fires. For. Sci. 2016, 62, 141–153. [Google Scholar] [CrossRef]
- Sims, A.; Mändma, R.; Laarmann, D.; Korjus, H. Assessment of tree mortality on the Estonian Network of Forest Research Plots. For. Stud. 2014, 60, 57–68. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fule, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread Increase of Tree Mortality Rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Rouvinen, S.; Kuuluvainen, T.; Siitonen, J. Tree mortality in a Pinus sylvestris dominated boreal forest landscape in Vienansalo wilderness, eastern Fennoscandia. Silva Fenn. 2002, 36, 127–145. [Google Scholar] [CrossRef]
- Hulsmann, L. Tree Mortality in Central Europe: Empirically-Based Modeling Using Long-Term Datasets. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2016; 217p. [Google Scholar] [CrossRef]
- Monserud, R.A.; Sterba, H. Modelling individual tree mortality for Austrian forest species. For. Ecol. Manag. 1999, 113, 109–123. [Google Scholar] [CrossRef]
- Bugmann, H.; Seidl, R.; Hartig, F.; Bohn, F.; Brůna, J.; Cailleret, M.; François, L.; Heinke, J.; Henrot, A.-J.; Hickler, T.; et al. Tree mortality submodels drive simulated long-term forest dynamics: Assessing 15 models from the stand to global scale. Ecosphere 2019, 10, e02616. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C. Woody plant mortality algorithms: Description, problems, and progress. Ecol. Model. 2000, 126, 225–248. [Google Scholar] [CrossRef]
- Morin, R.S.; Randolph, K.C.; Steinman, J. Mortality rates associated with crown health for eastern forest tree species. Environ. Monit. Assess. 2015, 187, 87. [Google Scholar] [CrossRef] [PubMed]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sanchez, G.; Penuelas, J. Widespread crown condition decline, food web distruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef]
- Jurkonis, N.; Juknys, R.; Venclovienë, J. Relationship between Scots Pine Individual Tree Mortality and Tree Vigor Indicators in the Polluted Environment. Balt. For. 2006, 12, 184–191. [Google Scholar]
- Dobbertin, M.; Brang, P. Crown defoliation improves tree mortality models. For. Ecol. Manag. 2001, 141, 271–284. [Google Scholar] [CrossRef]
- Langström, B.; Erkki, A.; Hellqvist, C.; Varama, M.; Niemelä, P. Tree Mortality, Needle Biomass Recovery and Growth Losses in Scots Pine Following Defoliation by Diprion pini (L.) and Subsequent Attack by Tomicus piniperda (L.). Scand. J. For. Res. 2001, 16, 342–353. [Google Scholar] [CrossRef]
- Gillner, S.; Rüger, N.; Roloff, A.; Berger, U. Low relative growth rates predict future mortality of common beech (Fagus sylvatica L.). For. Ecol. Manag. 2013, 302, 372–378. [Google Scholar] [CrossRef]
- Das, A.J.; Battles, J.J.; Stephenson, N.L.; Van Mantgem, P.J. The relationship between tree growth patterns and likehood of mortality: A study of two tree species in the Sierra Nevada. Can. J. For. Res. 2007, 37, 580–597. [Google Scholar] [CrossRef]
- Bigler, C.; Bugmann, H. Growth-dependent tree mortality models based on tree rings. Can. J. For. Res. 2003, 33, 210–221. [Google Scholar] [CrossRef]
- Wyckoff, P.H.; Clark, J.S. The relationship between growth and mortality for seven co-occurring tree species in the southern Appalachian Mountains. J. Ecol. 2002, 90, 604–615. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368. [Google Scholar] [CrossRef]
- Klein, T.; Hartmann, H. Climate change drives tree mortality. Science 2018, 362, 758. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshaers, D.D.; Hogg, E.H.; et al. Global overview of drought and heat induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- George, J.-P.; Sanders, T.G.M.; Neumann, M.; Cammalleri, C.; Vogt, J.V.; Lang, M. Long-term forest monitoring unravels constant mortality rise in European forests. Plant Biol. 2022, 24, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, L.; Santini, A.; Luchi, N. Globalization, invasive forest pathogen species, and forest tree health. For. Microbiol. 2022, 2, 61–76. [Google Scholar] [CrossRef]
