Effects of Priestia aryabhattai on Phosphorus Fraction and Implications for Ecoremediating Cd-Contaminated Farmland with Plant–Microbe Technology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Strain Identification
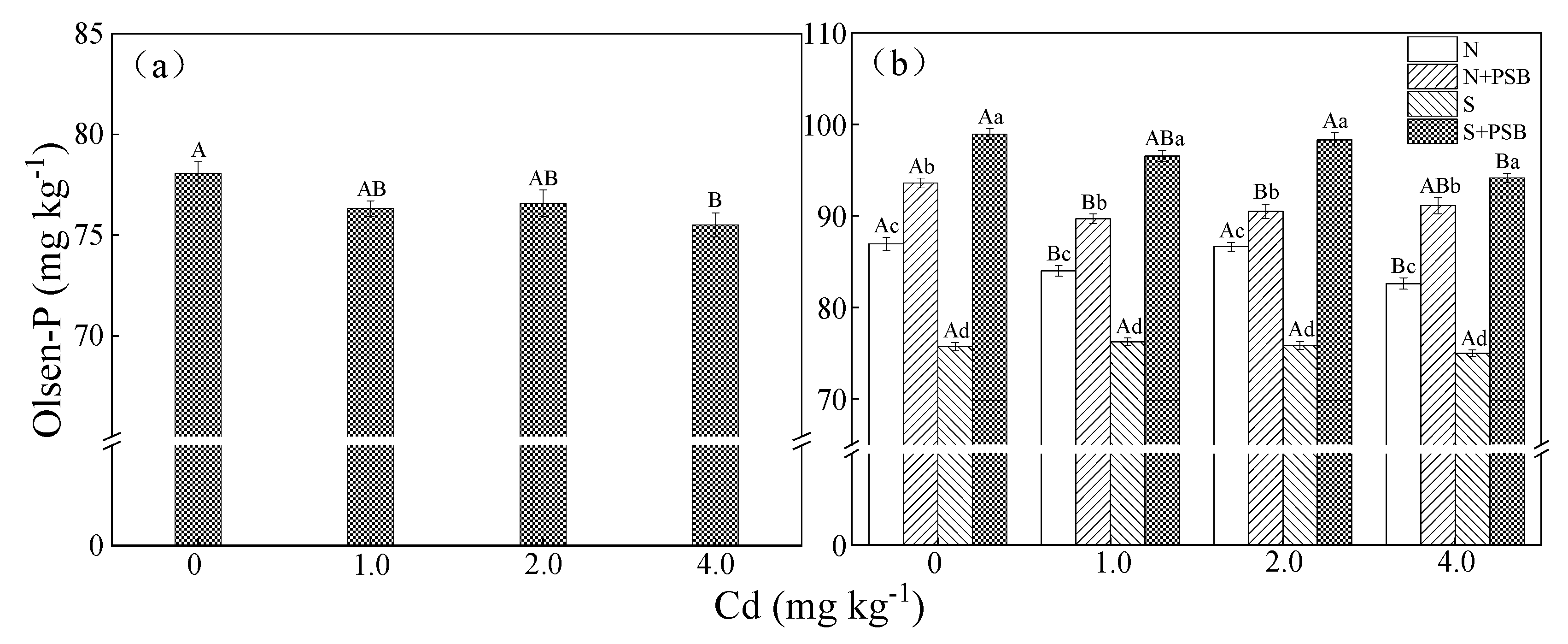
2.2. Effect of Priestia aryabhattai on Available Phosphorus in Cd-Contaminated Soil
2.3. Effect of Priestia aryabhattai on the Inorganic Phosphorus Fractions in Cd-Contaminated Soil
2.4. Effect of Priestia aryabhattai on the Organic Phosphorus Fractions in Cd-Contaminated Soil
2.5. Relationship between Soil Inorganic Phosphorus, Organic Phosphorus Fractions and Available Cd
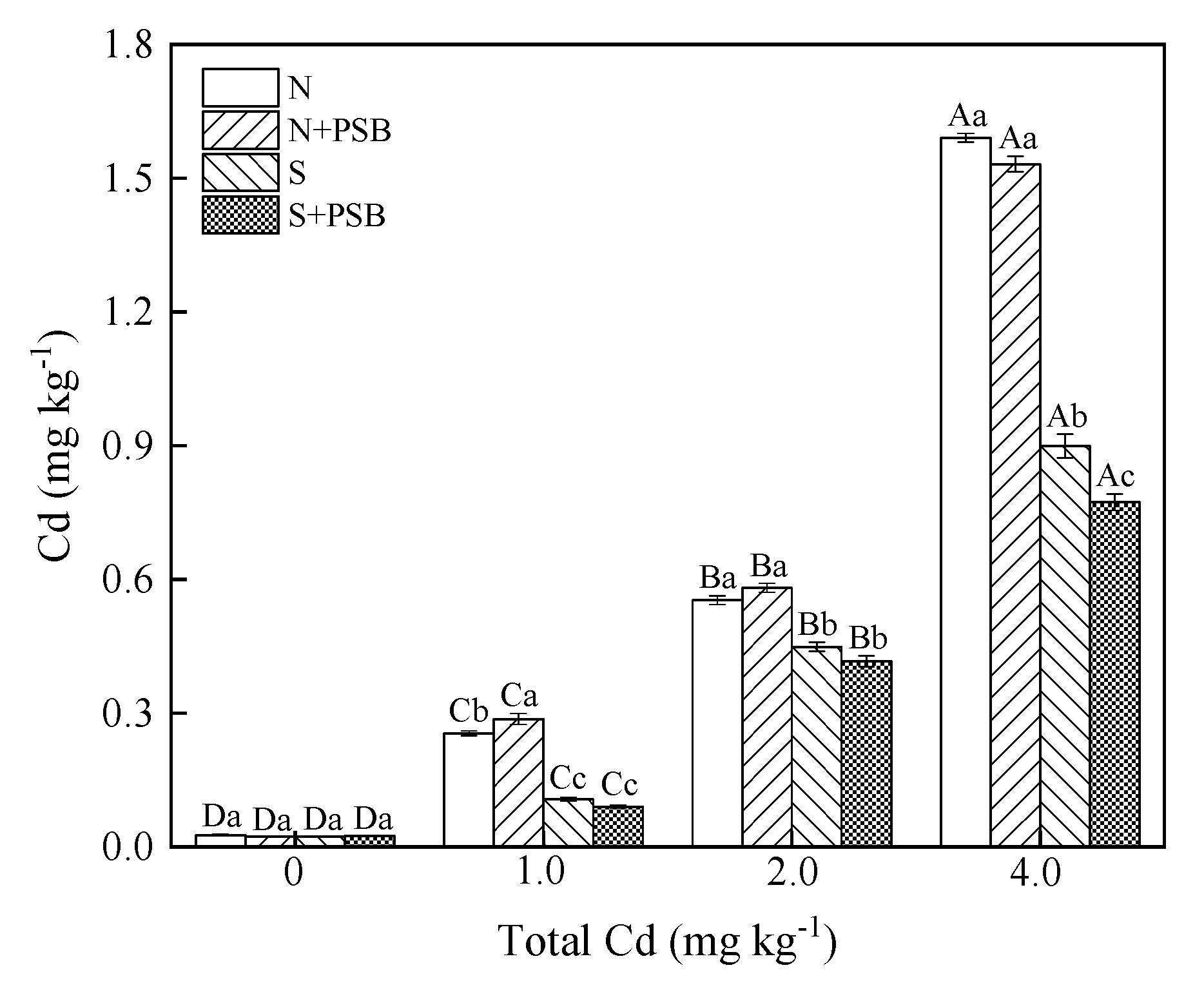
2.5.1. Effect of Priestia aryabhattai on Available Cd Content in Soil
2.5.2. Correlation Analysis between Soil Organic Phosphorus, Inorganic Phosphorus Fractions, and Available Cd
2.6. Effect of Priestia aryabhattai on Microbial Diversity and Community Composition of Soils
2.6.1. Soil Bacterial Alpha Diversity
2.6.2. Changes in Bacterial Community Composition
3. Materials and Methods
3.1. Materials
3.2. Culture Medium
