Antioxidant and Anti-Melanogenesis Effects of Teucrium chamaedrys L. Cell Suspension Extract and Its Main Phenylethanoid Glycoside in B16-F10 Cells
Abstract
:1. Introduction
2. Results and Discussion
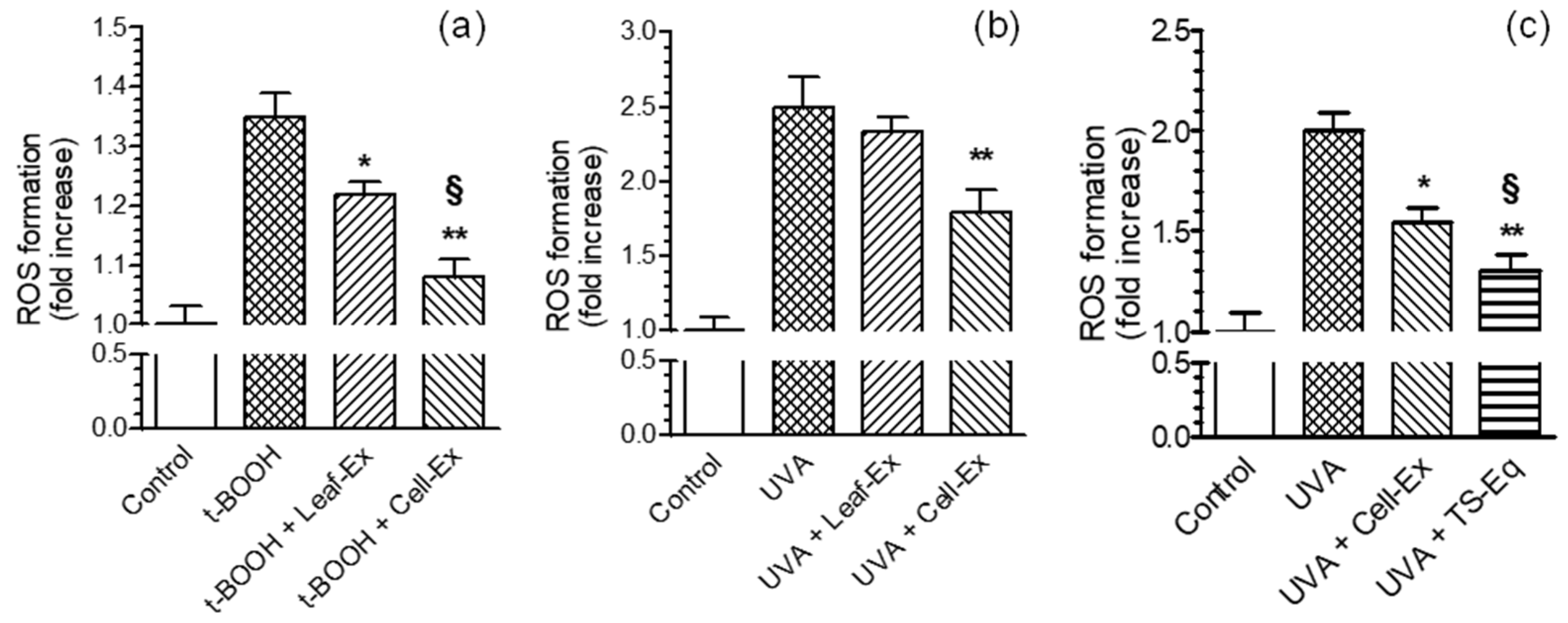
2.1. Antioxidant Activity of Leaf-Ex, Cell-Ex and TS
2.2. Anti-Melanogenesis Activity of Cell-Ex and TS
3. Materials and Methods
3.1. Chemicals
3.2. Cell Culture and Stock Solutions
3.3. Determination of Cell Viability
3.4. UVA Irradiation
3.5. Determination of Antioxidant Activity
3.6. Determination of the Melanin Levels
3.7. Determination of Cellular Tyrosinase Activity
3.8. Ultrastructural Analysis by Transmission Electron Microscopy (TEM)
3.9. Determination of Mushroom Tyrosinase Activity
3.10. Establishment of Suspension Cultures from Plant Leaves
3.11. Extract Preparation and HPLC-DAD Analysis
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frezza, C.; Venditti, A.; Matrone, G.; Serafini, I.; Foddai, S.; Bianco, A.; Serafini, M. Iridoid glycosides and polyphenolic compounds from Teucrium chamaedrys L. Nat. Prod. Res. 2018, 32, 1583–1589. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Brewer, C.; Chen, T. Hepatotoxicity of Herbal Supplements Mediated by Modulation of Cytochrome P450. Int. J. Mol. Sci. 2017, 18, 2353. [Google Scholar] [CrossRef]
- Piccolella, S.; Scognamiglio, M.; D’Abrosca, B.; Esposito, A.; Fiorentino, A.; Pacifico, S. Chemical Fractionation Joint to In-Mixture NMR Analysis for Avoiding the Hepatotoxicity of Teucrium chamaedrys L. subsp. chamaedrys. Biomolecules 2021, 11, 690. [Google Scholar] [CrossRef]
- Sticher, O.; Lahloub, M. Teucrioside, a New Phenylpropanoid Glycoside from Teucrium chamaedrys. Planta Med. 1982, 45, 157. [Google Scholar] [CrossRef]
- Gross, G.-A.; Lahloub, M.F.; Anklin, C.; Schulten, H.-R.; Sticher, O. Teucrioside, a phenylpropanoid glycoside from Teucrium chamaedrys. Phytochemistry 1988, 27, 1459–1463. [Google Scholar] [CrossRef]
- Avula, B.; Manyam, R.B.; Bedir, E.; Khan, I.A. Rapid Separation and Determination of Four Phenylpropanoid Glycosides from T. chamaedrys by Capillary Electrophoresis Method. Chromatographia 2003, 58, 751–755. [Google Scholar] [CrossRef]
- Pacifico, S.; D’Abrosca, B.; Pascarella, M.T.; Letizia, M.; Uzzo, P.; Piscopo, V.; Fiorentino, A. Antioxidant efficacy of iridoid and phenylethanoid glycosides from the medicinal plant Teucrium chamaedris in cell-free systems. Bioorg. Med. Chem. 2009, 17, 6173–6179. [Google Scholar] [CrossRef]
- Antognoni, F.; Iannello, C.; Mandrone, M.; Scognamiglio, M.; Fiorentino, A.; Giovannini, P.P.; Poli, F. Elicited Teucrium chamaedrys cell cultures produce high amounts of teucrioside, but not the hepatotoxic neo-clerodane diterpenoids. Phytochemistry 2012, 81, 50–59. [Google Scholar] [CrossRef]
- Fu, G.; Pang, H.; Wong, Y. Naturally Occurring Phenylethanoid Glycosides: Potential Leads for New Therapeutics. Curr. Med. Chem. 2008, 15, 2592–2613. [Google Scholar] [CrossRef]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef]
- Liu, M.-J.; Li, J.-X.; Guo, H.-Z.; Lee, K.-M.; Qin, L.; Chan, K.-M. The effects of verbascoside on plasma lipid peroxidation level and erythrocyte membrane fluidity during immobilization in rabbits: A time course study. Life Sci. 2003, 73, 883–892. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Shu, P.; Fei, Y.; Li, J.; Liu, A.; Zhang, L.; Niu, H.; Liu, W.; Wei, X.; Xiao, F.; Xu, Z. Two new phenylethanoid glycosides from Ginkgo biloba leaves and their tyrosinase inhibitory activities. Carbohydr. Res. 2020, 494, 108059. [Google Scholar] [CrossRef]