- Primack, R.B.; Ashton, P.S.; Chai, P.; Lee, H.S. Growth rates and population structure of Moraceae trees in Sarawak, East Malaysia. Ecology 1985, 66, 577–588. [Google Scholar] [CrossRef]
- Ulanova, N.G. The effects of windthrow on forests at different spatial scales: A review. For. Ecol. Manag. 2000, 135, 155–165. [Google Scholar] [CrossRef]
- Peterken, G.F. Natural Woodland: Ecology and Conservation in Northen Temperate Regions; Cambridge University Press: Cambridge, MA, USA, 1996; 540p. [Google Scholar]
- Hulsmann, L.; Bugmann, H.K.M.; Commarmot, B.; Meyer, P.; Zimmermann, S.; Brang, P. Does one model fit all? Patterns of beech mortality in natural forests of three European regions. Ecol. Appl. 2016, 26, 2465–2479. [Google Scholar] [CrossRef]
- Dobbertin, M. Sterberate, Nutzungsrate und Einwuchsrate. In Sanasilva-Bericht 1997. Zustand und Gefährdung des Schweizer Waldes—Eine Zwischenbilanz nach 15 Jahren Waldschadenforschung; Brang, P., Ed.; Berichte Eidg. Forschungsanstalt für Wald, Schnee und Landschaft: Birmensdorf, Switzerland, 1998; Volume 345, pp. 24–27. [Google Scholar]
- Eichhorn, J.; Roskams, P. Assessment of tree condition. In Forest Monitoring: Methods for Terrestrial Investigations in Europe with Overview of North America and Asia Developments in Environmental Science; Ferretti, M., Fischer, R., Eds.; Elsevier: London, UK, 2013; Volume 12, pp. 139–168. [Google Scholar]
- Tkacz, B.; Ritters, K.; Percy, K.E. Forest Monitoring Methods in the United States and Canada: An Overview. In Forest Monitoring: Methods for Terrestrial Investigations in Europe with Overview of North America and Asia Developments in Environmental Science; Ferretti, M., Fischer, R., Eds.; Elsevier: London, UK, 2013; Volume 12, pp. 49–76. [Google Scholar]
- Reineke, L.H. Perfecting a Stand-Density Index for Even-Aged Forests. J. Agric. Res. 1933, 46, 627–638. [Google Scholar]
- Yoda, K.; Kira, T.; Ogawa, H.; Hozumi, K. Self-thinning in overcrowded pure stands under cultivated and natural conditions. J. Biol. 1963, 14, 107–129. [Google Scholar]
- Nilson, A. Modeling dependence between the number of trees and mean tree diameter of stands, stand density and stand sparsity. In Proceedings of the 2nd International Conference on Forest Measurements and Quantitative Methods and Management & the 2004 Southern Mensurationists Meeting, Hot Springs, AR, USA, 15–18 June 2004; Cieszewski, C.J., Strub, M., Eds.; Warnell School of Forestry and Natural Resources, University of Georgia: Tbilisi, Georgia, 2018; pp. 74–94. [Google Scholar]
- Assmann, E. Waldertragskunde. Organische Produktion, Struktur, Zuwachs und Ertrag von Waldbeständen; BLV Verlagsgesellschaft München: Bonn, Germany, 1961; 490p. [Google Scholar]
- Potočić, N.; Timmermann, V.; Ognjenović, M. Tree Crown Condition in 2017. In Forest Condition in Europe: 2018 Technical Report of ICP Forests; Michael, A., Seidling, W., Prescher, A.-K., Eds.; Report under UNECE Convention on Long-Range Transboundary Air Pollution (CLRTAP), BFW-Dokumentation 24/2018; BFW Austrian Research Centre for Forests: Vienna, Austria, 2018; 88p. [Google Scholar]
- Woodall, C.W.; Grambsch, P.L.; Thomas, W. Applying survival analysis to a large-scale forest inventory for assessment of tree mortality in Minnesota. Ecol. Model. 2005, 189, 199–208. [Google Scholar] [CrossRef]
- Tymińska-Czabańska, L.; Hawryło, P.; Janiec, P.; Socha, J. Tree height, growth rate and stand density determined by ALS drive probability of Scots pine mortality. Ecol. Indic. 2022, 145, 109643. [Google Scholar] [CrossRef]