- NBRIP inorganic phosphorus medium: The agar medium was made from 10.0 g of C6H12O6, 5.0 g of Ca3(PO4)2 or Hydroxyapatite, 5.0 g of MgCl2·6H2O, 0.25 g of MgSO4·7H2O, 0.20 g of KCl, 0.10 g of (NH4)2SO4, and 18 g–20 g of agar [52]. The pH of the medium ranges from 7.0 to 7.5. For the aqueous medium, 18–20 g of agar was replaced by 1000 mL of distilled water. The agar medium and aqueous medium were sterilized in an autoclave at 121 °C for 30 min.
- NBRIP organophosphorus medium: The agar medium was made from 10.0 g of C6H12O6, 5.0 g of calcium phytate, 5.0 g of MgCl2·6H2O, 0.25 g of MgSO4·7H2O, 0.20 g of KCl, 0.10 g of (NH4)2SO4, and 18–20 g of agar [52]. The pH of the medium ranges from 7.0 to 7.5. For the aqueous medium, 18–20 g of agar was replaced by 1000 mL of distilled water. The agar medium and aqueous medium were sterilized in an autoclave at 121 °C for 30 min.
- The seed growth medium was an LB medium (lysogeny broth), which was made from 10 g of peptone (biochemistry), 5.0 g of yeast plaster,10 g of NaCl, and 1000 mL of distilled water. The pH of the medium ranges from 7.2 to 7.4. The medium was sterilized in an autoclave at 121 °C for 20 min.