- Song, H.S.; Sim, S.S. Acetoside inhibits α-MSH-induced melanin production in B16 melanoma cells by inactivation of adenyl cyclase. J. Pharm. Pharmacol. 2009, 61, 1347–1351. [Google Scholar] [CrossRef]
- Dal Monte, R.; Dal Toso, R.; Minghetti, A.; Crespi Perellino, N.; Pressi, G. Extracts from Ajuga reptans Cell Lines, Their Preparation and Use. EP1736166B1, 24 September 2008. [Google Scholar]
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a Bifunctional Antioxidant Prevents and Counteracts the Oxidative Stress and Neuronal Death Induced by Amyloid Protein in SH-SY5Y Cells. Antioxidants 2020, 9, 551. [Google Scholar] [CrossRef]
- Campos, H.M.; da Costa, M.; da Silva Moreira, L.K.; da Silva Neri, H.F.; Branco da Silva, C.R.; Pruccoli, L.; dos Santos, F.C.A.; Costa, E.A.; Tarozzi, A.; Ghedini, P.C. Protective effects of chrysin against the neurotoxicity induced by aluminium: In vitro and in vivo studies. Toxicology 2022, 465, 153033. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting Effect of Kojic Acid Esters in Hyperpigmented B16F1 Melanoma Cells. J. Biomed. Biotechnol. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Masamoto, Y.; Lida, S.; Kubo, M. Inhibitory Effect of Chinese Crude Drugs on Tyrosinase. Planta Med. 1980, 40, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Harnly, J.M.; Upton, R. Comparison of the phenolic component profiles of skullcap (Scutellaria lateriflora) and germander (Teucrium canadense and T. chamaedrys), a potentially hepatotoxic adulterant. Phytochem. Anal. 2009, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, B.; Xing, S.; Chen, Y.; Liao, Q.; Mo, J.; Chen, Y.; Li, Q.; Sun, H. Medicinal Prospects of Targeting Tyrosinase: A Feature Review. Curr. Med. Chem. 2023, 30, 2638–2671. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruccoli, L.; Nicolini, B.; Lianza, M.; Teti, G.; Falconi, M.; Tarozzi, A.; Antognoni, F. Antioxidant and Anti-Melanogenesis Effects of Teucrium chamaedrys L. Cell Suspension Extract and Its Main Phenylethanoid Glycoside in B16-F10 Cells. Plants 2024, 13, 808. https://doi.org/10.3390/plants13060808
Pruccoli L, Nicolini B, Lianza M, Teti G, Falconi M, Tarozzi A, Antognoni F. Antioxidant and Anti-Melanogenesis Effects of Teucrium chamaedrys L. Cell Suspension Extract and Its Main Phenylethanoid Glycoside in B16-F10 Cells. Plants. 2024; 13(6):808. https://doi.org/10.3390/plants13060808
Chicago/Turabian StylePruccoli, Letizia, Benedetta Nicolini, Mariacaterina Lianza, Gabriella Teti, Mirella Falconi, Andrea Tarozzi, and Fabiana Antognoni. 2024. "Antioxidant and Anti-Melanogenesis Effects of Teucrium chamaedrys L. Cell Suspension Extract and Its Main Phenylethanoid Glycoside in B16-F10 Cells" Plants 13, no. 6: 808. https://doi.org/10.3390/plants13060808
APA StylePruccoli, L., Nicolini, B., Lianza, M., Teti, G., Falconi, M., Tarozzi, A., & Antognoni, F. (2024). Antioxidant and Anti-Melanogenesis Effects of Teucrium chamaedrys L. Cell Suspension Extract and Its Main Phenylethanoid Glycoside in B16-F10 Cells. Plants, 13(6), 808. https://doi.org/10.3390/plants13060808