- Grodzki, W.; McManus, M.; Knizek, M.; Meshkova, V.; Mihalciuc, V.; Novotny, J.; Turcani, M.; Slobodyan, Y. Occurrence of spruce bark beetles in forest stands at different levels of air pollution stress. Env. Poll. 2004, 130, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Nagel, T.A.; Mikac, S.; Dolinar, M.; Klopcic, M.; Keren, S.; Svoboda, J.; Diaci, J.; Boncina, A.; Paulic, V. The Natural Disturbance Regime in Forests of the Dinaric Mountains: A Synthesis of Evidence. For. Ecol. Manag. 2017, 388, 29–42. [Google Scholar] [CrossRef]
- Canham, C.D.; Papaik, M.J.; Latty, E.F. Interspecific variation in susceptibility to windthrow as a function of tree size and storm severity for northern temperate tree species. Can. J. For. Res. 2001, 31, 1–10. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Gazda, A.; Dobrowolska, D.; Chećko, E.; Zaremba, J.; Tomski, A. Tree mortality after wind disturbance differs among tree species more than among habitat types in a lowland forest in northeastern Poland. For. Ecol. Manag. 2017, 398, 174–184. [Google Scholar] [CrossRef]
- Schütz, J.-P.; Götz, M.; Schmid, W.; Mandallaz, D. Vulnerability of spruce (Picea abies) and beech (Fagus sylvatica) forest stands to storms and consequences for literature. Eur. J. Forest Res. 2006, 125, 291–302. [Google Scholar] [CrossRef]
- Dobbertin, M. Influence of stand structure and site factors on wind damage comparing to the storm Vivian and Lothar. For. Snow Landscaspe Res. 2002, 77, 187–205. [Google Scholar]
- Mitchell, S.J. Wind as a natural disturbance agent in forests: A synthesis. Forestry 2013, 86, 147–157. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2015: How Are the World’s Forest Changing; FAO: Rome, Italy, 2016; 54p. [Google Scholar]
- Gessler, A.; Ferretti, M.; Schaub, M. Editorial: Forest monitoring to assess forest functioning under air pollution and climate change. Front. For. Glob. Chang. 2022, 5, 952232. [Google Scholar] [CrossRef]
- Anttonen, S.; Piispanen, R.; Ovaska, J.; Mutikainen, P.; Saranpää, P.; Vapaavuori, E. Effects of defoliation on growth, biomass allocation, and wood properties of Betula pendula clones grown at different nutrient levels. Can. J. For. Res. 2002, 32, 498–508. [Google Scholar] [CrossRef]
- Drobyshev, I.; Linderson, H.; Sonesson, K. Relationship Between Crown Condition and Tree Diameter Growth in Southern Swedish Oaks. Environ. Monit. Assess. 2007, 128, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Juknys, R.; Vencloviene, J.; Stravinskiene, V.; Augustatis, A.; Bartkevicius, E. Scots pine (Pinus sylvestris L.) growth and condition in a polluted environment: From decline to recovery. Env. Poll. 2003, 125, 205–212. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J. Growth patterns and sensitivity to climate predict silver fir decline in the Spain Pyrenees. Eur. J. Forest Res. 2012, 131, 1001–1012. [Google Scholar] [CrossRef]
- Solberg, S. Crown Condition and Growth Relationships within Stands of Picea abies. Scand. J. For. Res. 1999, 14, 320–327. [Google Scholar] [CrossRef]
- Eichhorn, J.; Roskams, P.; Potočić, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schröck, H.-W.; et al. Part IV: Visual Assessment of Crown Condition and Damaging Agents. Version 2020-3. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-Ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; 49p, Available online: https://www.icp-forests.org/pdf/manual/2020/ICP_Manual_part04_2020_Crown_version_2020-3_update_06-2023.pdf (accessed on 19 November 2023).
- Chiang, C.L. Life Table and Mortality Analysis; World Health Organization: Geneva, Switzerland, 1979; Available online: https://iris.who.int/bitstream/handle/10665/62916/15736_eng.pdf?sequence=1&isAllowed=y (accessed on 19 November 2023).
- Gehan, E.A. A Generalized Wilcoxon Test for Comparing Arbitrarily Singly-censored Samples. Biometrika 1965, 52, 203–223. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13.3.0; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. Available online: https://docs.tibco.com/products/tibco-statistica/archive (accessed on 19 November 2023).