3.3. Soil Culture Experiment Design and Sample Collection
3.4. Measurement Methods
3.5. Analysis of Microbial Community Structure
3.6. Statistical Analysis of Data
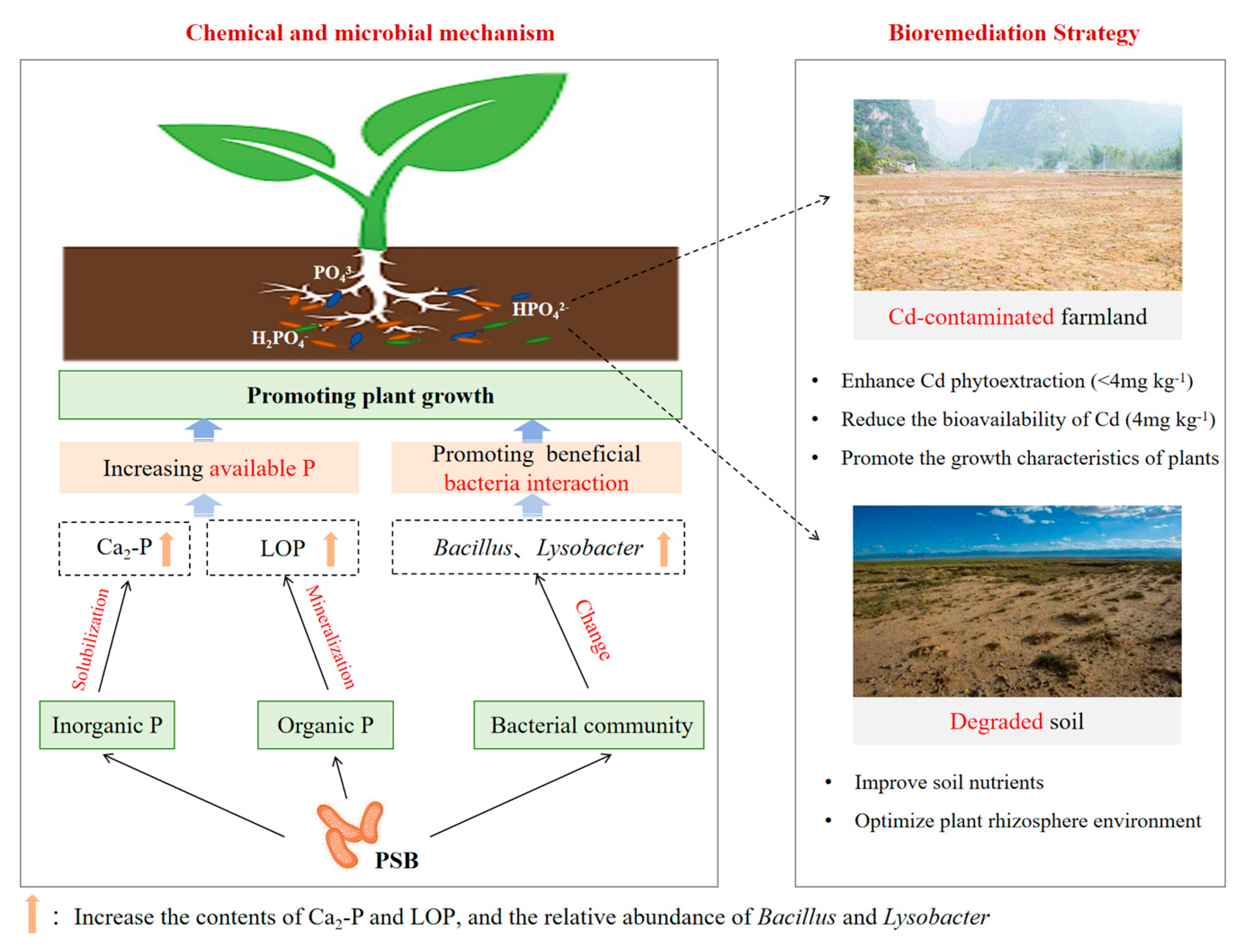
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baltas, H.; Sirin, M.; Gökbayrak, E.; Ozcelik, A.E. A case study on pollution and a human health risk assessment of heavy metals in agricultural soils around Sinop province, Turkey. Chemosphere 2019, 241, 125015. [Google Scholar] [CrossRef]
- Yuan, X.; Xue, N.D.; Han, Z.G. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef]
- Vasilachi-Mitoseru, I.C.; Stoleru, V.; Gavrilescu, M. Integrated Assessment of Pb(II) and Cu(II) Metal Ion Phytotoxicity on Medicago sativa L., Triticum aestivum L., and Zea mays L. Plants: Insights into Germination Inhibition, Seedling Development, and Ecosystem Health. Plants 2023, 12, 3754. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, P.Y.; Ye, J.P.; Zhang, G.M.; Cai, Y.J. Simultaneous in-situ remediation and fertilization of Cd-contaminated weak-alkaline farmland for wheat production. J. Environ. Manag. 2019, 250, 109528. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Nan, Z.; Hu, Y.; Chen, S.; Yang, X.; Su, J. Phosphorus supply level is more important than wheat variety in safe utilization of cadmium-contaminated calcareous soil. J. Hazard. Mater. 2022, 424, 127224. [Google Scholar] [CrossRef]
- Bilal, S.; Hazafa, A.; Ashraf, I.; Alamri, S.; Siddiqui, M.H.; Ramzan, A.; Qamar, N.; Sher, F.; Naeem, M. Biochemical and Molecular Responses Underlying the Contrasting Phosphorus Use Efficiency in Ryegrass Cultivars. Plants 2023, 12, 1224. [Google Scholar] [CrossRef]
- Ma, H.M.; Yu, X.; Yu, Q.; Wu, H.H.; Zhang, H.L.; Pang, J.Y.; Gao, Y.Z. Maize/alfalfa intercropping enhances yield and phosphorus acquisition. Field Crops Res. 2023, 303, 109136. [Google Scholar] [CrossRef]
- Su, N.; Xie, G.X.; Mao, Z.W.; Li, Q.R.; Chang, T.; Zhang, Y.P.; Peng, J.W.; Rong, X.M.; Luo, G.W. The effectiveness of eight-years phosphorus reducing inputs on double cropping paddy: Insights into productivity and soil-plant phosphorus tradeoff. Sci. Total Environ. 2023, 866, 161429. [Google Scholar] [CrossRef]
- Zhang, T.R.; Li, T.; Zhou, Z.J.; Li, Z.Q.; Zhang, S.R.; Wang, G.Y.; Xu, X.X.; Pu, Y.L.; Jia, Y.X.; Liu, X.J. Cadmium-resistant phosphate-solubilizing bacteria immobilized on phosphoric acid-ball milling modified biochar enhances soil cadmium passivation and phosphorus bioavailability. Sci. Total Environ. 2023, 877, 162812. [Google Scholar] [CrossRef]
- Duan, X.Y.; Zou, C.L.; Jiang, Y.F.; Yu, X.J. Effects of Reduced Phosphate Fertilizer and Increased Trichoderma Application on the Growth, Yield, and Quality of Pepper. Plants 2023, 12, 2998. [Google Scholar] [CrossRef]
- Qi, W.Y.; Chen, H.; Wang, Z.; Xing, S.F.; Song, C.; Yan, Z.; Wang, S.G. Biochar-immobilized Bacillus megaterium enhances Cd immobilization in soil and promotes Brassica chinensis growth. J. Hazard. Mater. 2023, 458, 131921. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Chaturvedi, P.; Begum, Z.; Pindi, P.K.; Manorama, R.; Padmanaban, D.A.; Shouche, Y.S.; Pawar, S.; Vaishampayan, P.; Dutt, C.B.S. Janibacter hoylei sp. nov. Bacillus isronensis sp. nov. and Bacillus aryabhattai sp. nov. isolated from cryotubes used for collecting air from the upper atmosphere. Int. J. Syst. Evol. Microbiol. 2009, 59, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Silva, P.; Brito, P.H.; Serrano, M.; Henriques, A.O.; Pereira-Leal, J.B. Rethinking the Niche of Upper-Atmosphere Bacteria: Draft Genome Sequences of Bacillus aryabhattai C765 and Bacillus aerophilus C772, Isolated from Rice Fields. Genome Announc. 2015, 3, e00094-15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, Y.; Zhang, Q.; Fan, H.Z.; Wang, X.Y.; Wang, J.A.; Zhou, Y.; Chen, Z.Y.; Sun, F.J.; Cui, X.Y. Saline-Alkali Soil Property Improved by the Synergistic Effects of Priestia aryabhattai JL-5, Staphylococcus pseudoxylosus XW-4, Leymus chinensis and Soil Microbiota. Int. J. Mol. Sci. 2023, 24, 7737. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.T.; Yen, J.H.; Liao, C.S.; Chen, W.C.; Chao, Y.T. Screening of Rice Endophytic Biofertilizers with Fungicide Tolerance and Plant Growth-Promoting Characteristics. Sustainability 2019, 11, 1133. [Google Scholar] [CrossRef]
- Ahmad, M.; Adil, Z.; Hussain, A.; Hussain, A.; Mumtaz, M.Z.; Jamil, M. Potential of phosphate solubilizing bacillus strains for improving growth and nutrient uptake in mungbean and maize crops. Pak. J. Agric. Sci. 2019, 56, 283–289. [Google Scholar]
- Song, C.; Wang, W.J.; Gan, Y.F.; Wang, L.F.; Chang, X.L.; Wang, Y.; Yang, W.Y. Growth Promotion Ability of Phosphate Solubilizing Bacteria from the Soybean Rhizosphere under Maize-Soybean Intercropping Systems. J. Sci. Food Agric. 2022, 102, 1430–1442. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Chen, Y.L.; Zhang, L.P.; Zhang, Y.; Wang, S.; Shi, X.; Li, L.; Liang, J.S. Effects of Phosphate Solubilizing Bacteria on the Growth, Photosynthesis, and Nutrient Uptake of Camellia oleifera Abel. Forests 2019, 10, 348. [Google Scholar] [CrossRef]
- Qin, S.M.; Zhang, H.Y.; He, Y.H.; Chen, Z.J.; Yao, L.Y.; Han, H. Improving radish phosphorus utilization efficiency and inhibiting Cd and Pb uptake by using heavy metal-immobilizing and phosphatesolubilizing bacteria. Sci. Total Environ. 2023, 868, 161685. [Google Scholar] [CrossRef]
- Adhikari, A.; Lee, K.E.; Khan, M.A.; Kang, S.M.; Adhikari, B.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.J. Effect of silicate and phosphate solubilizing rhizobacterium Enterobacter ludwigii GAK2 on Oryza sativa L. under cadmium stress. Environ. Microbiol. Biotechnol. 2020, 30, 118–126. [Google Scholar] [CrossRef]
- Wang, Z.W.; Zhang, Y.T.; Peng, J.; Zhou, F.; Yu, J.X.; Chi, R.A. Mechanisms of combined bioremediation by phosphate-solubilizing fungus and plants and its effects on cadmium contamination in phosphate-mining wastelands. J. Environ. Manag. 2023, 346, 118983. [Google Scholar] [CrossRef]
- Chidambaram, R.; Rajagopal, R.D.; Sagayaraj, I.R.; Pazhamalai, V. Plant-Microbe associations in remediation of contaminants for environmental sustainability. In Omics Insights in Environmental Bioremediation; Springer: Singapore, 2022; pp. 73–102. [Google Scholar] [CrossRef]
- Maqbool, A.; Rizwan, M.; Yasmeen, T.; Arif, M.S.; Hussain, A.; Mansha, A.; Ali, S.; Alshaya, H.; Okla, M.K. Phosphorus Fertilizers Enhance the Phytoextraction of Cadmium through Solanum nigrum L. Plants 2022, 11, 236. [Google Scholar] [CrossRef]
- Li, W.L.; Wang, J.F.; Lv, Y.; Dong, H.J.; Wang, L.L.; He, T.; Li, Q.S. Improving cadmium mobilization by phosphate-solubilizing bacteria via regulating organic acids metabolism with potassium. Chemosphere 2020, 244, 125475. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, B.; Bolan, N.S.; Wijesekara, H.; Kunhikrishnan, A.; Thangarajan, R.; Matheyarasu, R.; Rocco, C.; Kenneth, M.; Naidu, R. Phosphorus–cadmium interactions in paddy soils. Geoderma 2016, 270, 43–59. [Google Scholar] [CrossRef]