- Michael, A.; Kirchner, T.; Prescher, A.-K.; Schwärzel, K. (Eds.) Forest Condition in Europe: The 2022 Assessment; ICP Forests Technical Report under the UNECE Convention on Long-range Transboundary Air Pollution (Air Convention); Thünen Institute: Eberswalde, Germany, 2022. [Google Scholar] [CrossRef]
- Wawrzoniak, J.; Lech, P. System monitoringu lasów w Polsce—Nowoczesne narzędzie wspomagające decyzje w leśnictwie i ochronie środowiska. Postępy Tech. W Leśnictwie 2011, 113, 22–27. [Google Scholar]
- Yan, W.; Rajcan, I. Biplot analysis of test sites and trait relations of soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef]
- Kruskal, W.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 10 December 2019).
- Das, A.; Battles, J.; Stephenson, N.L.; Van Mantgem, P.J. The contribution of competition to tree mortality in old-growth coniferous forests. For. Ecol. Manag. 2011, 261, 1203–1213. [Google Scholar] [CrossRef]
- Peet, R.K.; Christensen, N.L. Competition and Tree Death. BioScience 1987, 37, 586–595. [Google Scholar] [CrossRef]
- Lutz, J.A.; Halpern, C.B. Tree mortality during early forest development: A long-term study of rates, causes, and consequences. Ecol. Monogr. 2006, 76, 257–275. [Google Scholar] [CrossRef]