- Wang, X.R.; Ai, S.Y.; Liao, H. Deciphering Interactions between Phosphorus Status and Toxic Metal Exposure in Plants and Rhizospheres to Improve Crops Reared on Acid Soil. Cells 2023, 12, 441. [Google Scholar] [CrossRef]
- Zhang, S.S.; Liu, Y.; Cao, K.K.; Huang, Y.; Li, C.C.; Guo, X.; Hu, X.Y. Effect of Biochar on the Morphological Transformation of Phosphorus and Cadmium in Phosphorus-cadmium Enriched Soil. Environ. Sci. Technol. 2019, 42, 16–22. [Google Scholar] [CrossRef]
- Jia, H.; Lei, Y.Z.; Pan, S.Z.; Zhu, J.; Shrn, Z.T.; Tang, L.Y.; Hou, D.Y. The impacts of exogenous phosphorus on Cd absorption in perennial ryegrass root cell: Kinetic and mechanism study. Plant Physiol. Biochem. 2024, 206, 108220. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhan, Y.B.; Zhang, H.; Wang, R.H.; Tao, X.L.; Zhang, L.P.; Zuo, Y.L.; Zhang, L.; Wei, Y.Q.; Li, J. Inoculation of phosphate-solubilizing bacteria (Bacillus) regulates microbial interaction to improve phosphorus fractions mobilization during kitchen waste composting. Bioresour. Technol. 2021, 340, 125714. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, A.; Han, F.; Chen, S.G.; Zhou, W.Z. Integrated application of phosphorus-accumulating bacteria and phosphorus-solubilizing bacteria to achieve sustainable phosphorus management in saline soils. Sci. Total Environ. 2023, 885, 163971. [Google Scholar] [CrossRef]
- Khourchi, S.; Elhaissouf, W.; Loum, M.; Ibnyasser, A.; Haddine, M.; Ghani, R.; Barakat, A.; Zeroual, Y.; Rchiad, Z.; Delaplace, P.; et al. Phosphate solubilizing bacteria can significantly contribute to enhance P availability from polyphosphates and their use efficiency in wheat. Microbiol. Res. 2022, 262, 127094. [Google Scholar] [CrossRef] [PubMed]
- Zha, Q.N.; Shen, H.; Li, H.Y.; Xiong, Q.Z.; Xu, G.; Tian, D.; Li, J.L.; Gao, H.J.; Ye, X.X. High-P wheat straw increases the availability and turnover of phosphorus in lime concretion black soil. J. Plant Nutr. Fertitizer 2022, 28, 2001–2010. [Google Scholar] [CrossRef]
- Chen, H.M.; Min, F.F.; Hu, X.; Ma, D.H.; Huo, Z.L. Biochar assists phosphate solubilizing bacteria to resist combined Pb and Cd stress by promoting acid secretion and extracellular electron transfer. J. Hazard. Mater. 2023, 452, 131176. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Bonilla, G.A.; Lopes, C.M.; Durrer, A.; Alves, P.R.L.; Passaglia, N.; Cardoso, E.J.B.N. Effect of phosphate-solubilizing bacteria on phosphorus dynamics and the bacterial community during composting of sugarcane industry waste. Syst. Appl. Microbiol. 2017, 40, 308–313. [Google Scholar] [CrossRef]
- Cao, D.; Chen, W.; Yang, P.; Lan, Y.; Sun, D.Q. Spatio-temporal variabilities of soil phosphorus pool and phosphorus uptake with maize stover biochar amendment for 5 years of maize. Environ. Sci. Pollut. Res. 2020, 27, 36350–36361. [Google Scholar] [CrossRef]
- Liu, Y.H.; Neaas, A.; Zheng, Q.Y.; Hu, D.N.; Zhang, W.Y.; Zhang, M.Y. Inoculations of phosphate-solubilizing bacteria alter soil microbial community and improve phosphorus bioavailability for moso bamboo (Phyllostachys edulis) growth. Appl. Soil Ecol. 2023, 189, 104911. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2020, 21, 49–68. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.Y.; Deng, S.Q.; Liu, X.W. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Plants 2021, 10, 158. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Narayanan, M.; Shi, X.J.; Chen, X.P.; Li, Z.L.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, X.; Wang, L.; Li, Q.S.; Zhou, T.; Chen, Y.K.; Zhao, Z.Y.; He, B.Y. Effect of Phosphate-Solubilizing Bacteria on the Mobility of Insoluble Cadmium and Metabolic Analysis. Int. J. Environ. Res. Public Health 2018, 15, 1330. [Google Scholar] [CrossRef]
- Qi, X.; Xiao, S.; Chen, X.; Ali, I.; Gou, J.L.; Wang, D.; Zhu, B.; Zhu, W.K.; Shang, R.; Han, M.W. Biochar-based microbial agent reduces U and Cd accumulation in vegetables and improves rhizosphere microecology. J. Hazard. Mater. 2022, 436, 129147. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.J.; Xu, Z.N.; Sun, J.Y.; Li, D.R.; Cheng, Z.; Niu, Y.L.; Guo, H.; Zhou, J.; Wang, T.C. Microplastics affect C, N, and P cycling in natural environments: Highlighting the driver of soil hydraulic properties. J. Hazard. Mater. 2023, 459, 132326. [Google Scholar] [CrossRef]
- Dai, Z.M.; Su, W.Q.; Chen, H.H.; Barberánc, A.; Zhao, H.C.; Yu, M.J.; Yu, L.; Brookes, P.C.; Schadtb, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Rodríguez, N.; Amils, R.; Sanz, J.L. Microbial diversity in anaerobic sediments at Rio Tinto, a naturally acidic environment with a high heavy metal content. Appl. Environ. Microbiol. 2011, 77, 6085–6093. [Google Scholar] [CrossRef] [PubMed]
- Sitte, J.; Akob, D.M.; Kaufmann, C.; Finster, K.; Banerjee, D.; Burkhardt, E.M.; Kostka, J.E.; Scheinost, A.C.; Büchel, G.; Küesel, K. Microbial links between sulfate reduction and metal retention in uranium-and heavy metal-contaminated soil. Appl. Environ. Microbiol. 2010, 76, 3143–3152. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Zhao, Q.; Xie, B.X.; Lu, X.; Guo, Q.; Liu, G.X.; Zhou, M.; Tian, J.H.; Lu, W.G.; Chenn, K.; et al. Soybean (Glycine max) rhizosphere organic phosphorus recycling relies on acid phosphatase activity and specific phosphorus-mineralizing-related bacteria in phosphate deficient acidic soils. J. Integr. Agric. 2023; in press. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Luo, G.; Ling, N.; Nannipieri, P.; Chen, H.; Raza, W.; Wang, M.; Guo, S.W.; Shen, Q.R. Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fertil. Soils 2017, 53, 375–388. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Li, J.; Wang, Y.; Xu, H.; Zhao, Y. Bioreduction of hexavalent chromium using a novel strain CRB-7 immobilized on multiple materials. J. Hazard. Mater. 2019, 368, 412–420. [Google Scholar] [CrossRef]
- Teng, Z.D.; Zhao, X.; Yuan, J.J. Phosphate functionalized iron based nanomaterials coupled with phosphate solubilizing bacteria as an efficient remediation system to enhance lead passivation in soil. J. Hazard. Mater. 2021, 419, 126433. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. Fems Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Chinese Agricultural Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Wang, Q.; Duan, C.J.; Liang, H.X.; Ren, J.W.; Geng, Z.T.; Xu, C.Y. Phosphorus acquisition strategies of wheat are related to biochar types added in cadmium-contaminated soil: Evidence from soil zymography and root morphology. Sci. Total Environ. 2023, 856, 159033. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhao, R.; Yang, Y.; Zhou, Y.Y.; Zhu, Y.C.; Qin, P.F.; Wang, M.; Huang, H.L. Effect of the Combination of Phosphate-Solubilizing Bacteria with Orange Residue-Based Activator on the Phytoremediation of Cadmium by Ryegrass. Plants 2023, 12, 2727. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Esteban, J.; Escolástico, C.; Ruiz-Fernández, J.; Masaguer, A.; Moliner, A. Bioavailability and extraction of heavy metals from contaminated soil by Atriplex halimus. Environ. Exp. Bot. 2013, 88, 53–59. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Chen, L.; Wang, L.; Ji, L.; Xiao, Y. Influences of arbuscular mycorrhizae, phosphorus fertiliser and biochar on alfalfa growth, nutrient status and cadmium uptake. Ecotoxicol. Environ. Saf. 2020, 196, 110537. [Google Scholar] [CrossRef]