- Greenwood, D.L.; Weisberg, P.J. Density-dependent tree mortality in pinyon-juniper woodlands. For. Ecol. Manag. 2008, 255, 2129–2137. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Małachowska, J. Zmiany stanu zdrowotnego monitorowanych gatunków drzew w latach 2012–2021. In Stan Zdrowotny Lasów w Polsce w Roku 2021 Roku na Podstawie Badań Monitoringowych; Lech, P., Ed.; Instytut Badawczy Leśnictwa Sękocin Stary: Sękocin Stary, Poland, 2022; pp. 76–107. [Google Scholar]
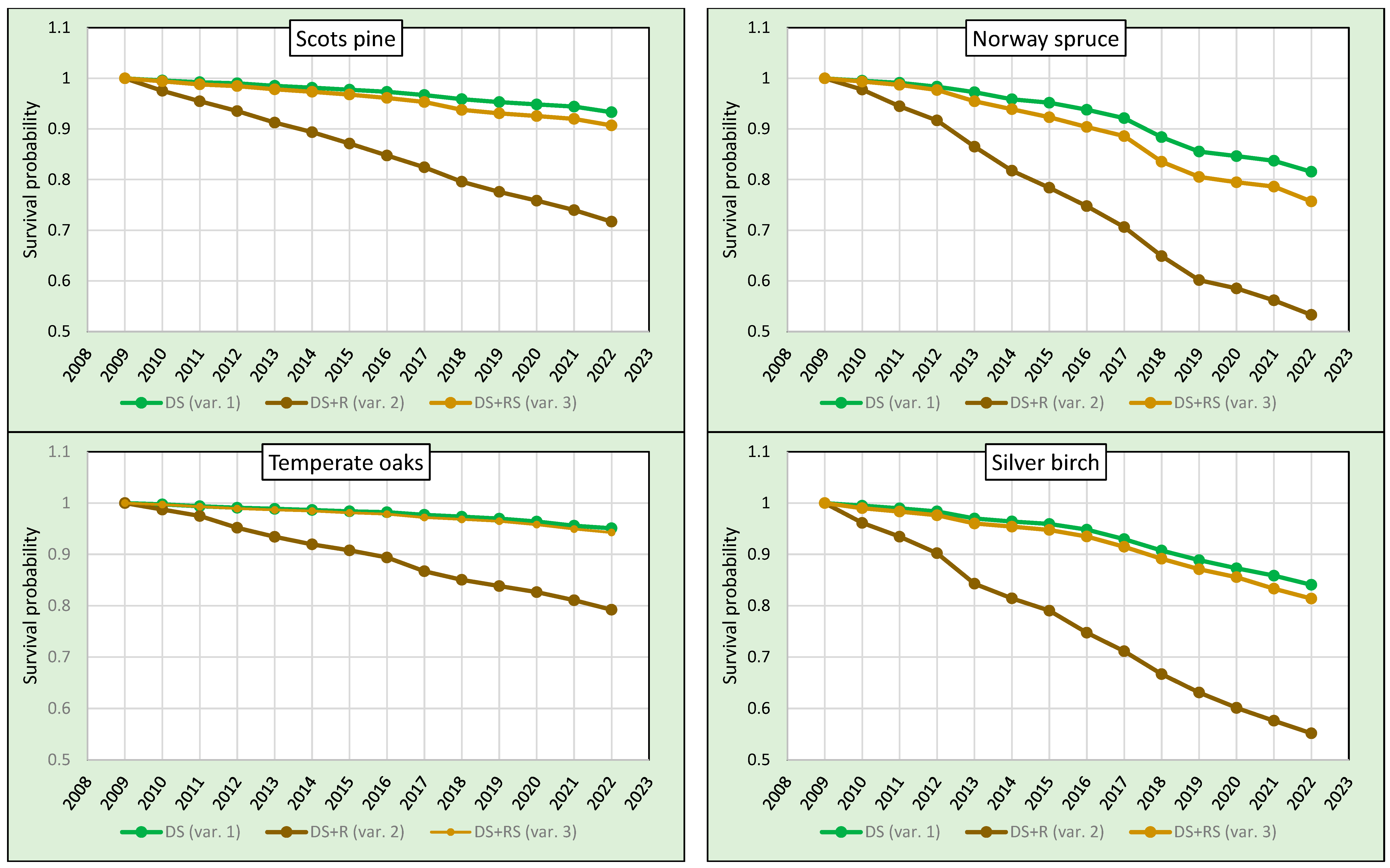
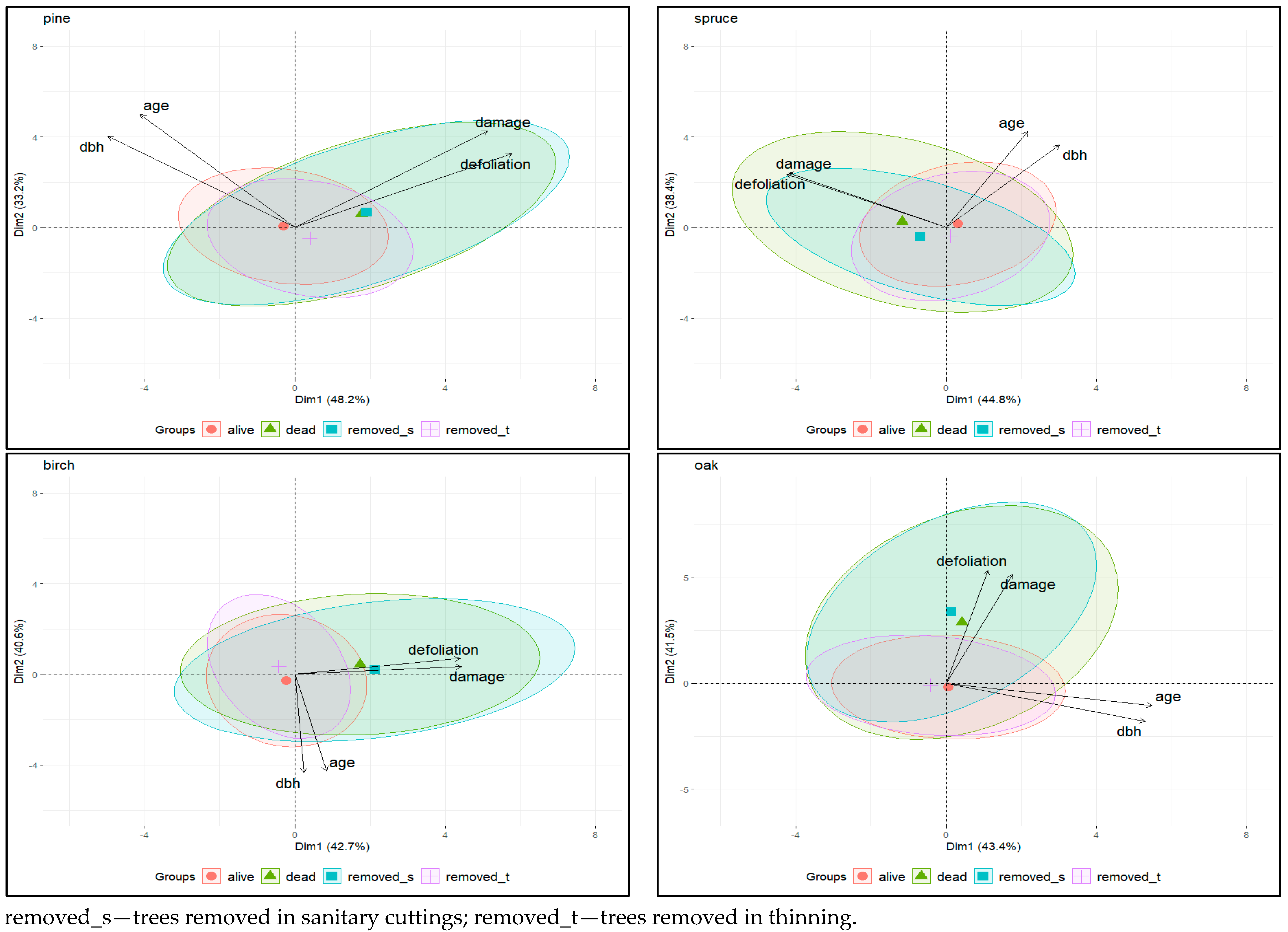

| Category of Tree | Year/Period | Units | Scots Pine | Norway Spruce | Temperate Oaks | Silver Birch |
|---|---|---|---|---|---|---|
| Alive | 2009 | Number | 16,271 | 1284 | 2491 | 3521 |
| % of 2009 | 100 | 100 | 100 | 100 | ||
| Alive | 2022 | Number | 11,767 | 707 | 1982 | 1997 |
| % of 2009 | 72.3 | 55.1 | 79.6 | 56.7 | ||
| Dead | 2009–2022 | Number | 918 | 177 | 108 | 419 |
| % of 2009 | 5.6 | 13.8 | 4.3 | 11.9 | ||
| Removed in thinning | 2009–2022 | Number | 3202 | 332 | 382 | 1023 |
| % of 2009 | 19.7 | 25.9 | 15.3 | 29.1 | ||
| Removed in sanitary cuts | 2009–2022 | Number | 384 | 68 | 19 | 82 |
| % of 2009 | 2.4 | 5.3 | 0.8 | 2.3 | ||
| Removed total | 2009–2022 | Number | 3586 | 400 | 401 | 1105 |
| % of 2009 | 22.0 | 31.2 | 16.1 | 31.4 |