- Gu, Y.C.; Jiang, B.P. Method for determination of inorganic phosphorus classification in calcareous soils. Soils 1990, 22, 101–102. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. An exploratory method for factionation of organic phosphorus from grassland soils. Soil Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Fan, Y.K.; Li, S.J. A modification of Bowman-cole’fractionation method of soil organic phosphorus. Chin. J. Soil Sci. 2004, 35, 743–749. [Google Scholar] [CrossRef]



| Inorganic Phosphorus Fractions | Ca2-P | Ca8-P | Al-P | Fe-P | O-P | Ca10-P |
|---|---|---|---|---|---|---|
| phosphorus content (mg kg−1) | 31.03 ± 0.86 | 127.36 ± 3.15 | 19.68 ± 0.28 | 32.59 ± 0.66 | 116.67 ± 1.91 | 323.75 ± 4.45 |
| Total Cd (mg kg−1) | Treatment | Ca2-P | Ca8-P | Al-P | Fe-P | O-P | Ca10-P |
|---|---|---|---|---|---|---|---|
| 0 | N0 | 40.72 ± 0.83 Ab | 114.64 ± 2.44 Bc | 18.12 ± 0.50 Aa | 26.54 ± 0.46 Cb | 112.99 ± 1.98 Aa | 297.18 ± 4.44 Aa |
| N0-PSB | 48.80 ± 1.01 Aa | 110.04 ± 3.04 Ac | 15.90 ± 0.39 Ab | 27.38 ± 0.42 Bb | 112.50 ± 1.43 Aa | 287.76 ± 5.19 Ba | |
| S0 | 30.40 ± 1.33 Ac | 126.48 ± 3.63 Aa | 19.05 ± 0.39 Aa | 30.85 ± 0.51 Ba | 113.92 ± 1.26 Aa | 324.23 ± 4.98 Aa | |
| S0-PSB | 49.20 ± 0.68 Aa | 119.85 ± 2.55 Aab | 18.16 ± 0.31 Ba | 29.99 ± 0.41 Ba | 112.76 ± 2.07 a | 285.59 ± 5.53 Ba | |
| 1.0 | N1 | 41.00 ± 0.68 Ab | 115.25 ± 1.79 Bb | 16.49 ± 0.41 Bb | 27.17 ± 0.58 Cb | 113.02 ± 1.49 Aa | 296.95 ± 5.92 Ab |
| N1-PSB | 45.99 ± 0.89 Ba | 116.20 ± 2.66 Ab | 18.84 ± 0.31 Ba | 27.32 ± 0.54 Bb | 112.48 ± 1.92 Aa | 291.29 ± 7.06 ABb | |
| S1 | 29.93 ± 1.09 Ac | 132.56 ± 2.05 Aa | 19.63 ± 0.43 Aa | 30.58 ± 0.44 Ba | 113.79 ± 2.11 Aa | 326.19 ± 5.69 Aa | |
| S1-PSB | 45.25 ± 0.73 Ba | 118.79 ± 2.60 Aa | 18.73 ± 0.47 ABa | 28.19 ± 0.45 Ab | 112.63 ± 1.84 Aa | 296.93 ± 5.25 Bb | |
| 2.0 | N2 | 39.43 ± 0.43 Ab | 117.48 ± 0.86 ABb | 18.89 ± 0.37 Aa | 30.15 ± 0.46 Bb | 112.95 ± 1.38 Aa | 297.67 ± 5.74 Aa |
| N2-PSB | 44.52 ± 0.82 Ba | 118.00 ± 3.55 Ab | 18.80 ± 0.49 Ba | 30.72 ± 0.67 Ab | 112.03 ± 1.87 Aa | 298.55 ± 6.66 ABa | |
| S2 | 28.85 ± 0.92 Ac | 133.51 ± 3.36 Aa | 19.32 ± 0.37 Aa | 32.63 ± 0.39 Aa | 113.64 ± 2.09 Aa | 325.62 ± 5.60 Aa | |
| S2-PSB | 41.13 ± 0.77 Cb | 116.02 ± 2.79 Ab | 18.34 ± 0.51 Ba | 30.59 ± 0.49 Bb | 112.76 ± 1.61 Aa | 301.60 ± 6.89 ABa | |
| 4.0 | N4 | 36.93 ± 0.84 Ba | 121.81 ± 2.46 Aab | 18.43 ± 0.43 Aa | 32.39 ± 0.68 Aa | 112.50 ± 0.85 Aa | 311.54 ± 4.69 Aa |
| N4-PSB | 38.82 ± 0.42 Ca | 119.53 ± 3.04 Ab | 19.13 ± 0.42 Ba | 31.39 ± 0.61 Aab | 111.75 ± 1.21 Aa | 307.41 ± 4.90 Aa | |
| S4 | 28.18 ± 1.03 Ab | 128.87 ± 3.56 Aa | 19.40 ± 0.55 Aa | 30.34 ± 0.51 Bb | 113.83 ± 1.62 Aa | 327.90 ± 6.32 Aa | |
| S4-PSB | 37.75 ± 0.78 Da | 118.54 ± 2.64 Ab | 19.86 ± 0.40 Aa | 30.97 ± 0.45 Bab | 112.80 ± 1.75 Aa | 314.58 ± 4.31 Aab |
| Organic Phosphorus Fractions | LOP | MLOP | MROP | HROP |
|---|---|---|---|---|
| Phosphorus content (mg kg−1) | 22.43 ± 0.35 | 101.28 ± 1.28 | 18.44 ± 0.51 | 22.34 ± 0.32 |
| Total Cd (mg kg−1) | Treatment | LOP | MLOP | MROP | HROP |
|---|---|---|---|---|---|
| 0 | N0 | 32.30 ± 0.87 Ac | 86.43 ± 1.81 Bb | 17.04 ± 0.66 Ab | 22.34 ± 0.47 Bb |
| N0-PSB | 35.02 ± 1.18 Ab | 87.22 ± 1.88 Bb | 16.59 ± 0.67 Ab | 24.07 ± 0.54 Aa | |