| Species (Age of Trees) | Results of Wilcoxon–Gehan Test | |||||
|---|---|---|---|---|---|---|
| Var. 1 versus Var. 2 | Var. 1 versus Var. 3 | Var. 2 versus Var. 3 | ||||
| Stat. Z | p | Stat. Z | p | Stat. Z | p | |
| Pine (21–80) | 49.226 | 0.00000 | 8.216 | 0.00000 | −42.568 | 0.00000 |
| Spruce (21–80) | 12.089 | 0.00000 | 3.436 | 0.00059 | −12.063 | 0.00000 |
| Oaks (21–120) | 16.315 | 0.00000 | 1.244 | 0.21363 | −15.328 | 0.00000 |
| Birch (21–80) | 25.972 | 0.00000 | 2.814 | 0.00490 | −23.611 | 0.00000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lech, P.; Kamińska, A. “Mortality, or not mortality, that is the question …”: How to Treat Removals in Tree Survival Analysis of Central European Managed Forests. Plants 2024, 13, 248. https://doi.org/10.3390/plants13020248
Lech P, Kamińska A. “Mortality, or not mortality, that is the question …”: How to Treat Removals in Tree Survival Analysis of Central European Managed Forests. Plants. 2024; 13(2):248. https://doi.org/10.3390/plants13020248
Chicago/Turabian StyleLech, Paweł, and Agnieszka Kamińska. 2024. "“Mortality, or not mortality, that is the question …”: How to Treat Removals in Tree Survival Analysis of Central European Managed Forests" Plants 13, no. 2: 248. https://doi.org/10.3390/plants13020248