| S0 | 22.88 ± 0.56 ABd | 103.98 ± 1.14 Aa | 19.85 ± 0.32 Aa | 22.69 ± 0.42 Bb | |
| S0-PSB | 37.55 ± 0.53 Aa | 87.12 ± 2.13 Bb | 17.29 ± 0.86 Ab | 23.00 ± 0.32 Aab | |
| 1.0 | N1 | 33.56 ± 1.48 Aa | 89.52 ± 2.12 Bb | 17.22 ± 0.65 Ab | 23.93 ± 0.46 Aab |
| N1-PSB | 34.69 ± 1.14 Aa | 88.43 ± 3.13 Bb | 17.35 ± 0.39 Ab | 23.01 ± 0.72 ABb | |
| S1 | 21.79 ± 1.23 Bb | 104.43 ± 4.17 Aa | 19.16 ± 0.51 ABa | 24.89 ± 0.46 Aa | |
| S1-PSB | 36.05 ± 1.77 Aa | 86.15 ± 1.51 Bb | 16.79 ± 0.75 Ab | 20.31 ± 0.42 Bc | |
| 2.0 | N2 | 28.74 ± 0.52 Ba | 98.48 ± 2.03 Ab | 18.28 ± 0.47 Aa | 22.21 ± 0.46 Bb |
| N2-PSB | 29.61 ± 0.57 Ba | 92.90 ± 3.31 ABb | 17.11 ± 0.62 Aa | 24.38 ± 0.47 Aa | |
| S2 | 24.79 ± 0.51 Ab | 107.65 ± 3.80 Aa | 17.67 ± 0.46 Ba | 21.11 ± 0.58 Cbc | |
| S2-PSB | 29.60 ± 0.87 Ba | 95.12 ± 3.15 Ab | 17.92 ± 0.67 Aa | 20.04 ± 0.27 Bc | |
| 4.0 | N4 | 27.53 ± 0.51 Ba | 103.12 ± 2.66 Aa | 17.55 ± 0.33 Aa | 22.16 ± 0.57 Ba |
| N4-PSB | 28.77 ± 0.34 Ba | 97.16 ± 1.98 Aa | 15.74 ± 0.59 Ab | 22.09 ± 0.53 Bb | |
| S4 | 24.46 ± 0.76 Ab | 106.28 ± 3.92 Aa | 17.76 ± 0.64 Ba | 23.05 ± 0.46 Ba | |
| S4-PSB | 29.18 ± 0.58 Ba | 102.19 ± 3.77 Aa | 18.47 ± 0.64 Aa | 22.73 ± 0.66 Aa |
| Total Cd (mg kg−1) | Treatment | Chao1 | Pielou Evenness | Shannon | Simpson |
|---|---|---|---|---|---|
| 0 | N0 | 3710.53 ± 147.37 a | 0.8695 ± 0.0011 a | 10.22 ± 0.04 a | 0.99773 ± 0.00005 a |
| N0-PSB | 3367.33 ± 111.69 a | 0.8648 ± 0.0011 a | 10.05 ± 0.02 a | 0.99722 ± 0.00009 a | |
| 1.0 | N1 | 3394.54 ± 94.37 a | 0.8651 ± 0.0003 a | 10.06 ± 0.03 a | 0.99748 ± 0.00004 a |
| N1-PSB | 3672.31 ± 74.51 a | 0.8594 ± 0.0029 a | 10.08 ± 0.02 a | 0.99705 ± 0.00027 a | |
| 2.0 | N2 | 3607.65 ± 97.56 a | 0.8704 ± 0.0015 a | 10.19 ± 0.05 a | 0.99781 ± 0.00009 a |
| N2-PSB | 3748.70 ± 25.88 a | 0.8627 ± 0.0045 a | 10.13 ± 0.05 a | 0.99719 ± 0.00056 a | |
| 4.0 | N4 | 3817.68 ± 61.15 a | 0.8733 ± 0.0027 a | 10.28 ± 0.03 a | 0.99792 ± 0.00007 a |
| N4-PSB | 3425.09 ± 193.32 a | 0.8206 ± 0.0227 b | 9.54 ± 0.32 b | 0.98690 ± 0.00580 b |
| Total Cd (mg kg−1) | 0 | 1.0 | 2.0 | 4.0 | |
|---|---|---|---|---|---|
| processing conditions | unpasteurized soil | N0 | N1 | N2 | N4 |
| unpasteurized soil + PSB | N0-PSB | N1-PSB | N2-PSB | N4-PSB | |
| sterilized soil | S0 | S1 | S2 | S4 | |
| sterilized soil + PSB | S0-PSB | S1-PSB | S2-PSB | S4-PSB |
| Projects | Numerical Value |
|---|---|
| pH | 8.67 ± 0.05 |
| Total nitrogen (g kg−1) | 1.324 ± 0.082 |
| Organic matter (g kg−1) | 6.427 ± 0.071 |
| Total phosphorus (mg kg−1) | 715.761 ± 8.930 |
| Available phosphorus (mg kg−1) | 76.828 ± 1.399 |
| Available potassium (mg kg−1) | 149.953 ± 5.648 |
| Total Cd (mg kg−1) | 0.056 ± 0.003 |
| Available Cd (mg kg−1) | 0.027 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Ning, Y.; Li, H.; Zhu, Y. Effects of Priestia aryabhattai on Phosphorus Fraction and Implications for Ecoremediating Cd-Contaminated Farmland with Plant–Microbe Technology. Plants 2024, 13, 268. https://doi.org/10.3390/plants13020268
Yang S, Ning Y, Li H, Zhu Y. Effects of Priestia aryabhattai on Phosphorus Fraction and Implications for Ecoremediating Cd-Contaminated Farmland with Plant–Microbe Technology. Plants. 2024; 13(2):268. https://doi.org/10.3390/plants13020268
Chicago/Turabian StyleYang, Shenghan, Yiru Ning, Hua Li, and Yuen Zhu. 2024. "Effects of Priestia aryabhattai on Phosphorus Fraction and Implications for Ecoremediating Cd-Contaminated Farmland with Plant–Microbe Technology" Plants 13, no. 2: 268. https://doi.org/10.3390/plants13020268





